Treatment of newly diagnosed severe aplastic anemia in children: Evidence-based recommendations
Abstract
Severe aplastic anemia (SAA) is a rare potentially fatal hematologic disorder. Although overall outcomes with treatment are excellent, there are variations in management approach, including differences in treatment between adult and pediatric patients. Certain aspects of treatment are under active investigation in clinical trials. Because of the rarity of the disease, some pediatric hematologists may have relatively limited experience with the complex management of SAA. The following recommendations reflect an up-to-date evidence-based approach to the treatment of children with newly diagnosed SAA.
Abbreviations
-
- AA
-
- aplastic anemia
-
- AIEOP
-
- Pediatric Haemato-Oncology Italian Association
-
- ANC
-
- absolute neutrophil count
-
- ATG
-
- antithymocyte globulin
-
- CNI
-
- calcineurin inhibitor
-
- CR
-
- complete response
-
- CSA
-
- cyclosporine
-
- Cy
-
- cyclophosphamide
-
- EBMT
-
- European Group for Blood and Marrow Transplantation
-
- EFS
-
- event-free survival
-
- FFS
-
- failure-free survival
-
- Flu
-
- fludarabine
-
- GCSF
-
- granulocyte colony-stimulating factor
-
- GVHD
-
- graft versus host disease
-
- hATG
-
- horse-ATG
-
- HLA
-
- human leukocyte antigens
-
- HSCT
-
- hematopoietic stem cell transplant
-
- IST
-
- immunosuppressive therapy
-
- MDS
-
- myelodysplastic syndrome
-
- MSD
-
- matched sibling donor
-
- NAPAAC
-
- North American Pediatric Aplastic Anemia Consortium
-
- NR
-
- nonresponder
-
- OR
-
- overall response
-
- OS
-
- overall survival
-
- PIGA
-
- phosphatidylinositol glycan anchor biosynthesis class A
-
- PNH
-
- paroxysmal nocturnal hemoglobinuria
-
- PR
-
- partial response
-
- QOL
-
- quality of life
-
- rATG
-
- rabbit-ATG
-
- RCT
-
- randomized controlled trial
-
- RD
-
- refractory disease
-
- SAA
-
- severe aplastic anemia
-
- TPO
-
- thrombopoietin
-
- TPO-RA
-
- thrombopoietin receptor agonist
-
- UCB
-
- umbilical cord blood
1 INTRODUCTION
Severe aplastic anemia (SAA) is an acquired bone marrow failure disorder caused by immune-mediated destruction of hematopoietic cells. It is characterized by pancytopenia and bone marrow hypoplasia as defined using the modified Camitta criteria, without evidence of underlying inherited bone marrow failure or myelodysplastic syndrome (MDS) (Table 1).1 The North American Pediatric Aplastic Anemia Consortium (NAPAAC) recently published recommendations for evaluation of children with suspected SAA.2
| Marrow cellularity |
<25%, or 25−50% with <30% residual hematopoietic cells |
| Peripheral cytopenias (at least two of three) | |
| Absolute neutrophil count (ANC) | <0.5 × 109/La |
| Platelets | <20 × 109/L |
| Absolute Reticulocyte Count | <60 × 109/L |
- a ANC < 0.2 × 109/L defines very severe aplastic anemia (vSAA).
Aplastic anemia was first described based on the marrow findings at autopsy in a patient by pathologist Paul Ehrlich in 1888.4 Treatments with androgens were reported in the 1960s, but median survival with supportive care, steroids, and androgens was less than 6 months.5 A syngeneic transplant for aplastic anemia was first performed in 1962, and allogeneic transplants were performed starting in the early 1970s.6 Based on evidence for an immune-mediated etiology, the use of antilymphocyte globulin (ATG) was trialed in the late 1970s. Cyclosporine was reported as a successful treatment in 1984.7
Today, for pediatric patients with newly diagnosed SAA, curative treatment options include hematopoietic stem cell transplant (HSCT) and immunosuppressive therapy (IST), with excellent overall survival (OS). NAPAAC previously published survey results from member institutions regarding diagnosis and management of SAA.8 Areas of controversy included the definition of treatment failure, use of hematopoietic growth factors, and preferred rescue therapy. The Marrow Failure Study Group of the Pediatric Haemato-Oncology Italian Association (AIEOP) published diagnostic and management guidelines in 2015.9 In 2017, UK-based pediatric aplastic anemia (AA) experts amended the 2016 adult British Society of Hematology AA guidelines.10, 11 With recent changes in treatment approaches for SAA in adults, there is a need for North American pediatric-specific recommendations.12 This paper addresses the management of newly diagnosed SAA. A companion publication addresses the management of relapsed and refractory SAA.
2 METHODS
NAPAAC membership includes pediatric hematologists, hematopoietic stem cell transplanters, and hematopathologists representing 56 institutions across North America where patients are treated for bone marrow failure. A subgroup of 19 members (representing 18 institutions) of the NAPAAC Acquired AA Working Group, all with significant experience caring for patients with SAA, identified important treatment issues to be addressed, then conducted a literature review using PubMed, supplemented with additional relevant sources, using search terms pertinent to each section of the recommendations. Summary recommendations using the GRADE process were graded as “strong” or “weak,” based on “high,” “moderate,” or “low” quality evidence (Table 2).13, 14 When existing data from the literature were insufficient, recommendations were based upon available evidence in combination with expert opinion. An iterative process was used for formulation of recommendations and evidence grading until unanimous concordance amongst authors was reached. Reflecting the complexity of disease management, multiple areas where evidence is lacking, and rapidly evolving management approaches, acceptable alternative options were provided with the recommendations. Table 3 lists the summary of recommendations.
| Grade | Definition |
|---|---|
| 1A | Strong recommendation, high quality evidence |
| 1B | Strong recommendation, moderate quality evidence |
| 1C | Strong recommendation, low quality evidence |
| 2A | Weak recommendation, high quality evidence |
| 2B | Weak recommendation, moderate quality evidence |
| 2C | Weak recommendation, low quality evidence |
| Quality of evidence | Definition |
|---|---|
| High quality | Well-designed RCTs |
| Moderate quality | RCTs with limitations; large, multiple, or well-designed observational studies |
| Low quality | Few or small observational studies, case reports; expert opinion weighing risks/benefits in absence of data |
- Abbreviation: RCT, randomized controlled trial.
| Recommendation | Grade |
|---|---|
| First-line treatment | |
| We recommend the use of MSD stem cell transplant for first-line therapy for pediatric patients with SAA. | 1A |
| Until data from ongoing trials are available, we recommend IST for first-line therapy for pediatric patients with SAA without an available MSD. | 2A |
| In some clinical settings, MUD HSCT may be a reasonable alternative to IST. | 2C |
| Matched sibling donor transplant | |
| We recommend the use of MSD HSCT with a nonmyeloablative conditioning regimen. | 1B |
| We recommend the use of bone marrow over other stem cell sources. | 1B |
| We recommend the use of fresh over cryopreserved stem cell product. | 1B |
| Immunosuppressive therapy | |
| We recommend the use of hATG in combination with CSA for first-line immunosuppressive therapy. | 1A |
| rATG may be considered in settings where hATG and HSCT are not available options. | 2B |
| We recommend beginning administration of CSA on day one of ATG treatment. | 1B |
| We recommend a CSA goal trough level of 200–350 ng/mL, monitoring serum creatinine and bilirubin levels, and adjusting the dose and goal levels based on response and toxicities. | 1C |
| For patients unable to tolerate CSA, we recommend tacrolimus as an alternative. | 2C |
| We recommend use of steroids with ATG for prevention of serum sickness. | 1C |
| We recommend a short (4–14 day) course of 2 mg/kg/day steroids followed by a taper over a minimum of 2 weeks to prevent serum sickness and other ATG-related reactions. | 2C |
| Thrombopoietin receptor agonists | |
| We do not recommend the routine addition of eltrombopag to IST for pediatric patients with newly diagnosed SAA. Further research is needed to identify a sub-population who may benefit. | 2B |
| Supportive care | |
| We recommend against the routine use of GCSF. | 2A |
| Tapering calcineurin inhibitor | |
| We recommend tapering after a minimum of 6 months treatment and a CR with stable counts for 3−6 months | 1C |
| For patients who tolerate the CSA taper, we recommend tapering the daily dose at a rate of 5−15% per month, with some patients requiring a longer taper. The tapering schedule should be adjusted based on the patient's ongoing blood counts in response to the taper and CSA toxicity. | 1C |
| Paroxysmal nocturnal hemoglobinuria | |
| For patients without a MSD, we recommend IST for SAA with a PNH clone, regardless of clone size. | 2B |
| In some clinical settings, MUD HSCT may be a reasonable alternative to IST to both treat SAA and eliminate any PNH clone. | 2C |
| We recommend considering the use of complement inhibition concurrently with therapy for SAA in children with large (≥ 30%) or symptomatic PNH clone. | 2C |
- Abbreviations: ATG, anti-thymocyte globulin; CR, complete response; CSA, cyclosporine; GCSF, granulocyte colony stimulating factor; hATG, horse-ATG; HSCT, hematopoietic stem cell transplant; IST, immunosuppressive therapy; MSD, matched sibling donor; SAA, severe aplastic anemia; MUD, matched unrelated donor; rATG, rabbit-ATG; PNH, paroxysmal nocturnal hemoglobinuria.
3 FIRST-LINE TREATMENT
A retrospective analysis of children undergoing human leukocyte antigens (HLA) matched sibling donor (MSD) HSCT or IST between 1992 and 2009 showed similar OS (92 vs. 88%, no statistical difference) but with significantly better failure-free survival (FFS) in the MSD HSCT group (87 vs. 56%, p < .0001).15 The European Group for Blood and Marrow Transplantation (EBMT) similarly reported lower 3-year event-free survival (EFS) when IST was compared with MSD HSCT in both younger children (33% IST vs. 87% HSCT) as well as adolescents (64% IST vs. 83% HSCT).16, 17 Therefore, MSD SCT remains the preferred treatment for patients newly diagnosed with SAA.
- We recommend the use of MSD stem cell transplant for first-line therapy for pediatric patients with SAA (grade 1A).
- Until data from ongoing trials are available, we recommend IST for first-line therapy for pediatric patients with SAA without an available MSD (grade 2A).
- In some clinical settings, MUD HSCT may be a reasonable alternative to IST (grade 2C).
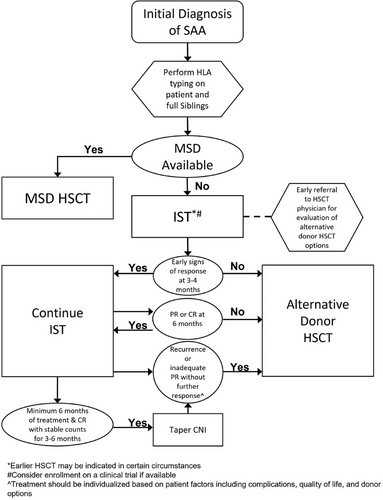
3.1 MSD transplant
Outcomes in the MSD setting are excellent with OS rates approaching 90% or greater.16, 28 Choice of conditioning regimen is dependent upon donor availability and stem cell source. In the setting of a MSD, cyclophosphamide (Cy), and antithymocyte globulin (ATG) with or without fludarabine (Flu) are the most commonly used regimens, with similar excellent outcomes (91% 5-year OS).29-31 Alemtuzumab-based conditioning regimens with Flu and Cy also have excellent OS.32
- We recommend the use of MSD HSCT with a nonmyeloablative conditioning regimen (grade 1B).
- We recommend the use of bone marrow over other stem cell sources (grade 1B).
- We recommend the use of fresh over cryopreserved stem cell product (grade 1B).
3.2 Immunosuppressive therapy
Standard IST for SAA includes ATG in combination with CSA (Table 4).
| Drug | Dose | Administration | Duration/comments |
|---|---|---|---|
| Treatment | |||
| Antithymocyte globulin (horse) | 40 mg/kg/dose every 24 h |
Initial dose: IV over 8−10 h Subsequent doses: IV over 6−8 h Rate may be slowed based on patient tolerance of infusion |
Days 1−4 Patients should be hospitalized during hATG infusions and monitored closely for infusion reactions and/or serum sickness. There is no evidence supporting a role for of intradermal skin testing prior to ATG administration. Monitor platelet count daily during ATG |
| Methylprednisolone | 1 mg/kg/dose every 12 h | IV |
Days 1−4 One dose can be given daily as hATG premedication or infused simultaneously with hATG. On Day 5, can change to PO prednisone to complete a 4−14 day course, followed by a minimum 2-week taper off. |
| Cyclosporine A |
Starting dose 5–6 mg/kg every 12 h [Lower starting dose if patient on concomitant medications metabolized through CYP3A4 pathway, e.g., azoles] |
PO |
Start on Day 1 of ATG and continue until taper indicated or starting alternative treatment (see Treatment Response section). Monitor drug levels—adjust dose for goal level 200–350 ng/mL. |
| Supportive care | |||
| Acetaminophen | 15 mg/kg/dose (650 mg max dose) | PO/IV |
Days 1−4 Give daily as hATG premedication then every 4−6 h through infusion and as needed. |
| H1 antihistamine (diphenhydramine or hydroxyzine) | 1 mg/kg/dose (50 mg max dose) | PO/IV |
Days 1−4 Give daily as hATG premedication then every 6 h through infusion and as needed. |
| GI prophylaxis with H2 blocker or proton pump inhibitor | Standard dosing | PO/IV | Start on Day 1 of ATG and continue until steroid wean completed. |
3.2.1 Antithymocyte globulin
ATG treats the autoimmune T-cell-mediated process underlying SAA. In 1978, several case reports documented efficacy of ATG in treating patients with AA, which was then followed by subsequent case series.39, 40 An RCT of patients with moderate to severe AA showed that 11 of 21 patients who received ATG had sustained improvement in hematopoiesis within 3 months of treatment, compared with no patients on the control/supportive care arm (p = .0005).41, 42 Since these initial studies, ATG has remained the backbone of IST regimens.
ATG is available in two formulations, horse- (hATG) and rabbit-derived (rATG), which have different immunosuppressive profiles.43-46 In a large prospective RCT, 120 patients aged 2–77 years were treated with either upfront hATG/CSA or rATG/CSA.47 hATG led to superior 6-month response rates (68% hATG vs. 37% rATG) and increased OS (94–96% hATG vs. 70−76% rATG). Findings from additional pediatric retrospective studies were consistent.48-50
- We recommend the use of hATG in combination with CSA for first-line IST (grade 1A).
- rATG may be considered in settings where hATG and HSCT are not available options (grade 2B).
3.2.2 Cyclosporine
Cyclosporine is a calcineurin inhibitor that suppresses T-lymphocyte activation. The combination of CSA and hATG for the treatment of AA has repeatedly been shown to be superior to treatment with ATG or CSA alone.51, 54-58 An RCT in adults and children demonstrated improvement in median time to response (60 days for hATG + CSA vs. 82 days for hATG, p = .019), overall response (OR) (65% for hATG + CSA vs. 31% for hATG, p = .011), and improved FFS (39% for hATG + CSA vs. 24% for hATG, p = .04).56 While alternative timing of cyclosporine with respect to ATG treatment has been evaluated, simultaneous administration has been effective, well tolerated, and is widely accepted.57
- We recommend beginning administration of CSA on day one of ATG treatment (grade 1B).
- We recommend a CSA goal trough level of 200–350 ng/mL, monitoring serum creatinine and bilirubin levels, and adjusting the dose and goal levels based on response and toxicities (grade 1C).
3.2.3 Cyclosporine alternatives
Tacrolimus is increasingly used instead of CSA to prevent rejection and GVHD in solid organ and HSCT.61-65 While CSA is a nonribosomal peptide that binds cyclophilins, tacrolimus is a macrolide antibiotic that binds FK-binding protein. The overall side effects of both drugs are similar, though tacrolimus treatment is associated with less gingival hyperplasia and hirsutism.
There are no published RCTs comparing CSA to tacrolimus. A single center, prospective cohort study of pediatric patients conducted between 2003 and 2008 compared eight patients receiving IST with tacrolimus to 13 historical controls receiving IST with CSA between 1990 and 2003. Tacrolimus was dosed at 0.15 mg/kg orally twice daily with no trough levels specified. Cohorts demonstrated similar complete response (CR) rates (88% for tacrolimus and 85% for CSA). Tacrolimus was well tolerated and none of the patients developed gingival hyperplasia or hirsutism. In contrast, all patients receiving CSA developed these two side effects. Data regarding renal toxicity were not collected systematically.66
Similarly, results from a single center prospective study of adult patients treated with rATG and tacrolimus for 12 months were compared with historical controls treated with rATG and CSA. Tacrolimus trough levels were 8–12 ng/mL. Thirteen subjects with a median age of 26 years (range 15−53) were enrolled. The OR and CR rates of the tacrolimus group were 84% and 53%, respectively. The OR and CR were comparable in the CSA group at 77 and 42%, respectively.67
These results suggest that tacrolimus has lower rates of hirsutism and gingival hyperplasia, but no clear benefit for other CSA-related side effects. The overall disease response for tacrolimus is noninferior to CSA. Therefore, we recommend tacrolimus as an alternative to CSA for pediatric patients with SAA unable to tolerate CSA. Further study is recommended prior to utilizing tacrolimus for first line IST therapy.
- For patients unable to tolerate CSA, we recommend tacrolimus as an alternative (grade 2C).
3.2.4 Steroids
Corticosteroids have a supportive care role in the standard IST regimen for SAA. They are used to reduce the risk of serum sickness since both rATG and hATG can cause serum sickness in 25−75% of patients and, more rarely, anaphylaxis.44, 70, 71 As a foreign protein, ATG elicits immune response in humans with immune complexes usually forming by 10 days after initiation of ATG. The most common manifestations are fever, arthralgia, malaise, rashes, lymphadenopathy, proteinuria and, in severe cases, acute renal failure.72, 73 Feng et al.44 reported serum sickness in 25% of patients treated with rATG versus 15% of those treated with hATG. A multicenter study using ATG in renal transplantation reported a 7.5% incidence of serum sickness at a median onset of 11 days, despite the administration of steroids.74
- We recommend use of steroids with ATG for prevention of serum sickness (grade 1C).
- We recommend a short (4–14 day) course of 2 mg/kg/day steroids followed by a taper over a minimum of 2 weeks to prevent serum sickness and other ATG-related reactions (grade 2C).
3.3 Thrombopoietin receptor agonists
Thrombopoietin receptor agonists (TPO-RA), eltrombopag and romiplostim, have emerged as promising therapeutic options for AA as their actions extend beyond megakaryopoiesis to affect trilineage responses.79, 80 Preclinical studies show that thrombopoietin (TPO) induces hematopoietic stem cell progenitors through binding its receptor, c-mpl.81-83
Eltrombopag is United States Food and Drug Administration approved for treatment of newly diagnosed SAA in conjunction with IST in patients over 2 years old.84 The approval was based on an NIH study, which included both pediatric and adult patients and showed a significant increase in response at 6 and 12 months for patients who received IST plus eltrombopag compared with historical controls.85 A subsequent RCT by the EBMT confirmed benefit of eltrombopag when added to hATG/CSA in patients greater than 15 years old, with significantly shorter median time to first hematologic response and CR compared with patients who received hATG/CSA alone.86, 87 EFS was higher in the eltrombopag group, although OS was not different. Toxicity, including development of somatic mutations or cytogenetic changes, was not increased by the addition of eltrombopag.
Despite these significant results in adults, the benefit of adding eltrombopag to standard IST in children with SAA is less clear. A subgroup analysis of the 40 pediatric patients (aged <18 years) in the NIH study did not show any improvement in outcomes versus historical controls for children who received eltrombopag in addition to hATG/CSA with OR at 6 months 70 versus 72% (p = .78), and 4-year EFS in responders 57 versus 69% (p = .0499).88 The discrepancy between pediatric and adult results may be due to better baseline response to IST in children. OR of pediatric historical controls was 72%, while that of adults was 58%. Additionally, there may be biologic differences in the SAA in children compared with adults that influences treatment response.89 In the EBMT study, the pediatric population (aged 15−18 years) made up only 5% of the trial population (n = 9; seven receiving IST alone and two receiving eltrombopag).
Two prospective pediatric trials evaluating the addition of eltrombopag to IST were recently completed. In a joint North American/ European multicenter single-arm phase 2 study (NCT03025698), children with de novo SAA (n = 37) treated with a combination of hATG/CSA/eltrombopag demonstrated an OR of 46% at 6 months.90 While the study was not powered for a formal statistical analysis to compare with historical controls, the OR was clearly no better than in previous extensive experience with hATG/CSA alone. Hepatotoxicity included elevated bilirubin in 43% and elevated ALT in 41% of patients with 7 patients discontinuing eltrombopag due to liver toxicity. Two of 37 patients developed new chromosomal abnormalities—one with a transient +Y at week 26 and another with a t(2;3) (p23;q12) at week 182.
A Russian RCT (NCT03413306) compared hATG/CSA with hATG/CSA/eltrombopag.91 For the primary endpoint of OR at 4 months, there was no difference between the groups (65% for eltrombopag arm vs. 53% for IST only arm; p = .28). There was a higher CR rate of 31% for the patients who received eltrombopag versus 12% for the IST only arm (p = .027) at 4 months. Three-year EFS (41% IST, 53% IST/eltrombopag; p = .326) and OS (91% IST, 89% IST/eltrombopag; p = .673), were the same for both arms, though an atypical cross-over design at 6 months makes that difficult to interpret. Two patients on the IST only arm developed monosomy 7. One patient treated with IST/eltrombopag developed a transient der(9) del(9q). Grade 3−4 hepatotoxicity was observed in 33% patients receiving IST alone compared with 61% of patients who received eltrombopag (p = .005). While eltrombopag may have benefited certain subgroups, study limitations make it difficult to draw specific conclusions.
- We do not recommend the routine addition of eltrombopag to IST for pediatric patients with newly diagnosed SAA. Further research is needed to identify a sub-population who may benefit (grade 2B).
3.4 Supportive care
3.4.1 Granulocyte colony-stimulating factor
Historically, granulocyte colony-stimulating factor (GCSF) has been used in patients with SAA.
In 2011, the EBMT published results from a RCT of 192 patients with SAA who were treated with ATG/CSA and then randomized to ±GCSF (dose of 150 mcg/m2/d). There was no difference in OS or EFS, remission, relapse rates or mortality. However, a post hoc analysis found that patients receiving GCSF had fewer infections compared with controls, especially for patients with very SAA (vSAA).92 Patients treated with GCSF also had fewer hospitalization days. Long-term follow-up published in 2020 showed no significant difference in OS, EFS, or the incidence of MDS, acute myeloid leukemia, isolated cytogenetic abnormalities, solid cancer, clinical paroxysmal nocturnal hemoglobinuria (PNH), aseptic necrosis, or relapse.92, 93
In 2014, a Chinese group published results from a RCT looking at four different regimens for pediatric SAA with IST with or without GCSF. GCSF neither contributed to early response nor reduced the infection rate, infection-related death rate, or the patients' long-term survival. There were no significant differences in OS among the four groups.94
- We recommend against the routine use of GCSF (grade 2A).
3.4.2 Other supportive care
Other supportive care issues include transfusion criteria, central line preferences, fever management, infection prophylaxis, oral hygiene, immunization practices, management of iron overload, and long-term follow-up. These are beyond the scope of this paper but will be the subject of future NAPAAC recommendations.
4 TREATMENT RESPONSE
4.1 Definition of response
Quantitative hematologic response: In 2000, Camitta95 defined CR as both transfusion independence and complete normalization for age of all three peripheral blood cell lines. Many have adapted pediatric SAA response criteria to accept lesser peripheral blood values that simply no longer meet blood count criteria of SAA.18 More recent manuscripts define CR by specific criteria for each cell line (Table 5).52 Quantitative assessment should occur off all colony-stimulating factors. Response should be sustained by two or more CBCs at least four weeks apart before assigning an IST response category.
| Transfusion status | ANC | Hemoglobin | Platelet count | |
|---|---|---|---|---|
| Complete response (CR) | Independent | ≥1 × 109/L | and ≥10 g/dL | and ≥ 100 × 109/L |
| Partial response (PR) | Independent | ≥0.5 × 109/L | and ≥8 g/dL | and ≥ 20 × 109/L |
| Nonresponder (NR) or refractory disease (RD) | Dependent | or <0.5 × 109/L | or <8 g/dL | or <20 × 109/L |
Qualitative hematologic response: The quality of a response and the effect on a patient's quality of life (QOL) are important in assessing whether additional therapy is required after up-front IST. Independence from red blood cell and platelet transfusions and immunologic recovery that allows participation in daily activities are broad response categories influencing disease morbidity.
4.2 Tapering calcineurin inhibitor
Historically, CSA was discontinued shortly after response or after 6 months of treatment.51, 55 However, disease recurrence was common with abrupt discontinuation. The NIH eltrombopag trial had high rates of relapse with discontinuation of CSA after 6 months, so was amended to continue CSA for a total of 24 months. The EBMT eltrombopag trial used CSA at fixed dosing for a minimum of 12 months followed by tapering over 12 months.85, 87 A retrospective AIEOP study of 42 children with AA treated with IST including CSA 5 mg/kg/day examined CSA tapering schedules beginning after at least 6 months of therapy and a minimum of 3 months of stable blood counts. There was a 7.6% incidence of relapse in the slow taper group (<0.3–0.7 mg/kg/month) compared with 60% in the rapid taper group (0.8 mg/kg/month), p = .001.60
The optimal duration of CSA treatment for each patient should consider duration of treatment and response. Tapering may begin after a minimum of 6 months of treatment and CR with stable counts for 3−6 months. If patients have a PR by 6 months and are continuing to have steady improvement in counts, treatment should be continued without tapering. If counts have plateaued for 3 months with a PR, HSCT donor options, side effects and toxicity of the treatment, QOL, and other goals of care should be considered prior to initiating a taper. Of note, some patients are CSA dependent to maintain blood counts and will be unable to taper off the drug.60 As long as CSA is well tolerated, it can be continued indefinitely, with consideration of the risk: benefit analysis of indefinite CSA treatment versus definitive therapy, such as HSCT.
For patients using tacrolimus in lieu of CSA, we recommend a similar tapering approach.
- We recommend tapering CSA after a minimum of 6 months treatment and a CR with stable counts for 3−6 months (grade 1C).
- For patients who tolerate the CSA taper, we recommend tapering the dose at a rate of 5−15% per month, with some patients requiring a longer taper. The tapering schedule should be adjusted based on the patient's ongoing blood counts and CSA toxicity (grade 1C).
5 PAROXYSMAL NOCTURNAL HEMOGLOBINURIA
PNH is an acquired clonal stem cell disorder with clinical manifestations of hemolytic anemia, thrombosis, and progression to acquired AA. In PNH, somatic mutation of the phosphatidylinositol glycan anchor biosynthesis class A (PIGA) gene occurs in hematopoietic stem cell subclones resulting in loss of glycosylphosphatidylinositol-anchored proteins, including CD55 and CD59, on the surface of hematopoietic cells, leading to increased complement-mediated lysis. Treatment of classical PNH with eculizumab, a monoclonal humanized antibody which inhibits C5 complement, decreases intravascular hemolysis and reduces risk of thrombosis.96-98
In a multi-institutional retrospective study of children with SAA, NAPAAC reported that 39% (55 of 140) had a detectable PNH clone.52 In this study, the majority of patients had a small clone size, with only 5 children having a PNH clone >10% (range: 12.56−28.4%).
Reports indicate that patients with SAA who have a PNH clone respond better to IST than patients without a PNH clone, regardless of clone size.99-103 There are no randomized clinical trials to compare outcomes of patients with SAA and a PNH clone who are treated with IST versus HSCT. Likewise, there are no clear recommendations for treatment of patients with a rapidly increasing PNH clone without major symptomatology. Therefore, treatment decisions are based on the clinical presentation and symptoms.
There are no published guidelines for the treatment of AA and a concomitant PNH clone or symptomatic PNH disease. No studies define a clone size cutoff that would support adding eculizumab, but rather refer to patients with a “significant clone,” generally defined as causing clinically significant hemolysis and/or thrombosis. Moderate sized clones of 30−50% present in patients with marrow failure may cause symptoms.104
- For patients without a MSD, we recommend IST for SAA with a PNH clone, regardless of clone size (grade 2B).
- In some clinical settings, MUD HSCT may be a reasonable alternative to IST to both treat SAA and eliminate any PNH clone (grade 2C).
- We recommend considering the use of complement inhibition concurrently with therapy for SAA in children with large or symptomatic PNH clone (grade 2C).
6 CONCLUSION/FUTURE DIRECTIONS
SAA is a rare hematologic disorder in children. Here, we have evaluated available evidence, bolstered by expert opinion where evidence is lacking, to provide recommendations for different aspects of treatment of newly diagnosed SAA in North American children. Individual institutional approaches must consider local expertise as well as patient-specific clinical circumstances. We advocate for pediatric clinical trials in SAA to continue improving outcomes and provide data necessary for future development of exclusively evidence-based treatment guidelines. While supportive care recommendations are beyond the scope of this paper, they are a critical component of AA treatment that we plan to address in future publications.
CONFLICT OF INTEREST STATEMENT
Research funding—Novartis: KAS. Research funding—Amgen, Inc.: AS. Research funding—Incyte, Elixirgen Therapeutics: KM. Research funding—Sanofi, Roche/Genentech/Spark,Takeda. Consulting/Medical Advisory Board—Genentech, BPL, CSL, Hema Biologics, Bayer, Octapharma: CM. Consulting fees—RocketPharma: NJG.
FUNDING INFORMATION
None.




