Extracranial germ cell tumours: Mature and immature (1990–2015). First report by the South African Association of Paediatric Haematology Oncology (SAAPHO)
Abstract
Background and objectives
Outcomes of rare paediatric teratomas have not previously been reported nor treatment regimens standardised in low- and middle-income settings. We sought to evaluate treatment outcomes of children and adolescents with histologically confirmed extracranial germ cell tumours, both mature teratomas (MT) and immature teratomas (IT) in preparation for the development of the South African national treatment guideline.
Methods
Retrospective data by folder review were collated from nine South African paediatric oncology units. Kaplan–Meier analysis with Cox regression was performed to determine 5-year overall survival (OS) and prognostic factors.
Results
From January 1990 to December 2015, 60 patients were diagnosed with MTs; 14 males (median age 2 months; interquartile range [IQR]: 0–8.75 months) and 46 females (median age 9 months; IQR: 0–88.5 months). Forty patients were diagnosed with ITs; 10 males (median age less than 1 month; IQR: 0–1.75 months) and 30 females (median age 4.5 months; IQR: 1–162 months). There were high rates of upfront surgical resections in patients with MTs (58/60; 96.6%) and ITs (36/40; 90%), and similarly satisfactory rates of complete resection in patients with both MTs (55/60; 91.7%) and ITs (32/40; 80%). The 5-year OS for the whole group was 85.4%, significantly influenced by stage: Stage I (96.9%), Stage II (100%), Stage III (38.9%) (p < .001 [MT]; p = .013 [IT]). The event-free survival (EFS) ratio for the whole cohort was 78.7%.
Conclusions
Five-year OS for those with low-stage disease was excellent, but was poorer for patients with advanced disease. The implementation of a national treatment guideline will facilitate the standardising of surgical approaches, indications for chemotherapy and specifications for follow-up to improve survival and to collect more robust late effects data.
Abbreviations
-
- AFP
-
- alpha-fetoprotein
-
- EFS
-
- event-free survival
-
- EGCT
-
- extracranial germ cell tumours
-
- IQR
-
- interquartile range
-
- IT
-
- immature teratoma
-
- MT
-
- mature teratoma
-
- OS
-
- overall survival
1 INTRODUCTION
Teratomas may contain cellular components from any of the three germ cell layers1 and are rare, with a reported incidence of 0.24/100,000.2 Their biologic behaviour is the result of a combination of patient and tumour factors, which include age, site, sex, histology and cytogenetics.3 Common sites in older children include the testis and ovary, but extragonadal sites, which predominate in infancy and early childhood, can include the chest, head and neck and brain.2-5 Teratomas can be mature, immature or malignant, containing either germ cell tumour or non-germ cell tumour elements like sarcoma or carcinoma.2 It is universally accepted that surgical resection is the definitive instrument in the management of teratomas in children and that chemotherapy is not indicated.4 A pooled analysis in adult and paediatric patients with ovarian immature teratomas (ITs) revealed that adjuvant chemotherapy did not decrease relapses in paediatric patients and that even amongst the small number who relapsed, some patients were salvaged with surgery alone.6 Some studies have shown that pure ITs are in fact chemotherapy resistant, and therefore endorse the exclusive surgical management of all ovarian ITs, irrespective of age, given the occurrence of severe toxicity in those patients who receive chemotherapy, irrespective of the type of regimen. The study recommended the extension of these indications to adults where chemotherapy administration for higher grade and stage tumours are still common practice.7 There are also reports to suggest that the administration of chemotherapy in ITs may in fact be deleterious and precipitate growing teratoma syndrome.8
Although these broad principles have been followed in individual institutions in South Africa, nationally there has been no standard approach, particularly with respect to fertility-sparing surgery, the indications for chemotherapy and the use of standardised chemotherapy regimens. Instead, chemotherapy regimens have traditionally been chosen based on institutional preference. Our objective with this study was to review our national practice ahead of the development and implementation of a new national treatment guideline.
2 METHODS
2.1 Patients
We undertook a retrospective folder review of all children from birth up to and including 16 years of age with histologically confirmed mature teratoma (MT) and IT from nine paediatric oncology units (POUs) across South Africa in the public and private sector. Our ability to collect detailed data was limited by the retrospective nature of the study over an extended period of time. Histologically MTs9 were considered to be those with a mixture of mature/differentiated tissues from all three germ layers, whilst ITs10 were defined as those with variable amounts of immature/embryonal tissue, and were graded 1, 2 or 3 depending on the amount of immature neural tissue present. In the earlier part of the time period, grade was almost never assigned, leaving only a small minority of patients assigned with the relevant grade toward the latter end of the time period. Patients with teratomas containing malignant non-germ cell tumour elements were excluded. None of the remaining patients with ITs were reported to have malignant germ cell tumour elements present. Tumour marker elevations were checked for diagnostic significance using standard nomograms.2 All patients with ITs with elevated levels of beta-human chorionic gonadotropin (β-HCG) and alpha-fetoprotein (AFP) for age were excluded on suspicion that they may represent mixed germ cell tumours. Patients with metastatic disease were excluded under the same presumption. Deidentified demographic and presentation data included age, sex, nutritional status assessed by anthropometry, socio-economic status (SES) measured by annual household income, symptom profiles, co-morbidities, tumour stage, site and histology and tumour markers. Staging was carried out pre-operatively with the use of computed tomography or magnetic resonance imaging in conjunction with elevated tumour markers and/or a tissue biopsy in patients where a safe upfront resection was not possible. In patients with resectable tumours, post-operative notes in conjunction with pathology reports were used to assess microscopic or macroscopic residual disease, as it impacted stage in the context of a multidisciplinary discussion forum. Treatment-related and outcome data included timing and extent of tumour resections, chemotherapy regimens (neo-adjuvant and adjuvant) where relevant, the number of courses of chemotherapy, the use of radiotherapy, medical and surgical treatment-related sequelae and interventions, the nature of follow-up and disease outcomes. Lost to follow-up was defined as patients who did not return for consultations prior to 5 years from the end of treatment, and treatment abandonment was defined as failure to complete potentially curative therapy. Nutritional parameters were defined according to the WHO Nutritional Landscape Information System (NLIS) Interpretation Guide, 2010.11 SES was assigned using the Western Cape Government's Federal Acquisition Regulation No. 9 of 2017. Given the retrospective nature of the study, consent was waivered.
2.2 Statistical analysis
Stata Statistical Software v. 14 (StataCorp) was used for consolidation, uniform recording and descriptive statistics. Survival analyses and output graphics were conducted with R Statistical Environment v. 4.1.2 (R Core Team). The Kaplan–Meier procedure was used to estimate overall survival (OS) curves and survival at specific time points (1, 2 and 5 years), with associated 95% confidence intervals.12, 13 The log-rank test was applied to assess the existence of significant differences between survival curves in different subgroups. Multivariate Cox regression models were used to determine the association of the OS and event-free survival (EFS) relative to a series of potential prognostic factors.14 The proportional hazards assumption was computed using the scaled Schoenfeld Residuals test.15 A p-value less than 5% was used as a cut-off to define statistical significance.
2.3 Ethical approval
Ethical approval was obtained from the Human Research Ethics Committee of the University of Cape Town (HREC 002/2018) with reciprocal approval obtained from all participating centres nationally.
3 RESULTS
3.1 Demographics
There were 60 patients with MT and 40 with IT eligible for analysis. The median age in the MT group was 7 months (interquartile range [IQR]: 0–74.5 months). Male patients (n = 14) presented at a younger median age of 2 months (IQR: 0−8.75 months) compared to female patients (n = 46) at 9 months (IQR: 0–88.5 months). Similarly, male patients (n = 10) with ITs presented at a younger median age of less than 1 month (IQR: 0–1.75 months) compared to female patients (n = 30) at 4.5 months (IQR: 0–162months).
3.2 Tumour site
In the combined group, 31 patients presented with ovarian primaries and the remaining 69 were all extragonadal: 30 sacrococcygeal, 19 in the head and neck, 11 retroperitoneal (abdomen and pelvis) and nine in the mediastinum. Table 1 shows the disaggregation by histological subgroup.
| Site |
MTs (n = 60) |
ITs (n = 40) |
Total (n = 100) |
|---|---|---|---|
| Ovarian | 20 | 11 | 31 |
| Testicular | 0 | 0 | 0 |
| Extragonadal | 40 | 29 | 69 |
| Extragonadal | |||
| Sacrococcygeal | 21 | 9 | 30 |
| Head and neck | 8 | 11 | 19 |
| Retroperitoneal (abdomen/pelvis) | 7 | 4 | 11 |
| Mediastinum | 4 | 5 | 9 |
3.3 Stage
Stage 1 patients comprised the majority of MTs (n = 55; 91.6%) and 75% (n = 30) of the ITs. Table 2 shows the distribution of patients by stage in each group (Table 2).
| Stage | MTs (n = 60) | ITs (n = 40) |
|---|---|---|
| 1 | 55 (91.6%) | 30 (75.0%) |
| 2 | 2 (3.3%) | 4 (10.0%) |
| 3 | 3 (5.0%) | 6 (15.0%) |
3.4 Tumour markers
AFP was the most commonly elevated documented tumour marker at diagnosis in 55 (27 MTs and 28 ITs) patients. All patients had levels that plotted within normal ranges according to the standard age nomograms.
3.5 Treatment interventions and outcomes
3.5.1 Mature teratomas
Upfront resection was performed in 58/60 (96.6%) patients. Complete resections were achieved in 55/60 (91.7%) patients and four had partial resections, one a neonate with a head and neck primary. One patient had an inoperable sacrococcygeal tumour.
Two patients received JEb (carboplatin, etoposide, bleomycin) chemotherapy, one was lost to follow-up and one is a survivor.
Four patients died: two from surgical complications, the first from an intra-operative vascular transection. The second patient presented with a massive right flank mass, which filled the right hemi-abdomen adherent to the right kidney and liver and developed post-operative chylous ascites and fungal septicaemia. The third patient with the inoperable sacrococcygeal tumour presented with critical pelviureteric junction obstruction and post-obstructive acute kidney injury, and died as a result of an extended beta-lactam Gram-negative septicaemia. The final patient died from an unknown cause not related to treatment.
3.5.2 Immature teratomas
Upfront resection was performed in 36/40 (90%) patients. Complete resections were achieved in 32/40 (80%) patients. Six patients had partial resections: five who received chemotherapy are survivors and one died from post-operative wound sepsis and anaemia. Two patients never came to surgery and died: the first a 3-year-old with a Stage 3 mediastinal tumour who was treated with chemotherapy alone, and the second a preterm infant with a head and neck primary who died from airway compromise. Fifteen patients received chemotherapy, of whom three died, two with Stage 3 disease, with a head and neck and mediastinal primary, respectively, and a third from an unrelated cause. Amongst the eight patients who received cisplatin-based regimens (one PEb [cisplatin, etoposide, bleomycin] and seven BEP [bleomycin, etoposide, cisplatin]), complications included hearing loss,1 renal impairment1 and Fanconi syndrome.1 There were no chemotherapy-related deaths.
No information was available regarding ovarian-sparing surgery in patients with ovarian primaries in either subgroup.
3.6 Follow-up
3.6.1 Mature teratomas
Eleven patients had no documented follow-up: one patient suffered an early death, and four were lost and therefore could not be followed, whilst among the remaining eight, six came from households with the lowest income of less than USD 6600 per annum. Forty-nine (81.6%) patients with MTs had documented follow-up: 24 with a clinical examination and serum markers, and 25 with an examination, markers and radiological evaluation. There were 20/60 patients (33.3%) lost to follow-up: 18/20 (90%) were from the poorest socio-economic strata and 13/18 (72.2%) were female. Only two medically insured patients from the whole cohort did not return for follow-up.
3.6.2 Immature teratomas
Five patients had no documented follow-up: two suffered early deaths and one was lost early, with the remaining two both from poor socio-economic conditions. Thirty-six (90%) patients with ITs had documented follow-up: 16 with a clinical examination and serum markers and 20 with an examination, markers and radiological evaluation. There were six of 40 patients (15%) lost to follow-up: four of six were from the poorest socio-economic groups and three of four were female (see Table S2).
There were no documented pregnancies amongst female patients in the follow-up period in either subgroup.
3.6.3 Outcomes
None of the patients with MTs died of disease. There were three deaths from toxicity, and one death unrelated to treatment. Four patients with ITs died. There were no chemotherapy-related deaths in this group, and one death unrelated to treatment.
3.7 Statistical analysis
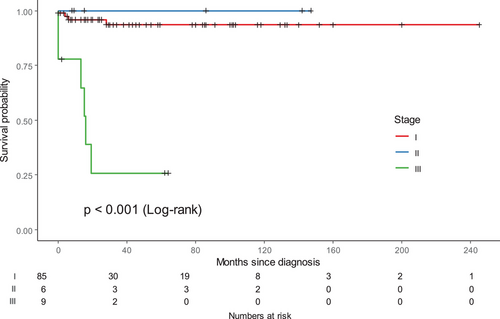
Age, sex, nutritional status, SES and tumour site did not impact outcome in a significant way. Stage was highly predictive of outcome, both on univariate and multivariate analysis for both groups (MT p < .001 and IT p = .013), particularly for patients with Stage 3 disease (p < .001). Patients with Stage 1 disease had a significantly higher chance of survival compared to those with Stage 2 and 3 disease (HR 19.32; p = .0031) (Table S1). The EFS by stage for MTs and ITs is shown in Figure 1 (p < .001). The EFS for MTs (p < .001) and ITs (p = .013) by stage disaggregated by group are shown in Figures S1 and S2.
3.8 Outcomes
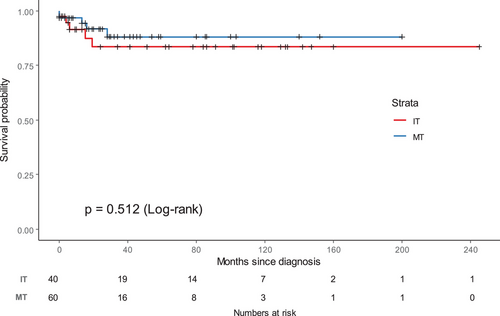
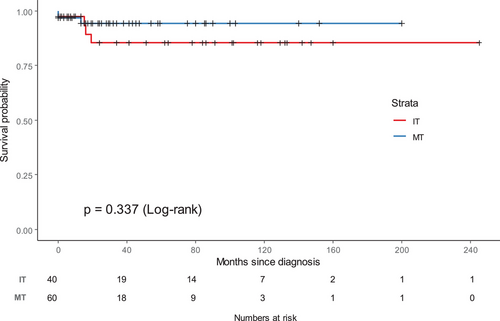
The 5-year estimated OS ratio for the whole group was 85.4% (95% CI: 79.1%–92.2%), and the 5-year EFS ratio for the whole group was 78.7% (95% CI: 71.1%–87.0%). The 5-year OS for patients with MTs was 80.8% (95% CI:71.9%–90.9%), while the EFS for patients with MTs was 74.0% (95% CI: 64.1%–85.5%). The 5-year OS for those with ITs was 92.8% (95% CI: 86.1%–100%) (p = .151), while the EFS for those with ITs was 86.7% (95% CI: 76.9%–97.9%) (p = .115). There was no significant difference in OS (p = .337) (Figure 2) or EFS (p = .512) (Figure 3) between MTs and ITs.
4 DISCUSSION
In this study we describe favourable outcomes in South African children with teratomas with a commendable 5-year OS of 85.4%. Additionally, only disease stage was found to be independently predictive of OS (p < .001). This aligns with findings from larger patient series that report the predictive value of stage on outcome in children and adults with ovarian ITs. As is the case in our cohort, the study showed that higher stage was associated with a poorer outcome.16
In our cohort, the M:F ratio was 1:3.2. Female patients comprised 76.7% (76/100) of the total. These high numbers may be explained by the high number of ovarian tumours (n = 31/100; 31%) as well as the much higher proportion of female patients with sacrococcygeal primaries (n = 21) compared to males (n = 9) (M:F = 1:2.3). Mann et al. reported a M:F ratio of 1:3.4 and found similarly that the high proportion of females in their cohort could be explained by higher numbers of patients with ovarian tumours and the preponderance of female patients in those with sacrococcygeal teratomas during infancy.4 An Italian study reported similar findings in a cohort of MTs but not in ITs, where in the former group there were twice as many ovarian tumours and sacrococcygeal tumours compared to the latter (45 vs. 19).5
Proportionately, we had higher numbers of extragonadal tumours 69/100 (69%). This is higher than those reported in well-resourced countries. A study from the United Kingdom4 reported that 47.8% of tumours arose at extragonadal sites. Similarly, an Italian group reported that extragonadal tumours comprised 53% of all teratomas in their series.5 The discrepancy is difficult to explain but may be due to the fact that gonadal tumours are diagnosed sooner, as masses at extragonadal sites may initially be ascribed to other pathologies.
Complete resection has also been shown to be a critical factor influencing survival.2, 4 In our study, rates of local surgical control were excellent in both groups with high rates of upfront resections. This is in contrast to other studies where the proportion of incomplete resections has been much higher.4 Unfortunately, no data were captured regards to ovarian-sparing procedures in children with ovarian primaries as a mechanism to safeguard fertility. A Polish study revealed low rates of ovarian-sparing surgery in the first 4 years of a 17-year retrospective study period, with rates increasingly above 40% in the latter part of the study. The authors suggested that in certain conditions, preservation of ovarian tissue could be considered in cases of localised MT, where clear planes of separation could be appreciated between tumour and normal ovarian tissue in which the suspicion of malignancy was low and in settings with access to a surgeon experienced in minimally invasive, laparoscopic surgery.17 This is an area worthy of interrogation in the prospective arm of the new South African guideline.
We recognise that mortality in patients with benign extracranial germ cell tumours (EGCT) is uncommon and that the use of chemotherapy in selected patients in this historical cohort was controversial in both groups, given that surgery is the definitive instrument and because it could be argued that over-treatment may have resulted in undue toxicity and additional avoidable costs to the patient and healthcare system. The use of chemotherapy reported in this cohort highlights the intersection of historical non-standardised practice and the structural, socio-economic and cultural barriers to treatment in low- and middle-income settings.
Although documented follow-up in both groups was good (84%), it still concerning that 33.3% of MTs were lost to follow-up compared to 15% in the IT group, probably reflecting less concern for relapse in patients with MTs. Interestingly, children and adolescents with resected mature cystic teratomas from a well-resourced setting reported almost identical rates (65%) of available follow-up data in their patients, although it is not specifically stated that the deficit was due to lost patients. Unlike our cohort, they reported seven pregnancies in their follow-up period, but their patient cohort had a much higher median age (16 years) compared to ours (2.7 years) and a median time of surgery to pregnancy of 70 months, which probably explains these differences.18 In our cohort in both patients with MTs and ITs, there was a concerning trend suggesting that patients with lower SES and female sex were more likely to be lost to follow-up.
4.1 Limitations
We acknowledge the limitations of this retrospective review inherent with the idiosyncrasies of local and often disparate historical, institutional decision-making in terms of pathology reporting, access to specialised surgery and the use of chemotherapy. Additionally, central histology review in our setting was not possible or available historically. Interpretation of data was made more challenging by differences in laboratory assessments of disease markers, differing timing and modalities of disease response assessments and the lack of clear details with respect to fertility-sparing surgery in those with ovarian tumours. The length of the review period is also complicated by the changes in definitions of disease and the evolving approaches to management over time, which may have generated inconsistencies in practice. We recognise that divergent practice is a reality of historical cohorts, but this only emphasises the necessity for a cohesive new national treatment approach as an imperative to improve survival going forward.
4.2 Implications for practice
This retrospective study is part of a larger project to harmonise treatment guidelines for children with malignant and benign EGCT in South Africa with the aim of improving survival rates by standardising risk stratification, surgical approaches and indications for chemotherapy. The guideline also provides an opportunity to identify and correct systemic inconsistencies in practice. To that end, central pathology review has been built in as a critical fall back to ensure accurate diagnosis and as a mechanism to standardise pathology reporting between centres. It also seeks to build increasingly collaborative networks whose components adhere to best practice surgical guidelines, and limit the administration of potentially harmful chemotherapy, often as a reflexive strategy in an environment where systemic delays in services and resource limitations cause worrying hiatuses in care. Appropriate treatment is built on accurate diagnosis, and multidisciplinary team meetings continue to play a critical role in the diagnostic and therapeutic evaluation of patients. Collaborative, well-communicated working plans, which include clinical reviews by both oncology and surgical staff, cast the foundations of integrated inter-disciplinary care, which ultimately serves the patient. The early identification of pre- and post-operative complications is the desirable endpoint of a system that blends continuous in-service training and heightened surveillance with shared responsibility. These inter-disciplinary assessments should happen as a matter of course, whether electively scheduled or done post-operatively in the case of children who require emergency surgical intervention. The ambulatory setting post discharge provides clinicians (oncologists and surgeons) with another opportunity to consolidate clinical reviews, contextualise pathology results for the patient and caregivers and to craft a plan for follow-up.
Consequently, the guideline also seeks to create more parity in structured follow-up, particularly for the children from low-income households where we found the attrition rates to be higher, evidenced by the fact that 84.6% of patients who were lost to follow-up were from low socio-economic households. This is aggravated by the ongoing lack of consensus in defining adult versus paediatric age cut-offs for care, inconsistencies in or the absence of supported transitional care for children into the adult service and the absence or minimal availability of combined adult-paediatric clinics. All of these factors conjointly increase the chances that patients are lost (especially the most socio-economically vulnerable), and prevent us from collecting reliable long-term survival and late effects fertility data. Instituting a national guideline creates an opportunity to bring allied disciplines like registry services, social workers and gynaecology and endocrine services into the fold and to develop new spaces that have historically been in deficit.
5 CONCLUSION
Given the trends seen in other related single-disease cohorts from South Africa, we recommend that it engages a discussion about formalising financial support for indigent families as a mandatory component to facilitate improved outcomes in economically vulnerable children.19 We advise more vigorous advocacy at a national level by the South African Association of Paediatric Haematology Oncology (SAAPHO) with the support of invested and independently funded parent support organisations to support access to national treatment guidelines at accredited centres in both the public and private sectors, especially in support of children from the most vulnerable sections of our society.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Professor David Reynders and Ms Judy Schoeman of the South African Children's Tumour Registry and Mrs Felicity Douglas from the Red Cross War Memorial Children's Hospital in Cape Town. Thanks to the staff from Paediatric Haematology-Oncology, Department of Paediatrics and Child Health, Pietermaritzburg Metropolitan Hospital Complex, University of Kwa-Zulu-Natal, Pietermaritzburg, South Africa for their participation. This work is funded by the National Research foundation (CSUR180429324830), the Harry Crossley Foundation at the University of Cape Town and the Professor Bongani Mayosi Netcare Clinical Scholarship.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data restricted due to ethical considerations, but reasonable requests will be considered by the corresponding author and only with the express consent of the South African Association of Paediatric Haematology Oncology (SAAPHO).




