Wilms tumor treatment protocol compliance and the influence on outcomes for children in Tanzania
Abstract
Background
Standardized Wilms tumor treatment protocols exist for low- and middle-income countries, but outcomes equivalent to high-income countries are not achieved outside of clinical trials. As Wilms tumor treatment protocols in Africa shift with increasing resource capacity, it is not known how treatment compliance to each stage of therapy affects outcomes and where the critical breakpoints are for protocol adherence in clinical practice.
Procedure
We describe both treatment outcomes and treatment protocol adherence in a retrospective single-center cohort study of pediatric Wilms tumor patients at a zonal cancer referral hospital in Tanzania from 2016 to 2019, treated per the International Society of Paediatric Oncology standard (2016–2017) or Tanzania adapted (2018–2019) therapy protocols.
Results
A total of 69 patients were evaluated. The two-year overall survival and event-free survival rates were 40% and 29%, respectively. Only 29% of patients completed recommended chemotherapy per protocol, and completion of preoperative and postoperative chemotherapy was predictive of two-year overall survival (odds ratio [OR] 14.4, p < .001). There were delays at almost every stage of treatment, especially time from preoperative chemotherapy to surgery (56 days), from surgery to pathology report (30 days), and from surgery to initiation of postoperative chemotherapy (38 days).
Conclusions
Nonadherence with recommended Wilms tumor treatment guidelines due to key health system delays correlated to reduced overall survival rates, with chemotherapy nonadherence due to abandonment, lack of surgery, and deaths on therapy as the strongest contributors. Future interventions targeting health system delays and reducing deaths during therapy are critical to improving protocol compliance and increasing overall survival for pediatric Wilms tumor patients in low-resource settings.
Abbreviations
-
- BMC
-
- Bugando Medical Centre
-
- IQR
-
- interquartile range
-
- LMICs
-
- low- and middle-income countries
-
- SIOP-WT
-
- International Society of Paediatric Oncology—Wilms Tumor
1 INTRODUCTION
Wilms tumor is the most common type of renal malignancy in pediatrics globally.1, 2 The majority of children diagnosed with Wilms tumor live in low- and middle-income countries (LMICs) where survival rates are often below 50%, compared to the over 90% survival rate seen in high-income countries.3-6 This is largely due to patients presenting later with more advanced disease, as well as problems with poor nutrition, difficulty completing treatment, and facility limitations, such as inability to fully support patients through treatment-related complications.4, 7
The International Society of Paediatric Oncology (SIOP) provides guidelines for the staging and treatment of Wilms tumor (SIOP-WT), and outside of North America, it is used as standard-of-care therapy.8 In Southern and Eastern European countries, registries totaling 300 Wilms tumor patients almost entirely on SIOP-based protocols demonstrated an 85% five-year event-free survival (EFS) rate and a 90% five-year overall survival (OS) rate.9 The protocol was adapted for use in low-income settings and evaluated in 201 patients with Wilms tumor in a multisite collaborative clinical trial across six sub-Saharan African medical centers.5, 10 The implementation of the adapted protocol resulted in an increase in end-of-treatment survival from 52% to 68%.11 These improved outcomes are mostly isolated to clinical trial settings, as usual care in the community outcomes are often affected by reduced patient support and poor protocol compliance.12
In Tanzania, the national pediatric hospital treated patients with Wilms tumor using a modified version of the Wilms tumor collaborative adapted protocol, increasing the postoperative chemotherapy course from 15 weeks to the standard SIOP-WT protocol of 27 weeks. A review of 73 patients treated with this protocol reported a 35% 18-month EFS rate, with 36% who relapsed or died, 19% abandoned, and 10% lost to follow-up.13 This is lower than the 46% two-year EFS rate reported in Malawi, a relatively resource-limited country compared to Tanzania, during the Wilms tumor collaborative clinical trial where there was more robust follow-up with only four patients lost to follow-up at two years (5%).6 It is unknown if the reduced outcomes are due to barriers of protocol implementation and reduced compliance outside of a supported clinical trial, or due to the modified duration of therapy.14
In the current study, we describe both treatment outcomes and treatment protocol compliance in a retrospective single-center cohort study of pediatric Wilms tumor patients at a zonal cancer referral hospital in Tanzania from 2016 to 2019. We further evaluated the timeliness of care at critical treatment decision points to better understand barriers to protocol compliance outside of clinical trials that can be targeted for future protocol implementation in lower resource hospital settings.
2 METHODS
2.1 Study design, setting, and sample
This retrospective cohort study included all patients under 18 years of age diagnosed with Wilms tumor at Bugando Medical Centre (BMC), the zonal referral hospital in northwest Tanzania, between January 2016 and April 2019. They were identified using hospital-based cancer registry and clinical records.
2.2 Treatment protocol
Wilms tumor diagnosis and staging were based on imaging using abdominal ultrasound and chest x-ray. Patients were treated per previously published SIOP-based Wilms tumor collaborative (2016–2017) or adapted Tanzania (2018–2019) protocols, including preoperative chemotherapy, followed by postoperative stage- and risk-stratified chemotherapy, with radiation as indicated.7, 10, 14, 15 Postoperative staging was defined per SIOP guidelines, which includes tumor location and intraoperative spillage.16
2.3 Clinical characteristics
Demographic information of age, sex, district, and region were obtained. Clinical characteristics obtained through chart review included if patient had metastatic disease, the number of preoperative chemotherapy weeks received, surgical complications (tumor spillage), postoperative staging, histology risk category, weeks of postoperative chemotherapy completed, and radiotherapy indication and compliance.
2.4 Process outcomes
To evaluate the diagnostic and treatment process, the number of days that elapsed between key milestones were calculated, and the ideal elapsed time was included in parentheses. These included days from presentation at BMC to diagnosis (14 days), from diagnosis to first day of preoperative chemotherapy treatment (7 days), from first day of chemotherapy treatment to surgery date (14 days from last preoperative chemotherapy infusion date), from surgery to pathology results (14 days), and from surgery to initiation of postoperative chemotherapy (7 days).
Preoperative and postoperative chemotherapy protocol adherence was determined based on the number of weeks given compared to recommended weeks per SIOP-WT protocol. Preoperative chemotherapy was adherent to protocol if 4-week long for patients without metastasis or if 9-week long for patients with metastasis or bilateral disease. Postoperative chemotherapy was adherent to protocol if 15-week long for patients treated from 2016 to 2017 or if 27-week long for patients treated from 2018 to 2019 due to changes in treatment guidelines. Major deviations from protocol were defined as more than 20% difference in the intended number of weeks of preoperative or postoperative chemotherapy. Radiotherapy adherence was determined if radiotherapy was given when indicated. Surgical quality was assessed based on whether intraoperative tumor spillage occurred.
2.5 Survival outcomes
OS outcome was categorized as alive, deceased, or unknown. For calculated EFS, events included treatment abandonment, disease progression, or death. Treatment outcomes were categorized as completion of therapy, abandonment of treatment, or incomplete therapy due to death. Abandonment of treatment was defined as missing four or more consecutive weeks of scheduled treatment; research suggests that families are unlikely to return after this period of time.17 Abandonment was further stratified by its timing relative to treatment phase: prior to preoperative chemotherapy, before completing surgery, after surgery but before starting postoperative chemotherapy, or during postoperative chemotherapy.
2.6 Statistical analysis
Descriptive statistics were determined for all demographic, patient clinical characteristics, and process outcomes. All clinical characteristics and process outcomes were compared for patients who lived and died using Wilcoxon rank-sum tests or Fisher's exact tests to calculate individual p-values. Only patients whose two-year survival status was known were included in mortality analyses, so patients who abandoned or were unknown if alive or dead at two years were excluded from Table analyses but described in overall percentages in the Results. Adjusted logistic regression analysis was done to determine independent predictors of two-year OS. Kaplan–Meier survival curves were created for the primary survival outcomes of one- and two-year OS and EFS rates. Concordance with stages of the treatment protocol were calculated based on process outcomes described above.
2.7 Ethics statement
The study was approved by the Catholic University of Health and Allied Sciences/BMC Research Ethical Committee (Mwanza, Tanzania), the National Institute for Medical Research, and the Duke University Institutional Review Board.
3 RESULTS
3.1 Study population and clinical characteristics
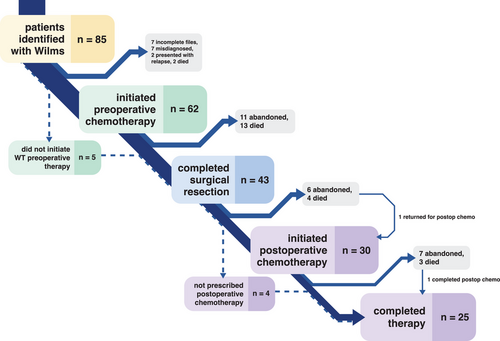
A total of 85 patients were initially identified as having Wilms tumor. Among these, 16 were excluded due to misdiagnosis (n = 7), unavailability of medical records for review (n = 7), or non-primary diagnosis presentation (n = 2). Thus, 69 patients were included in final analyses (Figure 1). The median age was 3 years (interquartile range [IQR]: 2–4 years), and 41% (n = 28) were female (Table 1). Most patients (80%, n = 55) came from the Lake Zone, with 25% (n = 17) from the immediate Mwanza region. Two patients (3%) had bilateral tumors, and 12 patients (19%) had metastatic disease at diagnosis. Metastatic or bilateral disease at presentation was associated with increased mortality (p = .04, Table 1). Metastasis was to the liver only (n = 6), lung only (n = 3), or multisite (n = 3).
| Demographic | Frequency [n] | Mortality ratea | p-Value |
|---|---|---|---|
| Age | 3 years median [IQR: 2–4] | 72% if ≤3 years old | .3 |
| 58% if >3 years old | |||
| Sex | .3 | ||
| Female | 41% [28] | 76% [19/25] | |
| Male | 59% [41] | 59% [22/37] |
| Tumor characteristic | Frequency [n] | Mortality ratea | p-Value |
|---|---|---|---|
| Preoperative mets | .04b | ||
| No metastases | 78% [50/64] | 57% [26/46] | |
| Bilateral disease | 3% [2/64] | 100% [1/1] | |
| Liver mets only | 9% [6/64] | 75% [3/4] | |
| Lung mets only | 5% [3/64] | 100% [3/3] | |
| Liver and lung mets | 3% [2/64] | 100% [2/2] | |
| Liver and other mets | 2% [1/64] | 100% [1/1] | |
| Postoperative stage | .02c | ||
| I | 20% [8/41] | 14% [1/7] | |
| II | 41% [17/41] | 41% [7/17] | |
| III | 39% [16/41] | 73% [11/15] | |
| Histology risk | .2 | ||
| Low | 20% [8/40] | 14% [1/7] | |
| Intermediate | 55% [22/40] | 57% [12/21] | |
| High | 25% [10/40] | 50% [5/10] |
- Abbreviation: IQR, interquartile range.
- a Patients who abandoned and it is unknown if they are alive or dead within 2 years of diagnosis are not included in mortality rate calculations.
- b Fisher's exact test of mortality for no metastasis versus metastatic or bilateral disease.
- c Fisher's exact test of mortality for low postoperative stage (I or II) versus high stage (III).
3.2 Treatment and process outcomes
The median time from presentation at BMC to diagnosis was 1 day (IQR: 0–6.5 days). A delay in diagnosis (>14 days from presentation) was not associated with a higher two-year mortality rate (p > .9) (Table 2). Preoperative staging was completed for 93% of patients (64/69) via chest x-ray and abdominal ultrasound. The other five patients either died prior to starting preoperative chemotherapy (n = 2), died early in their preoperative chemotherapy course after abdominal ultrasound but prior to a chest x-ray (n = 2), or had missing files on preoperative staging (n = 1).
| Treatment cascade | Median days [IQR] | Mortality rate [adherent]a | Mortality rate [nonadherent]a | Mortality p-value |
|---|---|---|---|---|
| Presentation to diagnosis | 1 [0–6.5] | 66% [33/50] if ≤14 | 67% [8/12] if >14 | >.9 |
| Diagnosis to pre-op chemo | 7 [2–14] | 71% [22/31] if ≤7 | 61% [19/31] if >7 | .6 |
| Pre-op chemo to surgery | 56 [48–81] | 44% [11/25] if ≤14 | 60% [6/10] if >14 | .5 |
| Surgery to pathology report | 30 [22.5–42.5] | 33% [1/3] if ≤14 | 52% [16/31] if >14 | >.9 |
| Surgery to post-op chemo | 38 [18.5–49] | 0% [0/1] if ≤7 | 46% [12/26] if >7 | >.9 |
| Process outcome | Frequency [n] | Mortality rate [adherent]a | Mortality rate [nonadherent]a | Mortality p-value |
|---|---|---|---|---|
| Pre-op chemo adherence | 83% [44/53] | 55% [23/42] | 100% [4/4] | .1 |
| No intraoperative spillage | 72% [28/39] | 37% [10/27] | 60% [6/10] | .3 |
| Post-op chemo adherence | 71% [22/31] | 29% [6/21] | 50% [4/8] | .4 |
| Radiotherapy adherence | 55% [6/11] | 33% [2/6] | 100% [4/4] | .08 |
| Treatment outcome | Frequency [n] | Mortality rate [adherent]a | Mortality rate [nonadherent]a | Mortality p-value |
|---|---|---|---|---|
| Overall survival status | – | |||
| Alive | 30% [21/69] | – | – | |
| Died | 59% [41/69] | – | – | |
| Unknown | 10% [7/69] | – | – | |
| Relapse | 24% [16/67] | 67% [30/45] if no relapse | 69% [11/16] if relapse | >.9 |
| Abandoned | 33% [23/69] | 60% [27/45] if no abandon | 82% [14/17] if abandon | .1 |
| Before pre-op chemo | 0% [0/63] | 66% [37/56] if no abandon | – | – |
| Before surgery | 21% [11/52] | 46% [18/39] if no abandon | 100% [6/6] if abandon | .02 |
| Before post-op chemo | 17% [6/36] | 38% [11/29] if no abandon | 67% [4/6] if abandon | .4 |
| During post-op chemo | 26% [7/27] | 20% [4/20] if no abandon | 83% [5/6] if abandon | .01 |
- Abbreviation: IQR, interquartile range.
- a Patients who abandoned and it is unknown if they are alive or dead within 2 years of diagnosis are not included in mortality rate calculations.
Preoperative chemotherapy was initiated a median of 7 days after diagnosis (IQR: 2–14 days) in 90% of patients (62/69). A delay in the start of preoperative chemotherapy (>7 days after diagnosis) was not associated with a higher mortality rate (p = .6) (Table 2). The other seven patients either passed away prior to initiating chemotherapy (n = 2), had surgery without preoperative chemotherapy (n = 4), or received a lymphoma regimen prior to surgery for Wilms tumor (n = 1) (Figure 1). Of the two patients who died before preoperative chemotherapy, one died at home, and one had insufficient records for location of death. During preoperative chemotherapy, nine patients passed away, and nine patients abandoned. After completing preoperative chemotherapy, an additional four patients passed away, and two abandoned (Figure 1). Thus, among patients who completed preoperative chemotherapy, 83% [44/53] had the recommended number of weeks. Adherence to the protocol for recommended preoperative chemotherapy duration was significantly higher for patients without metastatic disease (89%, 41/46, 4 weeks minimum) than for those with metastatic or bilateral disease (43%, 3/7, 8 weeks minimum; p = .01).
Surgery was completed for 62% of patients (43/69), as 12 patients abandoned and 14 patients died before surgery. Four patients had surgery without receiving preoperative chemotherapy at BMC: two had surgery elsewhere before presenting to BMC, and two had surgery upfront without preoperative chemotherapy. Having surgery was associated with a lower mortality rate (49% vs. 100%, p = .05). A median of 56 days elapsed from the start of preoperative chemotherapy to surgery (IQR: 48−81), and 30% [11/37] had surgery within 1 week of completing preoperative chemotherapy, with no difference in mortality between those who had surgery within 1 week and those who did not (p = .5, Table 2). For the 41 patients with surgical records available for postoperative staging, 20% [8/41] were Stage I, 41% [17/41] were Stage II, and 39% [16/41] were Stage III. Surgery was only done if the treatment team believed that disease was localized and that any preoperative metastatic disease had been addressed by chemotherapy. Postoperative Stage III was associated with higher mortality (p = .02, Table 1). Intraoperative tumor spillage occurred in 28% [11/39] of surgeries and was not associated with mortality (p = .3, Table 2).
Pathology reports were available a median of 30 days (IQR: 22.5–42.5 days) after surgery for 84% of patients [36/43], and only three patients had results within 14 days (Table 2). Pathology report within 14 days was not significantly associated with mortality (p > .9, Table 2). Of the 40 patients with known histology, 20% [8/40] were low risk, 55% [22/40] were intermediate risk, and 25% [10/40] were high risk. One patient with intermediate-risk histology was treated with high-risk chemotherapy, though, due to relapsed disease and splenic involvement. Histology risk was not significantly associated with mortality (p = .2, Table 1).
Postoperative chemotherapy was started a median of 38 days after surgery (IQR: 18.5–49 days) in 70% of patients who completed surgery [30/43], and only one patient started within 7 days, which was not significantly associated with mortality (p > .9, Table 2). Three patients did not require postoperative chemotherapy per protocol due to low-risk disease, and one patient was not told that postoperative chemotherapy was needed, and later the disease recurred. The other nine patients either abandoned (n = 5) or died (n = 4) prior to postoperative chemotherapy (Figure 1). One additional patient abandoned before postoperative chemotherapy but returned with relapsed disease (Figure 1). Of the 30 patients who received postoperative chemotherapy, 11 received SIOP-WT regimen (15 weeks minimum), 16 received the adapted Tanzania regimen (27 weeks minimum), and three received individualized regimens. There was no significant difference in two-year mortality between SIOP-WT and adapted Tanzania regimens (p = .7). During postoperative chemotherapy, three patients died, and seven patients abandoned, though one patient completed chemotherapy elsewhere after abandoning. In addition to the one patient who inadvertently skipped postoperative chemotherapy due to staff miscommunication and later relapsed, two more patients did not adhere to protocol due to staff miscalculations of the number of weeks. Thus, of those who survived postoperative therapy, 71% [22/31] adhered to postoperative chemotherapy protocol, and mortality was not statistically lower for those with protocol-adherent postoperative chemotherapy duration (29% vs. 50%, p = .4), though this is a clinically meaningful difference for patient care. Adherence to the protocol for postoperative chemotherapy duration was similar for patients regardless of preoperative metastasis status or postoperative chemotherapy regimen, even though the adapted Tanzania protocol is 12 weeks longer than the SIOP-WT regimen. Patients who abandoned received fewer weeks of postoperative chemotherapy (8 vs. 19 weeks, p = .008).
Only 29% [20/69] of patients completed both preoperative and postoperative chemotherapy per protocol, and those with metastatic or bilateral disease were slightly less likely to complete both regimens per protocol than those without metastasis at diagnosis (14% vs. 36%, p = .2). All but one patient who did not adhere with preoperative or postoperative chemotherapy was classified as a major deviation (>20% from intended number of weeks). For those who received postoperative therapy and in whom radiotherapy was indicated (excluding those who previously died or abandoned care), adherence was 55% [6/11], and their adherence was almost significantly associated with improved two-year survival (67% vs. 0%, p = .08). The five patients who did not adhere to radiotherapy when indicated either abandoned or died during postoperative chemotherapy.
3.3 Survival outcomes
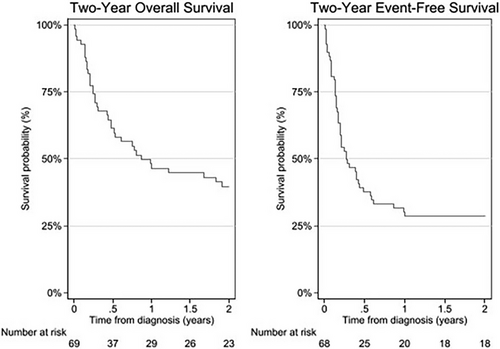
One-year, two-year, and median OS were a 46%, 40%, and 10 months, respectively (Figure 2). One-year, two-year, and median EFS were 29%, 29%, and 3 months, respectively (Figure 2). The only statistically significant predictor of two-year OS was protocol adherence to both preoperative and postoperative chemotherapy (OR = 14.4, 95% CI: 3.9–53.0, p < .001).
A total of 33% [23/69] of patients abandoned care, including one patient who abandoned twice (Table 2). Those who abandoned were less likely to have adhered to preoperative chemotherapy protocol (53% vs. 100%, p < .001), undergone surgery (55% vs. 100%, p < .001), or adhered to postoperative chemotherapy protocol (14% vs. 88%, p = .001).
Twelve patients relapsed within two years of diagnosis, five during therapy, six while abandoned, and one after completing therapy. An additional four patients relapsed after more than two years had elapsed since initial diagnosis. There were no significant differences in two-year relapse rate between those with and without metastasis or bilateral disease on presentation or those with high postoperative stage versus low postoperative stage.
4 DISCUSSION
Although published adapted treatment protocols for Wilms tumor have improved outcomes for children with Wilms tumor in sub-Saharan Africa in the context of clinical trials, when these protocols are implemented in a non-clinical trial setting, these same outcomes are not achieved. These disparities in treatment outcomes for Wilms tumor may be attributable, in part, to several treatment process implementation barriers and adherence failures. Given statistically significant associations with two-year OS and abandonment, adherence to preoperative and postoperative chemotherapy protocol is the most apparent areas of focus, but implementation barriers such as delays in surgery, pathology reports, and the initiation of postoperative chemotherapy and adherence failures, such as lack of surgery and radiotherapy nonadherence, also contributed to the differences in real-world data and clinical trial outcomes.
In the current study, patients with Wilms tumor treated at BMC had lower two-year EFS (29%) than patients treated in the Collaborative Wilms Tumor Africa Project (50%) including those treated in Wilms Collaborative clinical trial in Malawi (46%), almost entirely driven by higher abandonment at BMC (33%) than within the Collaborative (12%) or in Malawi (15%).5, 6 At the end of therapy, only 36% of BMC patients were alive with no evidence of disease compared to 68% of the Collaborative Wilms Tumor Africa Project patients, which was related to abandonment but also higher rates of death during treatment (29% vs. 13%).5 To reduce abandonment, BMC created a hostel for families to stay closer to the hospital and initiated supportive care services for patients and families.
Our one-year OS was equivalent to that of the national referral hospital in Tanzania (52% vs. 52%).13 It is curious that our outcomes are equivalent when the referral hospital saw patients with higher stage disease (88% vs. 45% Stage 3 or 4) and higher risk pathology (41% vs. 28% high risk) than we did at BMC and the referral hospital had lower radiotherapy compliance (34% vs. 67%) and lower postoperative chemotherapy compliance for high-risk pathology (45% vs. 75%).14 Our survival outcomes may have been affected by BMC having a higher abandonment rate than the referral hospital (33% vs. 29%) and a subsequently lower one-year EFS rate (29% vs. 37%).13
Timeliness of proceeding to the next step in the treatment cascade and completion of each step in the cascade were both process-level targets to improve clinical outcomes. This was especially true for preoperative (83%) and postoperative chemotherapy (71%) protocol adherence. There was also room for improvement in the timeliness almost every process step except for days from presentation to diagnosis and from diagnosis to initiating preoperative chemotherapy. Additional studies are needed to determine if these delays were due to patient-level barriers (e.g., financial constraints to travel to appointments or surgical costs) versus health system-level barriers (e.g., reduced operating room capacity, limited blood supply, or overwhelming the pathology lab's capacity) as reported in studies that evaluated implementation of adult cancer therapy guidelines in LMICs.18, 19
Adherence also dropped off with each step of the treatment cascade: 83% [44/53] adhered to protocol for preoperative chemotherapy duration, 81% [43/53] completed surgery, 71% [22/31] adhered to protocol for postoperative chemotherapy duration, and 55% [6/11] completed radiotherapy when indicated. Focus should specifically be placed on getting patients to surgery and adhering to radiotherapy when indicated as both were associated with lower two-year mortality (p = .05 and .08, respectively). Abandonment severely affected adequacy of preoperative and postoperative chemotherapy, and any efforts to reduce abandonment would invariably improve mortality, as those who adhered to preoperative and postoperative chemotherapy duration had much lower two-year mortality than those who did not (26% vs. 84%, p < .001).
There were several limitations in this study. These included the cohort size of only 69 patients all treated at a single center in Tanzania. Additionally, there were fair amount of missing data points due to incomplete records and the abandonment rate of 33%. Our analysis takes into consideration the overlapping factors that affect clinical outcome, such as metastasis requiring longer preoperative chemotherapy, which affects both preoperative chemotherapy adherence and subsequent survival, as well as metastasis itself affecting survival. The cause of death was also largely missing from our database, though we anticipate a similar distribution of causes of death as those reported in Blantyre, Malawi.6 Future interventions can target improving supportive care to reduce treatment-related toxicity and increasing community awareness to reduce stage at presentation to improve outcomes.
It is imperative that the ideal treatment regimen for Wilms tumor in Africa be further explored, particularly as radiotherapy availability expands. Despite an estimated incidence of 50,000 childhood cancers across Africa per year, capacity building will require higher political prioritization.20 We demonstrated survival outcomes in the upper range for LMICs and identified steps in the treatment cascade that can be targeted to improve outcomes.
ACKNOWLEDGMENTS
We acknowledge the contributions of the pediatric psychosocial support staff in Mwanza: Mastidia Maxmilian, Judith Mafwibo, and Jacque Kamanga.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




