Effect of voxelotor on cardiopulmonary testing in youths with sickle cell anemia in a pilot study
In Memoriam: Laura Caldarera passed away in early 2022 after a nearly year-long battle with aggressive cancer. As manager of the stress testing lab and preventive cardiology clinic at Child Cardiology Associates, she spent the majority of her career helping and assessing pediatric and adult congenital patients to improve their exercise capacity with a goal of improving their overall health and life expectancy. She was passionate, smart, and a true advocate for patients and families. She is greatly missed.
Abstract
Background
Individuals with sickle cell anemia (SCA) exhibit decreased exercise capacity. Anemia limits oxygen-carrying capacity and affects cardiopulmonary fitness. The drug voxelotor raises hemoglobin in SCA. We hypothesized that voxelotor improves exercise capacity in youths with SCA.
Methods
In a single-center, open-label, single-arm, longitudinal interventional pilot study (NCT04581356), SCA patients aged 12 and older, stably maintained on hydroxyurea, were treated with 1500 mg voxelotor daily, and performed cardiopulmonary exercise testing before (CPET#1) and after voxelotor (CPET#2). A modified Bruce Protocol was performed on a motorized treadmill, and breath-by-breath gas exchange data were collected. Peak oxygen consumption (peak VO2), anaerobic threshold, O2 pulse, VE/VCO2 slope, and time exercised were compared for each participant. The primary endpoint was change in peak VO2. Hematologic parameters were measured before each CPET. Patient Global Impression of Change (PGIC) and Clinician Global Impression of Change (CGIC) surveys were collected.
Results
Ten hemoglobin SS patients aged 12–24 completed the study. All demonstrated expected hemoglobin rise, with average +1.6 g/dL (p = .003) and P50 left shift of average −11 mmHg (p < .0001) with decreased oxygen off-loading at low pO2. The change in % predicted peak VO2 from CPET#1 to CPET#2 ranged from −12.8% to +11.3%, with significant improvement of more than 5% in one subject, more than 5% decrease in five subjects, and insignificant change of less than 5% in four subjects. All 10 CGIC and seven of 10 PGIC responses were positive.
Conclusion
In a plot study of 10 youths with SCA, voxelotor treatment did not improve peak VO2 in 9 out of 10 patients.
Abbreviations
-
- AT
-
- anaerobic threshold
-
- CGIC
-
- Clinician Global Impression of Change
-
- CPET
-
- cardiopulmonary exercise test
-
- Hgb F
-
- fetal hemoglobin
-
- HU
-
- hydroxyurea
-
- ODC
-
- oxygen dissociation curve
-
- PGIC
-
- Patient Global Impression of Change
-
- SCA
-
- sickle cell anemia
-
- SCT
-
- sickle cell trait
-
- VO2
-
- oxygen consumption
1 INTRODUCTION
Physical activity is limiting for patients with sickle cell anemia (SCA). Whereas people with sickle cell trait (SCT) engage in high-intensity athletics and military training, individuals with SCA, defined as genetically severe forms of sickle cell disease, including hemoglobin SS and hemoglobin S beta0 thalassemia, have not participated, due to patients’ own exercise intolerance or restrictions placed upon them by others, or both.1, 2 In the era of hydroxyurea (HU), children and teenagers can be physically active and participate in athletics. Sickle cell providers are often asked to provide sports clearance for students with SCA, but no athletic guidelines are available for SCA, as there are for SCT.3, 4 Additionally, regular exercise has been shown to benefit patients with SCA.1, 5-9 Thus, understanding exercise capacity in SCA is increasingly necessary.
Although limited, investigations of exercise in SCA have reassured that maximal exercise testing is safe and does not induce sickle cell crisis.1, 8, 10-12 Children and adults with SCA exhibit decreased cardiopulmonary fitness, most informatively demonstrated through cardiopulmonary exercise testing (CPET) and peak oxygen consumption (VO2).11-16 Peak VO2 in adolescents and adults with SCA were lower than controls by as much as 30%, even after adjusting for body mass index (BMI) and hemoglobin.11, 12, 16 In the Cooperative Study for Sickle Cell Disease, standard Balke treadmill exercise testing found decreased exercise capacity among more than 200 adults with SCA compared to the general African American population, with lower exercise capacity associated with lower hemoglobin, among other factors.17
SCA is a systemic disease, and decreased peak VO2 is likely multifactorial. Anemia directly affects oxygen-carrying capacity, compromising aerobic activity. Indeed, CPET before and after transfusion in patients with chronic anemia and in anemic presurgical patients showed approximately 10% rise in peak VO2 after transfusion.18, 19 In SCA patients, exercise studies improved after exchange transfusion, which partially replaced hemoglobin S blood with hemoglobin A blood.20, 21 These studies suggest low hemoglobin in SCA is likely one factor contributing to decreased exercise capacity, and increasing hemoglobin might improve VO2.
HU prevents many sickle cell complications and increases hemoglobin, mainly by increasing fetal hemoglobin (Hgb F) and preventing hemoglobin S polymerization. The newer drug voxelotor decreases hemolysis and increases hemoglobin by modifying alpha globin to keep hemoglobin in the oxygenated form that is not susceptible to polymerization.22 Voxelotor can further raise hemoglobin in patients already on HU. One would expect voxelotor to improve peak VO2, due to higher hemoglobin, as well as less sickling and hemolysis. The concern that the higher O2 affinity of voxelotor-modified hemoglobin could negatively impact VO2 was addressed in Global Blood Therapeutics (GBT) phase 1/2 voxelotor study, in which 16 adult SCA patients taking voxelotor showed no significant change in CPET VO2 max or ventilatory threshold.23 In that study, the voxelotor doses were 700–1000 mg/day, below GBT's currently recommended dose of 1500 mg/day, and most patients were not taking HU. Given the widespread use of HU, the effect of voxelotor, as currently recommended, on exercise capacity should be evaluated in the context of HU. We hypothesized that voxelotor can improve exercise capacity in youths with SCA.
2 METHODS
A single-center, open-label, single-arm, longitudinal interventional pilot study was conducted to evaluate the effect of voxelotor on exercise capacity in youths with SCA age 12 or older (NCT04581356). CPET was conducted at baseline and after voxelotor therapy for at least 8 weeks, which was when the change in hemoglobin stabilized in GBT's phase 3 trial.22 Participants were recruited from the sickle cell clinic of Pediatric Specialists of Virginia in Fairfax, Virginia. CPET was conducted at the exercise lab of Child Cardiology Associates in Rockville, Maryland, and red cell biochemical studies were performed at the University of California San Francisco (UCSF) Pediatric Hematology Red Blood Cell (RBC) Lab in Oakland, California. The study was approved by Western Institutional Review Board (WIRB; Tracking ID #20201909).
2.1 Patient enrollment
Patients with SCA, age 12 years and older, who had been on HU for more than 3 months, were eligible. Exclusion criteria included chronic transfusions, transfusion within 8 weeks of enrollment, hospitalization for vaso-occlusive crisis or acute chest syndrome within 30 days of enrollment, alanine aminotransferase four times the upper limit of normal, physical inability to exercise, and unavailability for timed study activities. To ensure adherence to study drug, patients with good HU adherence, evidenced by Hgb F level and hemogram, were offered participation.
Written IRB-approved informed consent was obtained. Participants age 12–17 provided written assent. Patients were compensated with gift cards for CPET completion and mileage reimbursement for travel to exercise lab.
2.2 Intervention and assessments
One month after enrollment, participants had baseline labs drawn and performed CPET#1. The following day, voxelotor 1500 mg daily was started. After 1 month of voxelotor, monitoring clinic visit and routine labs were done. After 2 months of voxelotor, routine and study labs were drawn, and CPET#2 was conducted. The final study visit was 1 month following CPET#2, when quality-of-life assessments were obtained. Weekly phone calls were made to monitor adverse events and answer questions.
2.3 Biochemical markers
Blood samples were obtained before each CPET. Routine labs included complete blood count, reticulocyte count, comprehensive metabolic panel, lactate dehydrogenase, indirect and direct bilirubin, and Hgb F. Blood samples were shipped on ice overnight to the UCSF RBC lab for study labs, including oxygen dissociation (Hemox Analyzer, TCS Scientific Corp), and percentage of cells containing Hgb F (% F cells) by flow cytometry (Fortessa, Becton Dickinson).
2.4 Exercise testing
CPET was performed using a motorized treadmill (Trackmaster Model TMX428, Full Vision, Inc.), and a modified Bruce Protocol, which includes 2-minute stages that increase in incline and speed incrementally to induce peak physical effort. Participants were asked to exercise for 8−12 minutes, but could terminate any time they exhibited signs of inability. Breath-by-breath gas exchange data were collected and analyzed using a VMax Encore 29C metabolic cart (CareFusion Corp, San Diego, CA, USA). A 12-lead electrocardiogram (ECG) and pulse oximetry were used to monitor participants during exercise. Peak heart rate (peak HR), peak blood pressure, peak oxygen consumption (peak VO2), oxygen pulse (O2 pulse), VE/VCO2 slope to the respiratory compensation point (minute ventilation/carbon dioxide production; a marker of ventilatory efficiency), VO2/HR slope, ventilatory anaerobic threshold (VAT; when the shift from aerobic to anaerobic metabolism begins), percent predicted VO2 at VAT, and time exercised were measured. A maximal exercise response is defined as a respiratory exchange ratio > 1.1, meaning excess CO2 was produced from O2 consumed for exercise, which is evidence of participant maximal effort. Prediction calculations included Cooper's equation for height or weight and Wasserman's equation; all were adjusted for treadmill.
2.5 Adherence monitoring
Participants were asked to bring back all voxelotor bottles to each clinic visit. Pill counts were used to calculate percent adherence. Hemoglobin electrophoresis detected voxelotor-modified Hgb. The distinct shift in the oxygen dissociation curve (ODC) curve at low PO2 as the result of voxelotor further confirmed adherence.
2.6 HRQoL
Health-related quality of life (HRQoL) was assessed by patients and clinicians via the Patient Global Impression of Change survey (PGIC) and the Clinical Global Impression of Change-Improvement survey (CGIC), respectively. The surveys rated patient overall improvement after voxelotor on a seven-point scale.
2.7 Statistical analysis
Stata/IC version 16 (StataCorp, College Station, TX, USA) was used for statistical analysis. All study parameters were subjected to descriptive statistics. Student paired t-test was used to compare CPET#1 and CPET#2 peak VO2. p-Values less than or equal to .05 were considered statistically significant. Power could not be calculated for this pilot study of only 10 subjects.
3 RESULTS
3.1 Study participants
Between October 9, 2020 and June 9, 2021, 14 patients were enrolled. Four subjects were withdrawn due to one screen failure, one adverse event on study drug with fever and chills, one hypersensitivity reaction with fever, hives, swelling, requiring steroid therapy, and one did not keep HU dose constant. Ten participants completed the study, including five females and five males aged 12−24, with average age 16.2 ± 3.9 (Table 1).
| Subject # | 1 | 2 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Average ± SD |
p-Value [95% CI] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 14 | 15 | 14 | 12 | 17 | 19 | 19 | 24 | 12 | 12 | ||
| Sex | M | F | F | M | F | F | M | M | F | M | ||
| Hgb number (g/dL) | ||||||||||||
| Baseline | 8.8 | 9.1 | 8.4 | 9.6 | 8.3 | 9.9 | 10.2 | 7.0 | 10.1 | 11.0 | ||
| After | 10.1 | 9.5 | 9.7 | 11.1 | 10.4 | 10.9 | 10.7 | 11.9 | 11.7 | 12.8 | ||
| Δ | 1.3 | 0.4 | 1.3 | 1.5 | 2.1 | 1.0 | 0.5 | 4.9 | 1.6 | 1.8 | 1.6 ± 1.3 |
.003 [0.74, 2.5] |
| Reticulocyte (%) | ||||||||||||
| Baseline | 2.6 | 9.4 | 5.1 | 4.8 | 3.6 | 4.2 | 13.6 | 3.3 | 7.7 | 2.1 | ||
| After | 1.6 | 5.3 | 3.6 | 3.4 | 1.8 | 2.7 | 6.2 | 5.3 | 4.7 | 1.8 | ||
| Δ | −1.0 | −4.1 | −1.5 | −1.4 | −1.8 | −1.5 | −7.4 | 2.0 | −3.0 | −0.3 | −2.0 ± 2.5 |
.03 [−3.8, −0.23] |
| Bilirubin (mg/dL) | ||||||||||||
| Baseline | 1.0 | 0.7 | 0.8 | 1.7 | 0.8 | 1.5 | 2.1 | 2.5 | 2.7 | 0.7 | ||
| After | 0.7 | 1.0 | 0.6 | 0.9 | 0.5 | 0.6 | 1.2 | 2.3 | 2.0 | 0.6 | ||
| Δ | −0.3 | 0.3 | −0.2 | −0.8 | −0.3 | −0.9 | −0.9 | −0.2 | −0.7 | −0.1 | −0.4 ± 0.40 |
.01 [−0.70, −0.13] |
| LDH (U/L) | ||||||||||||
| Baseline | 511 | 653 | 751 | 664 | 518 | 562 | 607 | 323 | 847 | 646 | ||
| After | 471 | 827 | 846 | 591 | 507 | 536 | 504 | 269 | 1233 | 651 | ||
| Δ | −40 | 174 | 95 | −73 | −11 | −26 | −103 | −54 | 386 | 5 | 35 ± 148 |
.47 [−71, 141] |
| % change | −7.8% | 27% | 13% | −11% | −2.1% | −4.6% | −17% | −17% | 46% | 0.8% | 2.6 ± 20 | |
| Hgb F (%) | ||||||||||||
| Baseline | 17.4 | 21.3 | 20.9 | 25.7 | 17.8 | 32.0 | 24.6 | 28.9 | 27.7 | 30.4 | ||
| After | 8.6 | 11.6 | 20.5 | 22.3 | 13.0 | 16.0 | 24.0 | 13.5 | 23.6 | 22.8 | ||
| Δ | −8.8 | −9.7 | −0.4 | −3.4 | −4.8 | −16 | −0.6 | −15.4 | −4.1 | −7.6 | −7.1 ± 5.5 |
.003 [−11, −3.1] |
| % change | −51% | −46% | −1.9% | −13% | −27% | −50% | −2.4% | −53% | −15% | −25% | −28 ± 20 | |
| % F cells | ||||||||||||
| Baseline | 71.4 | 81.9 | 79.0 | 93.4 | 74.5 | 90.1 | 79.7 | 83.5 | 85.7 | 91.7 | ||
| After | 56.9 | 70.9 | 70.7 | 85.3 | 63.2 | 66.0 | 82.6 | 65.3 | 76.2 | 80.5 | ||
| Δ | −14.5 | −11 | −8.3 | −8.1 | −11.3 | −24.1 | 2.9 | −18.2 | −9.5 | −11.2 | −11 ± 7.0 |
<.001 [−16, −6.3] |
| P50 (mmHg) | ||||||||||||
| Baseline | 32.0 | 31.8 | 40.1 | 30.5 | 29.5 | 31.0 | 32.5 | 35.3 | 34.6 | 36.7 | ||
| After | 24.0 | 19.7 | 23.3 | 22.4 | 21.5 | 20.4 | 23.0 | 26.7 | 28.4 | 13.7 | ||
| Δ | −8.00 | −12.1 | −16.8 | −8.1 | −8.0 | −10.6 | −9.5 | −8.6 | −6.2 | −23 | −11 ± 5.1 |
<.001 [−15, −7.4] |
- Abbreviations: CI, confidence interval; Hgb, hemoglobin; Hgb F, fetal hemoglobin; LDH, lactate dehydrogenase; P50, the partial pressure of O2 at which Hgb is 50% saturated with O2; SD, standard deviation; % F cells, % of cells containing Hgb F.
All participants had Hgb SS with stable Hgb F levels on HU, which was continued without dose change throughout the study. All voxelotor pill bottles were returned, and pill counts confirmed adherence at 84%–100% (average 98%).
3.2 Hematology and biochemical markers
All lab studies were collected at designated times and showed expected changes after voxelotor, confirming that the drug worked (Table 1, Figure 1A). Mean Hgb change was +1.6 ± 1.3 g/dL, range +0.4 to +4.9 g/dL. Mean reticulocyte change was −2.0% ± 2.5%, range −7.4% to +2%. Mean total bilirubin change was −0.4 ± 0.4 mg/dL, range −0.9 to +0.3 mg/dL.
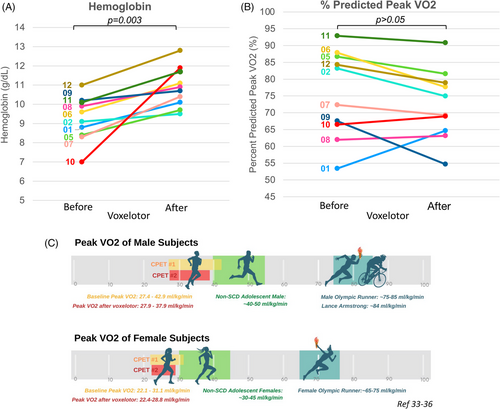
Accurate percent Hgb F quantitation was challenging after voxelotor due to drug modification causing variant hemoglobin species. Percent Hgb F was more reliably obtained from capillary zone electrophoresis (Labcorp), and changed by −0.4% to −16%, with an average decrease of −7.1% ± 5.5%. Hgb F % dropped significantly for all but two subjects, including decreasing by approximately half in four subjects (Table 1). Flow cytometry showed 71.4%–93.4% F cells at baseline. After voxelotor, % F cells decreased in all but one subject, ranging from −8.1% to −24.1%, with average change of −11.3% ± 7.0% (Table 1).
3.3 Oxygen dissociation curves
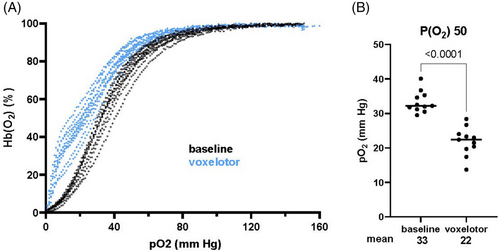
All participants demonstrated a leftward shift of P50, ranging from −23 to −6.2 mmHg, with mean and significant shift of −11 mmHg, from 33 to 22 mmHg (p < .0001) (Figure 2). For reference, P50 is 35 mmHg for Hgb S, 27 mmHg for Hgb A, and 20 mmHg for Hgb F. In contrast to the classical sigmoidal shape, all voxelotor-treated ODCs had a steeper slope at lower partial pressure of oxygen, reflecting proportionally less oxygen off-loading in more oxygen-deprived environments.
3.4 CPET
All subjects completed CPET#1 and CPET#2. All subjects attained respiratory quotient (VCO2/VO2) ≥ 1.1, confirming maximal exercise effort. Subject 10 showed ST depression on ECG during CPET#1, thought to be due to anemia, as his Hgb was 7 g/dL. He underwent cardiac workup that was ultimately negative, including echocardiogram and CT angiogram. This delayed his CPET#2 until 7 months later, at which time his Hgb rose by 4.9 to 11.9 g/dL, and no ECG changes occurred during CPET#2. No other adverse events occurred during exercise testing.
Reported reproducibility of CPET ranges from 2% to 10%; this exercise lab accepts ±5% for intra-individual variability, and a difference of more than 5% in either direction is considered significant. Oxygen consumption was measured as peak VO2 in L/min, in mL/kg/min, and as % predicted (Table 2). Peak VO2 changes (in L/min) before and after voxelotor ranged from −0.38 to +0.32 L/min. To account for sex, age, and other variables, peak VO2 was also compared as % predicted, and individual changes ranged from −12.8% to +11.3%. Subject 1 was the only participant with significant positive change of +11.3% in peak VO2. Four subjects showed insignificant change (Subjects 7, 8, 10, 11). Five subjects showed significant negative change (Subjects 2, 5, 6, 9, 12) (Table 2, Figure 1B).
| Subject # | 1 | 2 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Average ± SD |
p-Value [95% CI] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 14 | 15 | 14 | 12 | 17 | 19 | 19 | 24 | 12 | 12 | ||
| Sex | M | F | F | M | F | F | M | M | F | M | ||
| Measured peak VO2 per kg (mL/kg/min) | ||||||||||||
| Baseline | 27.4 | 31.1 | 25.4 | 42.9 | 26.2 | 22.1 | 32.2 | 32.6 | 29.1 | 40.5 | ||
| After | 32.5 | 28 | 24.6 | 37.9 | 27.3 | 22.4 | 27.2 | 34.8 | 28.8 | 37.9 | ||
| Δ | 5.1 | −3.1 | −0.8 | −5.0 | 1.1 | 0.3 | −5.0 | 2.2 | −0.3 | −2.6 | −0.81 ± 3.2 | .45 [−3.1, 1.5] |
| % Change | 19% | −10% | −3.1% | −12% | 4.2% | 1.4% | −16% | 6.7% | −1.0% | −6.4% | −1.7% ± 10% | |
| Measured peak VO2 (L/min) | ||||||||||||
| Baseline | 1.52 | 1.42 | 1.70 | 1.75 | 1.64 | 1.14 | 2.15 | 2.23 | 1.76 | 1.52 | ||
| After | 1.84 | 1.30 | 1.60 | 1.54 | 1.65 | 1.14 | 1.77 | 2.37 | 1.72 | 1.42 | ||
| Δ | 0.32 | −0.12 | −0.10 | −0.21 | 0.01 | 0.00 | −0.38 | 0.14 | −0.04 | −0.10 | −0.047 ± 0.19 | .46 [−0.18, 0.090] |
| % Change | 21% | −8% | −6% | −12% | 0.6% | 0.0% | −18% | 6% | −2% | −7% | −2.4 ± 11% | |
| % Predicted peak VO2 | ||||||||||||
| Baseline | 53.4 | 83.2 | 86.7 | 87.9 | 72.3 | 62.0 | 67.5 | 66.5 | 92.9 | 84.3 | ||
| After | 64.7 | 74.9 | 81.6 | 77.7 | 69.3 | 63.1 | 54.7 | 68.9 | 90.8 | 78.9 | ||
| Δ | 11.3 | −8.3 | −5.1 | −10.2 | −3.1 | 1.2 | −12.8 | 2.5 | −2.1 | −5.4 | −3.2 ± 7.0 | .18 [−8.2, 1.8] |
| VO2 at AT (% predicted peak VO2) | ||||||||||||
| Baseline | 47.0 | 40.0 | 49.0 | 45.0 | 45.0 | 42.0 | 43.0 | 43.0 | 44.0 | 50.0 | ||
| After | 39.0 | 36.0 | 51.0 | 42.0 | 41.0 | 34.0 | 36.0 | 57.0 | 48.0 | 56.0 | ||
| Δ | −8.0 | −4.0 | 2.0 | −3.0 | −4.0 | −8.0 | −7.0 | 14.0 | 4.0 | 6.0 | −0.8 ± 7.2 | .73 [−5.9, 4.3] |
| % Change | −17% | −10% | 4.1% | −7% | −9% | −19% | −16% | 33% | 9% | 12% | −2 ± 16% | |
| Peak HR (beats per minute) | ||||||||||||
| Baseline | 205 | 187 | 196 | 197 | 186 | 197 | 196 | 167 | 196 | 176 | ||
| After | 206 | 195 | 196 | 188 | 179 | 187 | 200 | 176 | 190 | 177 | ||
| Δ | 1.0 | 8.0 | 0.0 | −9.0 | −7.0 | −10.0 | 4.0 | 9.0 | −6.0 | 1.0 | −0.9 ± 7.8 | .69 [−5.8, 3.9] |
| % Change | 0.5% | 4% | 0% | −5% | −4% | −5% | 2% | 5% | −3% | 0.6% | −0.4 ± 4% | |
| % Predicted peak HR | ||||||||||||
| Baseline | 105 | 99 | 104 | 100 | 99 | 106 | 103 | 89 | 103 | 90 | ||
| After | 106 | 103 | 104 | 96 | 96 | 101 | 105 | 94 | 100 | 90 | ||
| Δ | 1.0 | 4.0 | 0 | −4.0 | −3.0 | −5.0 | 2.0 | 5.0 | −3.0 | 0 | −0.3 ± 3.4 | .79 [−2.7, 2.1] |
| O2 pulse (mL/beat) | ||||||||||||
| Baseline | 7.4 | 7.6 | 8.7 | 8.9 | 8.8 | 5.8 | 11.0 | 13.3 | 9.0 | 8.7 | ||
| After | 8.9 | 6.6 | 8.1 | 8.2 | 9.2 | 6.1 | 8.8 | 13.5 | 9.1 | 8.0 | ||
| Δ | 1.5 | −1.0 | −0.6 | −0.7 | 0.4 | 0.3 | −2.2 | 0.2 | 0.1 | −0.7 | −0.3 ± 1.0 | .42 [−0.99, 0.45] |
| % Change | 20% | −13% | −6.9% | −8% | 5% | 5% | −20% | 2% | 1% | −8% | 2.3 ± 11% | |
| % Predicted O2 pulse | ||||||||||||
| Baseline | 53.5 | 91.3 | 91.6 | 93.0 | 78.9 | 63.6 | 69.5 | 77.9 | 98.8 | 100.1 | ||
| After | 64.3 | 78.3 | 85.3 | 86.0 | 78.3 | 67.3 | 54.7 | 76.9 | 99.9 | 92.6 | ||
| Δ | 10.8 | −13.0 | −6.3 | −7.1 | −0.7 | 3.7 | −14.8 | −1.0 | 1.1 | −7.5 | −3.5 ± 7.8 | .19 [−9.0, −2.1] |
| VE/VCO2 slope | ||||||||||||
| Baseline | 27.1 | 25.7 | 22.6 | 28.6 | 24.7 | 21.4 | 20.1 | 31.7 | 24.5 | 29.6 | ||
| After | 26.1 | 28.4 | 23.5 | 27.7 | 25.7 | 20.8 | 20.8 | 26.1 | 25.1 | 30.9 | ||
| Δ | −1.0 | 2.7 | 0.9 | −0.9 | 1.0 | −0.6 | 0.7 | −5.6 | 0.6 | 1.3 | −0.09 ± 2.2 | .90 [−1.7, 1.5] |
| % Change | −3.7% | 10.5% | 4.0% | −3.1% | 4.0% | −2.8% | 3.5% | −18% | 2.4% | 4.4% | 0.16 ± 7.6% | |
| Time exercised (minutes) | ||||||||||||
| Baseline | 8.2 | 6.4 | 6.4 | 8.0 | 6.3 | 6.0 | 7.3 | 5.4 | 7.5 | 9.3 | ||
| After | 8.4 | 7.1 | 6.2 | 8.0 | 6.2 | 6.0 | 8.0 | 5.4 | 7.3 | 8.3 | ||
| Δ | 0.2 | 0.7 | −0.2 | 0.0 | −0.1 | 0.0 | 0.7 | 0.0 | −0.2 | −1.0 | 0.02 ± 0.48 | .89 [−0.32, 0.36] |
| % Change | 2.9% | 11% | −2.4% | 0% | −2.4% | 0% | 9.3% | −0.4% | −2.0% | −10% | −0.13 ± 3.4% | |
| Respiratory quotient | ||||||||||||
| Baseline | 1.24 | 1.23 | 1.36 | 1.35 | 1.37 | 1.35 | 1.50 | 1.21 | 1.35 | 1.09 | ||
| After | 1.28 | 1.35 | 1.22 | 1.33 | 1.33 | 1.41 | 1.69 | 1.17 | 1.40 | 1.12 | ||
| Δ | 0.04 | 0.12 | −0.14 | −0.02 | −0.04 | 0.06 | 0.19 | −0.04 | 0.05 | 0.03 | 0.03 ± 0.09 | .41 [−0.41, 0.091] |
- Abbreviations: SD, standard deviation; CI, confidence interval; VO2, respiratory oxygen uptake; AT, anaerobic threshold; HR, heart rate; O2, oxygen consumed; VE/VCO2, ventilatory efficiency (ratio of minute ventilation [VE] to carbon dioxide production [CO2]); bpm, beats per minute.
Peak VO2 calculated in mL/kg/min showed similar results. Change in peak VO2 expressed in L/min or in mL/kg/min both showed only one subject demonstrated significant improvement (Subject 1), and two subjects (Subjects 6, 9) showed significant reduction. The remaining seven subjects showed either minimal or no significant change in either direction (Table 2, Figure 1C).
Changes in individuals’ anaerobic threshold (AT), O2 pulse, VE/VCO2 slope, and time exercised were not consistent and did not correlate with changes in peak VO2. Individual changes in hematologic parameters also did not correlate with changes in peak VO2 or other CPET measurements.
3.5 PGIC/CGIC
On the seven-point questionnaires evaluating activity limitations, symptoms, emotions, and overall quality of life, PGIC and CGIC were concordantly positive for seven of 10 participants, and discordant for three of 10 participants reporting no change or almost the same, while clinicians reported improvement (Table 3). Clinicians reported basing their CGIC response mostly on Hgb change after voxelotor.
- Abbreviations: CGIC, Clinical Global Impression of Change; PGIC, Patient Global Impression of Change.
- a PGIC Rating Scale: 1 = no change (or worse); 2 = almost the same; 3 = a little better; 4 = somewhat better; 5 = moderately better; 6 = better and a definite improvement; 7 = a great deal better.
- b CGIC Rating Scale: 1 = very much worse; 2 = much worse; 3 = minimally worse; 4 = no change; 5 = minimally improved; 6 = much improved; 7 = very much improved.
4 DISCUSSION
4.1 Voxelotor and O2 consumption in study patients
Exercise tolerance is increasingly relevant in sickle cell disease with improving medical care and more SCA patients striving for lifestyles similar to their peers. Higher hemoglobin holds promise for improved exercise capacity. Our hypothesis that voxelotor, the primary clinical outcome for which is higher hemoglobin, could increase peak VO2 on CPET for adolescents with SCA, was not supported by data from this pilot study. Only one of 10 participants demonstrated significant positive change in peak VO2 after voxelotor, while two of 10 participants performed significantly worse. Notably, the participant with the largest hemoglobin increase (Subject 10) from 7 to 11.9 g/dL while on voxelotor for 7 months between CPET#1 and CPET#2, only demonstrated +6% change in peak VO2 and +2.5% change in % predicted peak VO2, which were not significant given ±5% cutoff for intra-individual variability (Table 2, Figure 1B). Although only one example, this participant demonstrated that even impressive correction of severe anemia with voxelotor for longer time did not result in higher peak VO2.
Study subjects demonstrated lower-than-normal O2 pulse (Table 2). O2 pulse is typically considered a surrogate for stroke volume, but based on the Fick equation, also includes O2 extraction, which is affected by hemoglobin and O2 saturations. No patient had significant O2 desaturation, and hemoglobin increased in all patients on voxelotor, suggesting impairment in either oxygen extraction or stroke volume as reasons for low O2 pulse. Peak HR was higher than 85% predicted for all patients, confirming no chronotropic incompetence. Cardiac physiology can be abnormal in SCA due to diastolic dysfunction, abnormal muscle function, and decreased pumping action.24, 25 Cardiac assessment was not included in this pilot study, but was normal for one subject when done outside of the study, which showed that lack of peak VO2 improvement in this subject (Subject 10, with hemoglobin rise of 4 g/dL) was not due to cardiac dysfunction. Abnormal vasculature can affect pulmonary and systemic vascular resistance, but VE/VCO2 was normal in all patients, suggesting no significant ventilation to perfusion (V/Q) mismatch or intrapulmonary shunting (patients did not have to excessively ventilate to remove CO2). This leaves limited oxygen extraction due to high-affinity voxelotor-modified hemoglobin as a plausible reason for low O2 pulse. In support of this concept, in a detailed study of voxelotor in normal volunteers showing oxygen saturation improvement during incremental exercise, oxygen extraction and consumption also did not change. The researchers suggested that differential O2 off-loading offsets the advantage of higher O2 saturation, leading ultimately to no change or decrease in peak VO2.26 Interestingly, GBT reported normal oxygen delivery in exercise testing of normal volunteers taking voxelotor, as assessed by resting and peak exercise heart rate, but no VO2 data were presented.27
Additionally, when assessing VO2 at AT, which represents the highest VO2 (aerobic capacity) before converting to anaerobic metabolic pathways, many patients had a lower value on the subsequent CPET, indicating less O2 was extracted. This includes Subject 1 who had a higher peak VO2 on the second test. VO2 at AT is typically approximately 50%–60% of the predicted peak VO2, but more than 40% is considered normal. Four of 10 patients on the second CPET had values lower than 40%, while none had values less than 40% on the first CPET (Table 2). This is an unusual finding and one could speculate whether voxelotor affected O2 extraction.
4.2 Voxelotor-modified Hgb and oxygen dissociation curve
Voxelotor directly increases oxy-hemoglobin, which should increase oxygen-carrying capacity. Indeed, detailed exercise studies with pulmonary gas exchange and arterial blood gas measurements by Stewart et al. showed higher arterial oxygen saturation during exercise after voxelotor, consistent with left-shifted ODC.26 However, peak VO2 decreased after voxelotor, which the authors suggested may be due to altered oxygen off-loading by voxelotor-modified Hgb. Using a sickling assay and structural modeling, Henry et al. concluded that potential oxygen delivery to tissues gained from reduced sickling is offset by less oxygen off-loading.28 Here, oxygen consumption did not improve in 9 of 10 SCA patients after voxelotor, while ODC assumed a hyperbolic shape and shifted leftward (Figure 2A), with a significant drop in average P50 of −11 mmHg (Figure 2B). Thus, live exercise data in SCA patients reported here are consistent with the model of decreased oxygen off-loading, although other explanations, such as cardiac limitations, cannot be excluded.
The usual sigmoidal shape of ODC ensures more oxygen off-loading from hemoglobin as oxygen tension decreases and tissue oxygen demand increases. Instead, ODC of hemoglobin containing voxelotor-modified species has the opposite hyperbolic shape, showing less proportional oxygen off-loading, that is, less oxygen delivery, when tissues are more hypoxic (Figure 2A). The need to balance voxelotor's ability to prevent sickling by increasing oxygen affinity against voxelotor's negative effect on oxygen delivery was ardently articulated in a commentary by Quinn and Ware,29 and the recent NEJM review by Bunn.30 High-affinity Hgb F also shifts ODC to the left, but the sigmoidal shape is maintained, as is shown in our patients with high Hgb F prior to starting voxelotor (Figure 2A). Although we have no data on in vivo fractional oxygen extraction or tissue oxygen status in the study subjects, the remarkably altered ODC of voxelotor-modified hemoglobin, low O2 pulse, and lower VO2 at AT are consistent with limited oxygen off-loading.
4.3 Peak VO2 in SCA patients on hydroxyurea
Peak VO2 at baseline for the participants ranged from 53.4% to 92.9% of predicted, with an average of 75.6%, which is roughly consistent with published reports that CPET performance is approximately 30% lower in patients with SCA compared to control populations (Table 2, Figure 1C).11, 12, 16 This is surprising in that HU increases Hgb and Hgb F, and prevents sickling, which might be expected to positively affect exercise tolerance. All study participants were long-term HU users, and some were athletically active, yet their peak VO2 overall fell in the range of untreated sickle cell patients. Baseline average P50 at 33 mmHg is to the right of P50 for Hgb A, which is 27 mmHg, thus oxygen off-loading is not a problem in patients with high Hgb F. An ancillary study from the Multicenter Study of Hydroxyurea tested 10 subjects on HU and 14 placebo subjects by cycle ergometry at 6, 12, and 18 months and found more increase in peak muscle power and more decrease in peak heart rate response to workload among HU subjects, reflecting improvements in anaerobic muscle performance and aerobic cardiovascular efficiency, suggesting improvement in exercise capacity associated with HU.31 Other publications reporting VO2 have not separated out HU-treated versus untreated SCA patients, perhaps because HU-treated patients did not stand out on exercise testing.
4.4 Improving exercise capacity in SCA patients
Studies that showed improvement in exercise capacity with higher Hgb in SCA patients have increased Hgb by transfusion with Hgb A blood.20, 21 In the exchange transfusion experiment attempting to keep Hgb the same (but actually Hgb increased by 1.4 g/dL), the effect of Hgb A was directly queried and results suggested improved exercise capacity was associated with Hgb A.21 In contrast, in a study of children on long-term exchange transfusion maintaining Hgb S approximately 30%, average peak VO2 was still less than 80% compared to healthy controls, but the transfused patients also had significantly lower Hgb (9.3 ± 0.5 g/dL) than healthy controls.32
Several studies documented improved exercise performance in SCA patients after moderate exercise training.1, 5, 7-9 Anecdotally, Subject 1 in this study, the only one who demonstrated increase in peak VO2, reported engaging in regular exercise after starting voxelotor, but this was not prospectively or objectively documented. At least in one study, muscle biopsy demonstrated altered skeletal microvasculature in SCA patients limiting oxygen extraction, which improved after a regular exercise program, along with improved peak VO2.6, 7 It is possible that improved Hgb along with guided exercise training could lead to sustained improvement in exercise capacity.
4.5 Voxelotor effect on Hgb F
A notable finding of voxelotor is its effect on Hgb F. Voxelotor treatment decreased % F cells, but due to higher RBC number after voxelotor, the absolute number of F cells remained largely unchanged. However, % Hgb F, the absolute amount of Hgb F, as well as the amount of Hgb F per F cell decreased in many subjects after voxelotor (Table 1 and Table S1). In addition, voxelotor modification of Hgb F would also alter ODC of Hgb F, raising the same concern that impairment of oxygen delivery from voxelotor-modified Hgb F counters the benefit of Hgb F in inhibiting Hgb S polymerization. Given that Hgb F thus far is the singularly most effective molecule in protecting against deleterious complications of sickle hemoglobin, potential interference with Hgb F level and function is another consideration in using voxelotor in combination with HU.
4.6 Limitations
Limitations of this study include small sample size, lack of cardiac function assessments, and lack of spirometry at baseline or post exercise to determine potential pulmonary limitation to exercise.
5 CONCLUSION
In this pilot CPET study, voxelotor treatment did not improve peak VO2 in 9 of 10 young SCA patients already on HU with relatively high Hgb F. Voxelotor raised hemoglobin as expected, but altered the hemoglobin oxygen dissociation curve. It is possible that limitation of oxygen delivery played a role in the lack of improvement in exercise capacity in patients treated with voxelotor.
ACKNOWLEDGMENTS
This study was funded by Global Blood Therapeutics, Inc., now a wholly owned subsidiary of Pfizer Inc. We acknowledge Karen E. Devine at Labcorp for supervising hemoglobinopathy profile results reporting in the study patients.
CONFLICT OF INTEREST STATEMENT
Robin Dulman owns Pfizer stock. The remaining authors have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




