Prevention of acute and delayed chemotherapy-induced nausea and vomiting in pediatric cancer patients: A clinical practice guideline
Abstract
This clinical practice guideline provides recommendations for preventing acute and delayed phase chemotherapy-induced nausea and vomiting (CINV) in pediatric patients. The recommendations are based on two systematic reviews of randomized controlled trials evaluating interventions to prevent (1) acute phase CINV and (2) delayed phase CINV. Recommendations for acute phase and delayed phase CINV prophylaxis are made for patients receiving chemotherapy of varying emetogenicity, as well as for patients not able to receive dexamethasone or a neurokinin-1 receptor antagonist. Evidence gaps, including antiemetic safety and optimal dosing, were identified.
Abbreviations
-
- (fos)aprepitant
-
- IV fosaprepitant or oral aprepitant
-
- (fos)netupitant
-
- IV fosnetupitant or oral netupitant
-
- 5HT3RA
-
- serotonin-3 receptor antagonist
-
- CIN
-
- chemotherapy-induced nausea
-
- CINV
-
- chemotherapy-induced nausea and vomiting
-
- CIV
-
- chemotherapy-induced vomiting
-
- CPG
-
- clinical practice guideline
-
- HEC
-
- highly emetogenic chemotherapy
-
- HSCT
-
- hematopoietic stem cell transplant
-
- IV
-
- intravenous
-
- LEC
-
- low emetogenic chemotherapy
-
- MEC
-
- moderately emetogenic chemotherapy
-
- minEC
-
- minimally emetogenic chemotherapy
-
- NK1RA
-
- neurokinin-1 receptor antagonist
-
- POGO
-
- Pediatric Oncology Group of Ontario
-
- RCT
-
- randomized controlled trial
-
- SR
-
- systematic review
1 INTRODUCTION
Chemotherapy-induced nausea and vomiting (CINV) are among the most common adverse effects related to cancer therapy and, when poorly controlled, impair patients’ health and quality of life.1-7 Clinical practice guideline (CPG)-consistent CINV prophylaxis improves CINV control for both adult and pediatric patients.8-10
This CPG is one of a series focused on CINV prevention and treatment in pediatric patients receiving cancer treatment or undergoing hematopoietic stem cell transplant (HSCT).11-13 Our objective was to provide evidence-based guidance on strategies for acute and delayed phase CINV prevention and thereby optimize the supportive care of children and adolescents with cancer or receiving chemotherapy for HSCT. This CPG will be of interest to health care professionals who care for patients up to 18 years of age with cancer or undergoing HSCT. It may also be useful for patients and their families, administrators, educators, supportive care researchers, and quality improvement leaders.
2 METHODS
2.1 CPG panel constitution
The panel included representatives from pediatric hematology/oncology, HSCT, nursing, pharmacy, and psychology; patient advocates; and CPG methodologists (Appendix SA1). Panel member selection was based upon expertise in CINV, pediatric supportive care, CPG methodology, and experience in managing CINV as a family caregiver. Invited panel members declared potential conflicts of interest; no panel member was excluded from participation due to a conflict (Appendix SA2).
This CPG was funded and developed through the Pediatric Oncology Group of Ontario (POGO) Guidelines Program. The development process and the CPG content are editorially independent from POGO.
2.2 General approach to CPG development
The Appraisal of Guidelines for Research and Evaluation II14 framed CPG development. Eight virtual CPG panel meetings were held between February 2021 and April 2022 to formulate the approach and recommendations.
Key health questions are shown in Table 1. Panel members identified key outcomes and rated their importance by consensus. Complete chemotherapy-induced vomiting (CIV) control, complete chemotherapy-induced nausea (CIN) control, complete CINV control, adverse effects of an intervention, and feasibility of intervention administration to children and adolescents were considered critical outcomes for both the acute and delayed phase.
| Health questions and recommendations | Recommendation strength/evidence quality |
|---|---|
| 1. In pediatric patients receiving highly emetogenic chemotherapy, | |
| 1.1 what strategies are recommended to prevent acute phase CINV? | |
| a. Use a 5HT3RA + dexamethasone + (fos)aprepitanta | Strong/high |
| b. Use palonosetron + dexamethasone in patients unable to receive (fos)aprepitanta | Strong/moderate |
| c. Use palonosetron + (fos)aprepitanta in patients unable to receive dexamethasone | Strong/low |
| d. Use palonosetron in patients unable to receive dexamethasone + (fos)aprepitanta | Strong/moderate |
| e. Consider adding olanzapine to other CPG-consistent antiemetics | Conditional/moderate |
| 1.2 what strategies are recommended to prevent delayed phase CINV? | |
| a. Use palonosetron in the acute phase as the preferred 5HT3RA in patients at high risk of delayed phase CINV | Strong/moderate |
| b. Use oral aprepitant in the delayed phase, if (fos)aprepitanta started in the acute phase | Strong/high |
| c. Add dexamethasone in the delayed phase in patients who received granisetron or ondansetron in the acute phase | Strong/moderate |
| d. Consider adding dexamethasone in the delayed phase in patients who received palonosetron in the acute phase | Conditional/moderate |
| e. Use dexamethasone in the delayed phase in patients unable to receive oral aprepitant | Strong/moderate |
| f. Continue olanzapine in the delayed phase, if started in the acute phase | Strong/moderate |
| g. Do not use 5HT3RAs in the delayed phase | Strong/low |
| 2. In pediatric patients receiving moderately emetogenic chemotherapy, | |
| 2.1 what strategies are recommended to prevent acute phase CINV? | |
| a. Use a 5HT3RA + dexamethasone | Strong/moderate |
| b. Use a 5HT3RA + (fos)aprepitanta in patients unable to receive dexamethasone | Strong/low |
| c. Use a 5HT3RA in patients unable to receive dexamethasone + (fos)aprepitanta | Strong/low |
| d. Consider using palonosetron as the preferred 5HT3RA in patients unable to receive dexamethasone + (fos)aprepitanta | Conditional/low |
| e. Consider adding olanzapine to other CPG-consistent antiemetics in patients unable to receive dexamethasone + (fos)aprepitanta | Conditional/low |
| 2.2 what strategies are recommended to prevent delayed phase CINV? | |
| a. Consider using dexamethasone in the delayed phase | Conditional/low |
| b. Continue oral aprepitant in the delayed phase in patients receiving single-day chemotherapy who received (fos)aprepitanta in the acute phase | Strong/moderate |
| c. Consider not using oral aprepitant in the delayed phase in patients receiving multi-day chemotherapy (≥3 days) who received (fos)aprepitanta in the acute phase | Conditional/low |
| d. Continue olanzapine in the delayed phase, if started in the acute phase | Strong/low |
| 3. In pediatric patients receiving low emetogenic chemotherapy, | |
| 3.1 what strategies are recommended to prevent acute phase CINV? | |
| a. Use a 5HT3RA | Strong/low |
| 3.2 what strategies are recommended to prevent delayed phase CINV? | |
| a. Do not use prophylaxis routinely in the delayed phase | Strong/very low |
| 4. In pediatric patients receiving minimally emetogenic chemotherapy, | |
| 4.1 what strategies are recommended to prevent acute phase CINV? | |
| a. Do not use prophylaxis routinely | Strong/very low |
| 4.2 what strategies are recommended to prevent delayed phase CINV? | |
| a. Do not use prophylaxis routinely in the delayed phase | Strong/very low |
- CINV, chemotherapy-induced nausea and vomiting; 5HT3RA, serotonin-3 receptor antagonist.
- a IV fosaprepitant or oral aprepitant.
The Grading of Recommendations Assessment, Development and Evaluation approach15 was used to rate the quality of evidence and formulate recommendations. Evidence quality was rated as high, moderate, low, or very low. The quality rating was lowered if there were study design flaws, inconsistent results, imprecision or if direct pediatric data were lacking. Strong or conditional recommendations were based on the panel's confidence in an intervention's effect, while also taking into consideration its safety profile. A strong recommendation was made when the benefit of an intervention clearly outweighed the potential harms. A conditional recommendation was made when the benefit or harm of an intervention were unclear or when the benefits and disadvantages were closely matched.
This CPG will be updated within 5 years or sooner if new evidence becomes available that would affect its recommendations.
2.3 Evidence base formulation
Although originally planned as an update of the 2013 and 2017 acute CINV prevention CPGs,16, 17 a new (rather than updated) acute phase CINV CPG was created so that recommendations for preventing both acute and delayed phase CINV could be developed as a single CPG using identical methodology. Two systematic reviews (SRs) were conducted to identify the evidence base that informed CPG recommendations (see Appendix SA3 for list of team members). Each SR included parallel arm randomized controlled trials (RCTs) evaluating interventions to prevent: (1) acute phase CINV or (2) delayed phase CINV. The complete methods for the SR evaluating acute phase CINV are published separately.18 The complete methods for the SR evaluating delayed phase CINV are presented in Appendix SA4. In brief, English-language RCTs published between January 1, 1989 (publication year of first ondansetron study) and January 21, 2022 that evaluated a strategy to prevent acute phase or delayed phase CINV were identified. RCTs conducted in patients of any age were included.
2.4 Definitions
A chemotherapy block was defined as consecutive days of chemotherapy. The acute phase was defined as starting with administration of the first chemotherapy dose and ending 24 h after administration of the last chemotherapy dose of a chemotherapy block. The delayed phase was defined as starting at the end of the acute phase and continuing for 96 h or until another chemotherapy block began, whichever occurred first.
2.5 Meta-analysis
The methods for synthesis of the acute phase data have been published elsewhere.18 The methods for meta-analysis of the delayed phase data were similar and are fully presented in Appendix SA4. Briefly, for the acute and delayed phase CINV SRs, synthesis was planned when three or more RCTs had the following characteristics: compared the same intervention against the same control, reported one of the designated key outcome measures and included chemotherapy of the same emetogenicity. Serotonin-3 receptor antagonists (5HT3RAs) and neurokinin-1 receptor antagonists (NK1RAs) were each evaluated as a group to evaluate class effects. For multi-arm studies, the two study arms of highest clinical interest that permitted synthesis were used; this decision was made by three authors (PP, PDR, and LLD) blinded to outcome data.
Three categories of studies were relevant to delayed phase CINV control: (1) RCTs where antiemetics given in the acute phase were the same in each study arm and randomization to different delayed phase antiemetics occurred; (2) RCTs where randomization to different antiemetics occurred in both the acute and delayed phase and where the randomized antiemetic was the same in both the acute and delayed phase; and (3) RCTs where randomization to different acute phase antiemetics occurred, delayed phase antiemetics were the same by study arm and delayed phase CINV control was evaluated. These categories are summarized with examples in Appendix SA5.
Intervention effects were described using the risk ratio (RR) with its 95% confidence interval (CI) and synthesis was conducted using the random effects model in Review Manager 5.4 (Cochrane Collaboration, Nordic Cochrane Centre, London, United Kingdom).19 A RR > 1 in analyses evaluating CIV, CIN, and CINV control suggests that the intervention improves patient outcomes (e.g., a higher proportion of patients receiving the intervention experienced complete CINV control versus the comparator). For the safety outcome of antiemetic discontinuation due to an antiemetic-related adverse effect, a RR < 1 suggests a benefit among patients receiving the intervention. In addition to evaluating antiemetic-related adverse effects, we narratively described adverse effects for antiemetics that were not included in the previous acute CINV CPG.
For the delayed phase meta-analysis, two stratified analyses were performed. First, we evaluated the effect of randomization to dexamethasone versus no dexamethasone in the delayed phase among those who systematically received a first-generation 5HT3RA (granisetron or ondansetron) or a second-generation 5HT3RA (palonosetron) in the acute phase (category 1). Second, we evaluated the effect of randomization to palonosetron versus granisetron or ondansetron in the acute phase among those who systematically received dexamethasone or no dexamethasone in the delayed phase (category 3). We used the p value for interaction (p int) to determine if heterogeneity in the effect of an intervention (e.g., 5HT3RA choice) could be explained by the use of another antiemetic (e.g., delayed phase dexamethasone).
Publication bias of meta-analyses was explored through visual examination of funnel plots when there were at least 10 studies included. When publication bias was suspected, “trim and fill” technique was to be applied to determine the impact of the potential bias. Outlying studies would be removed (trim) and hypothetical negative studies with equal weight would be added (fill).20
3 RESULTS
The literature search identified 65,172 citations, of which 74418 were retrieved for full-text screening for both the acute and delayed phase SRs. A total of 328 RCTs were included across both SRs. The characteristics of the RCTs included in the acute phase (296 RCTs) and delayed phase (212 RCTs) SRs are summarized in Appendix SA6. The study flow diagram for the acute phase SR has been published previously and the delayed phase SR study flow diagram can be found in Appendix SA7. The study-level data for the acute phase RCTs were previously published18 while the study-level data for the delayed phase RCTs are presented in Appendices SA8a and SA8b.
3.1 Acute phase CINV
The acute phase CINV SR has been published.18 Few RCTs were conducted exclusively in pediatric patients (25 out of 296; 8%). Almost all RCTs consisted of patients with cancer (284 out of 296; 96%). Almost half (133 out of 296; 45%) of the RCTs studied chemotherapy-naïve patients and 58% (173 out of 296) studied patients receiving single-day chemotherapy. Most RCTs studied patients receiving HEC (167 out of 296; 56%). Table 2 summarizes the meta-analyses that informed recommendations for preventing acute phase CINV. More detailed analyses are available elsewhere.18
| Comparison and outcomes | Number of studies | N | RR | 95% CI | I2 | p |
|---|---|---|---|---|---|---|
| Highly emetogenic chemotherapy | ||||||
| 5HT3RA prophylaxis | ||||||
| Granisetron vs ondansetron | ||||||
| Granisetron vs ondansetron | ||||||
| Complete acute phase CIV control | 11 | 3202 | 1.00 | 0.94–1.07 | 24% | .91 |
| Complete acute phase CIN control | 7 | 2923 | 1.00 | 0.93–1.08 | 9% | .97 |
| Complete acute phase CINV control | 2 | UTD | UTD | UTD | UTD | UTD |
| Granisetron + corticosteroid vs ondansetron + corticosteroid | ||||||
| Complete acute phase CIV control | 3 | 1047 | 0.99 | 0.93–1.06 | 0% | .84 |
| Complete acute phase CIN control | 2 | UTD | UTD | UTD | UTD | UTD |
| Complete acute phase CINV control | 2 | UTD | UTD | UTD | UTD | UTD |
| Palonosetron vs granisetron/ondansetron | ||||||
| Palonosetron vs granisetron/ondansetron | ||||||
| Complete acute phase CIV control | 6b | 1410 | 1.11 | 1.02–1.20 | 43% | .01 |
| Complete acute phase CIN control | 2b | UTD | UTD | UTD | UTD | UTD |
| Complete acute phase CINV control | 0 | UTD | UTD | UTD | UTD | UTD |
| Palonosetron + dexamethasone vs granisetron/ondansetron + dexamethasone | ||||||
| Complete acute phase CIV control | 7b | 4985 | 1.10 | 1.02–1.18 | 79% | .01 |
| Complete acute phase CIN control | 3b | 1784 | 1.53 | 1.00–2.34 | 94% | .05 |
| Complete acute phase CINV control | 2b | UTD | UTD | UTD | UTD | UTD |
| Palonosetron + dexamethasone + NK1RA vs granisetron/ondansetron + dexamethasone + NK1RA | ||||||
| Complete acute phase CIV control | 5b | 1301 | 1.01 | 0.97–1.05 | 15% | .71 |
| Complete acute phase CIN control | 2 | UTD | UTD | UTD | UTD | UTD |
| Complete acute phase CINV control | 1 | UTD | UTD | UTD | UTD | UTD |
| Corticosteroid prophylaxis | ||||||
| Corticosteroid vs no corticosteroid | ||||||
| Corticosteroid + metoclopramide vs metoclopramide | ||||||
| Complete acute phase CIV control | 3 | 226 | 1.03 | 0.70–1.52 | 0% | .89 |
| Complete acute phase CIN control | 3 | 226 | 1.02 | 0.75–1.39 | 0% | .88 |
| Complete acute phase CINV control | 0 | UTD | UTD | UTD | UTD | UTD |
| Corticosteroid + 5HT3RA vs 5HT3RA | ||||||
| Complete acute phase CIV control | 14 | 2410 | 1.32 | 1.18–1.47 | 78% | <.00001 |
| Complete acute phase CIN control | 8 | 1252 | 1.39 | 1.26–1.53 | 2% | <.00001 |
| Complete acute phase CINV control | 5 | 1301 | 1.36 | 1.23–1.50 | 15% | <.00001 |
| Dexamethasone + 5HT3RA vs 5HT3RA | ||||||
| Complete acute phase CIV control | 10 | 1855 | 1.29 | 1.12–1.49 | 82% | .0003 |
| Complete acute phase CIN control | 5 | 783 | 1.46 | 1.28–1.67 | 0% | <.00001 |
| Complete acute phase CINV control | 4 | 993 | 1.35 | 1.18–1.55 | 34% | <.00001 |
| NK1RA prophylaxis | ||||||
| NK1RA vs no NK1RA | ||||||
| (Fos)aprepitant c + ondansetron vs ondansetron | ||||||
| Complete acute phase CIV control | 3b | 371 | 1.20 | 1.00–1.44 | 0% | .06 |
| Complete acute phase CIN control | 0 | UTD | UTD | UTD | UTD | UTD |
| Complete acute phase CINV control | 0 | UTD | UTD | UTD | UTD | UTD |
| NK1RA + 5HT3RA + corticosteroid vs 5HT3RA + corticosteroid | ||||||
| Complete acute phase CIV control | 28b | 11,767 | 1.11 | 1.08–1.14 | 68% | <.00001 |
| Complete acute phase CIN control | 14 | 5739 | 1.05 | 1.01–1.08 | 0% | .01 |
| Complete acute phase CINV control | 7 | 2906 | 1.07 | 1.01–1.13 | 0% | .02 |
| NK1RA + 5HT3RA + dexamethasone vs 5HT3RA + dexamethasone | ||||||
| Complete acute phase CIV control | 27b | 11,666 | 1.11 | 1.07–1.14 | 69% | <.00001 |
| Complete acute phase CIN control | 13 | 5638 | 1.04 | 1.01–1.08 | 0% | .02 |
| Complete acute phase CINV control | 7 | 2906 | 1.07 | 1.01–1.13 | 0% | .02 |
| (Fos)aprepitantc + 5HT3RA + dexamethasone vs 5HT3RA + dexamethasone | ||||||
| Complete acute phase CIV control | 20b | 6376 | 1.13 | 1.08–1.18 | 70% | <.00001 |
| Complete acute phase CIN control | 7 | 2070 | 1.04 | 0.98–1.11 | 0% | .17 |
| Complete acute phase CINV control | 6 | 2322 | 1.07 | 1.00–1.14 | 0% | .04 |
| Fosaprepitant vs oral aprepitant | ||||||
| Fosaprepitant + 5HT3RA + dexamethasone vs oral aprepitant + 5HT3RA + dexamethasone | ||||||
| Complete acute phase CIV control | 3 | 3611 | 1.01 | 0.99–1.03 | 0% | .50 |
| Complete acute phase CIN control | 0 | UTD | UTD | UTD | UTD | UTD |
| Complete acute phase CINV control | 0 | UTD | UTD | UTD | UTD | UTD |
| (Fos)netupitantd vs (fos)aprepitantc | ||||||
| (Fos)netupitantd + 5HT3RA + dexamethasone vs (fos)aprepitantc + 5HT3RA + dexamethasone | ||||||
| Complete acute phase CIV control | 4 | 2653 | 1.00 | 0.97–1.02 | 0% | .70 |
| Complete acute phase CIN control | 2 | UTD | UTD | UTD | UTD | UTD |
| Complete acute phase CINV control | 1 | UTD | UTD | UTD | UTD | UTD |
| Olanzapine prophylaxis | ||||||
| Olanzapine vs no olanzapine | ||||||
| Olanzapine (+/- any concomitant antiemetics a ) vs no olanzapine (+/- any concomitant antiemetics a ) | ||||||
| Complete acute phase CIV control | 7b | 1659 | 1.23 | 1.05–1.44 | 91% | .01 |
| Complete acute phase CIN control | 7b | 1660 | 1.34 | 1.10–1.62 | 87% | .003 |
| Complete acute phase CINV control | 3 | 909 | 1.29 | 0.95–1.77 | 74% | .10 |
| Olanzapine + NK1RA + 5HT3RA + dexamethasone vs NK1RA + 5HT3RA + dexamethasone | ||||||
| Complete acute phase CIV control | 5b | 1520 | 1.25 | 0.99–1.57 | 93% | .06 |
| Complete acute phase CIN control | 5b | 1521 | 1.36 | 1.06–1.75 | 90% | .02 |
| Complete acute phase CINV control | 2 | UTD | UTD | UTD | UTD | UTD |
| Olanzapine vs NK1 receptor antagonist | ||||||
| Olanzapine + 5HT3RA + dexamethasone vs NK1RA + 5HT3RA + dexamethasone | ||||||
| Complete acute phase CIV control | 4 | 500 | 1.07 | 0.98–1.15 | 19% | .13 |
| Complete acute phase CIN control | 3 | 481 | 1.00 | 0.91–1.10 | 12% | .98 |
| Complete acute phase CINV control | 0 | UTD | UTD | UTD | UTD | UTD |
| Moderately emetogenic chemotherapy | ||||||
| 5HT3RA prophylaxis | ||||||
| Palonosetron vs granisetron/ondansetron | ||||||
| Palonosetron + dexamethasone vs granisetron/ondansetron + dexamethasone | ||||||
| Complete acute phase CIV control | 3b | 193 | 0.94 | 0.86–1.02 | 0% | .15 |
| Complete acute phase CIN control | 1 | UTD | UTD | UTD | UTD | UTD |
| Complete acute phase CINV control | 0 | UTD | UTD | UTD | UTD | UTD |
| Corticosteroid prophylaxis | ||||||
| Corticosteroid vs no corticosteroid | ||||||
| Dexamethasone + 5HT3RA vs 5HT3RA | ||||||
| Complete acute phase CIV control | 3 | 1002 | 1.29 | 1.21–1.39 | 0% | <.00001 |
| Complete acute phase CIN control | 2 | UTD | UTD | UTD | UTD | UTD |
| Complete acute phase CINV control | 1 | UTD | UTD | UTD | UTD | UTD |
| NK1RA prophylaxis | ||||||
| NK1RA vs no NK1RA | ||||||
| (fos)aprepitantc + 5HT3RA vs 5HT3RA | ||||||
| Complete acute phase CIV control | 3b | 344 | 1.15 | 0.96–1.37 | 80% | .12 |
| Complete acute phase CIN control | 2 | UTD | UTD | UTD | UTD | UTD |
| Complete acute phase CINV control | 0 | UTD | UTD | UTD | UTD | UTD |
| (fos)aprepitantc + 5HT3RA + dexamethasone vs 5HT3RA + dexamethasone | ||||||
| Complete acute phase CIV control | 11 | 2916 | 1.03 | 1.00–1.07 | 76% | .07 |
| Complete acute phase CIN control | 4 | 595 | 1.02 | 0.91–1.16 | 37% | .70 |
| Complete acute phase CINV control | 0 | UTD | UTD | UTD | UTD | UTD |
- N, number of patients included in analysis; RR, risk ratio; CI, confidence interval; I2, statistic describing percentage of variation across studies that is due to heterogeneity rather than chance; CIV, chemotherapy-induced vomiting; CIN, chemotherapy-induced nausea; CINV, chemotherapy-induced nausea and vomiting; UTD, unable to determine; OLZ, olanzapine; HEC, highly emetogenic chemotherapy; MEC, moderately emetogenic chemotherapy; LEC, low emetogenic chemotherapy; NR, not reported; 5HT3RA, serotonin-3 receptor antagonist; NK1RA, neurokinin-1 receptor antagonist.
- a Concomitant antiemetics identical in both study arms.
- b At least one pediatric study included.
- c IV fosaprepitant or oral aprepitant.
- d IV fosnetupitant or oral netupitant.
3.2 Delayed phase CINV
Few RCTs were conducted exclusively in pediatric patients (14 out of 212; 7%). Most studied patients with cancer (210 out of 212; 99%) receiving single-day chemotherapy (145 out of 212; 68%) and approximately half evaluated CIV, CIN, or CINV control in chemotherapy-naïve patients (99 out of 212; 47%). Administered chemotherapy was classified as HEC or MEC in 184 (87%) and 27 (13%) RCTs, respectively.
Table 3 summarizes the meta-analyses that informed recommendations for delayed phase CINV prevention. Detailed versions of this table are provided in Appendices SA9 and SA10. The results of the stratified analyses are presented in Appendix SA11. Details of RCTs included in meta-analyses of studies involving HEC and MEC are presented in Appendices SA12 and SA13. Sensitivity analyses undertaken for the meta-analyses where publication bias was suggested by visual inspection of funnel plots did not change the interpretation of the base analyses (Appendix SA14).
| Comparison and delayed phase outcomes | Number of studies | N | RR | 95% CI | I2 | p |
|---|---|---|---|---|---|---|
| Highly emetogenic chemotherapy | ||||||
| Category 1: Meta-analyses where acute phase antiemetics were the same in each study arm and randomization to different delayed phase antiemetics occurred | ||||||
| Delayed phase 5HT3RA prophylaxis | ||||||
| 5HT3RA vs no 5HT3RA | ||||||
| Granisetron/ondansetron (+/- any concomitant antiemeticsa) vs no granisetron/ondansetron (+/- any concomitant antiemeticsa) | ||||||
| Complete delayed phase CIV control | 4 | 1642 | 1.03 | 0.97–1.10 | 15% | .39 |
| Complete delayed phase CIN control | 3 | 1226 | 0.98 | 0.84–1.14 | 0% | .76 |
| Complete delayed phase CINV control | 1 | UTD | UTD | UTD | UTD | UTD |
| 5HT3RA vs metoclopramide | ||||||
| Granisetron/ondansetron + corticosteroid vs metoclopramide + corticosteroid | ||||||
| Complete delayed phase CIV control | 3 | 744 | 1.01 | 0.92–1.10 | 0% | .89 |
| Complete delayed phase CIN control | 2 | UTD | UTD | UTD | UTD | UTD |
| Complete delayed phase CINV control | 2 | UTD | UTD | UTD | UTD | UTD |
| Delayed phase corticosteroid prophylaxis | ||||||
| Corticosteroid vs no corticosteroid | ||||||
| Dexamethasone (+/- any concomitant antiemeticsa) vs no dexamethasone (+/- any concomitant antiemeticsa) | ||||||
| Complete delayed phase CIV control | 11 | 2980 | 1.11 | 1.02–1.20 | 74% | .01 |
| Complete delayed phase CIN control | 6 | 2050 | 1.22 | 1.10–1.35 | 22% | .0001 |
| Complete delayed phase CINV control | 3 | 1195 | 1.38 | 1.11–1.72 | 55% | .004 |
| Category 2: Meta-analyses where randomization to different antiemetics occurred in both the acute and delayed phase and where the antiemetic randomized was the same in both the acute and delayed phase | ||||||
| Acute and delayed phase oral aprepitant prophylaxis | ||||||
| Oral aprepitant vs no oral aprepitant | ||||||
| Oral aprepitant (+/- any concomitant antiemeticsa) vs no oral aprepitant (+/- any concomitant antiemeticsa) | ||||||
| Complete delayed phase CIV control | 11b | 2818 | 1.44 | 1.29–1.61 | 65% | <.00001 |
| Complete delayed phase CIN control | 5 | 1958 | 1.29 | 1.12–1.48 | 40% | .0003 |
| Complete delayed phase CINV control | 4 | 1858 | 1.34 | 1.14–1.56 | 42% | .0003 |
| Acute and delayed phase olanzapine prophylaxis | ||||||
| Olanzapine vs no olanzapine | ||||||
| Olanzapine (+/- any concomitant antiemeticsa) vs no olanzapine (+/- any concomitant antiemeticsa) | ||||||
| Complete delayed phase CIV control | 6b | 1507 | 1.53 | 1.25–1.89 | 76% | <.00001 |
| Complete delayed phase CIN control | 5b | 825 | 1.70 | 1.45–1.99 | 0% | <.00001 |
| Complete delayed phase CINV control | 3 | 889 | 1.19 | 0.70–2.05 | 82% | .52 |
| Olanzapine vs oral aprepitant | ||||||
| Olanzapine + dexamethasone vs oral aprepitant + dexamethasone | ||||||
| Complete delayed phase CIV control | 3 | 259 | 1.04 | 0.93–1.18 | 0% | .48 |
| Complete delayed phase CIN control | 2 | UTD | UTD | UTD | UTD | UTD |
| Complete delayed phase CINV control | 0 | UTD | UTD | UTD | UTD | UTD |
| Category 3: Meta-analyses where randomization to different acute phase antiemetics occurred and delayed phase antiemetics were the same in each study arm | ||||||
| Delayed phase 5HT3RA prophylaxis | ||||||
| Delayed phase: impact of day 1 5HT3RA | ||||||
| Palonosetron D1 (+/- any concomitant antiemeticsa) vs granisetron/ondansetron D1 (+/- any concomitant antiemeticsa) | ||||||
| Complete delayed phase CIV control | 10 | 4010 | 1.26 | 1.11–1.43 | 81% | .0004 |
| Complete delayed phase CIN control | 5 | 1684 | 1.68 | 1.16–2.43 | 74% | .006 |
| Complete delayed phase CINV control | 1 | UTD | UTD | UTD | UTD | UTD |
| Delayed phase NK1RA prophylaxis | ||||||
| Delayed phase: impact of day 1 NK1RA | ||||||
| NK1RA D1 (+/- any concomitant antiemeticsa) vs no NK1RA D1 (+/- any concomitant antiemeticsa) | ||||||
| Complete delayed phase CIV control | 9b | 5792 | 1.21 | 1.14–1.29 | 67% | <.00001 |
| Complete delayed phase CIN control | 6 | 3726 | 1.17 | 1.09–1.25 | 0% | <.00001 |
| Complete delayed phase CINV control | 3 | 1104 | 1.24 | 1.06–1.46 | 0% | .0009 |
| Moderately emetogenic chemotherapy | ||||||
| Category 1: Meta-analyses where acute phase antiemetics were the same in each study arm and randomization to different delayed phase antiemetics occurred | ||||||
| Delayed phase oral aprepitant prophylaxis | ||||||
| Dexamethasone vs no dexamethasone | ||||||
| Dexamethasone (+/- any concomitant antiemeticsa) vs no dexamethasone (+/- any concomitant antiemeticsa) | ||||||
| Complete delayed phase CIV control | 4 | 313 | 1.13 | 0.99–1.28 | 0% | .06 |
| Complete delayed phase CIN control | 1 | UTD | UTD | UTD | UTD | UTD |
| Complete delayed phase CINV control | 3 | 245 | 1.15 | 0.91–1.44 | 0% | .25 |
| Category 2: Meta-analyses where randomization to different antiemetics occurred in both the acute and delayed phase and where the antiemetic randomized was the same in both the acute and delayed phase | ||||||
| Acute and delayed phase oral aprepitant prophylaxis | ||||||
| Oral aprepitant vs no oral aprepitant | ||||||
| Oral aprepitant (+/- any concomitant antiemeticsa) vs no oral aprepitant (+/- any concomitant antiemeticsa) | ||||||
| Complete delayed phase CIV control | 8 | 1801 | 1.20 | 1.12–1.29 | 29% | <.00001 |
| Complete delayed phase CIN control | 2 | UTD | UTD | UTD | UTD | UTD |
| Complete delayed phase CINV control | 0 | UTD | UTD | UTD | UTD | UTD |
| Category 3: Meta-analyses where randomization to different acute phase antiemetics occurred and delayed phase antiemetics were the same in each study arm | ||||||
| Delayed phase NK1RA prophylaxis | ||||||
| Delayed phase: impact of oral aprepitant in acute phase | ||||||
| Oral aprepitant (+/- any concomitant antiemeticsa) vs no oral aprepitant (+/- any concomitant antiemeticsa) in acute phase | ||||||
| Complete delayed phase CIV control | 3b | 319 | 1.31 | 0.97–1.78 | 55% | .08 |
| Complete delayed phase CIN control | 1 | UTD | UTD | UTD | UTD | UTD |
| Complete delayed phase CINV control | 0 | UTD | UTD | UTD | UTD | UTD |
- N, number of patients included in analysis; RR, risk ratio; CI, confidence interval; I2, statistic describing percentage of variation across studies that is due to heterogeneity rather than chance; CIV, chemotherapy-induced vomiting; CIN, chemotherapy-induced nausea; CINV, chemotherapy-induced nausea and vomiting; UTD, unable to determine; HEC, highly emetogenic chemotherapy; 5HT3RA, serotonin-3 receptor antagonist; NK1RA, neurokinin-1 receptor antagonist.
- a Concomitant antiemetics identical in both study arms
- b At least one pediatric study included.
3.3 Safety
Synthesis of antiemetic discontinuation due to an antiemetic-related adverse effect was possible for one comparison in the acute phase SR18 and for none in the delayed phase most studies either did not report these data (176 out of 328; 54%) or had zero events in both study arms (70 out of 328; 21%). (Appendix SA15)
Fosaprepitant and olanzapine were the only antiemetics included in the recommendations of this CPG that had not appeared in earlier pediatric CPGs on acute phase CINV prevention. Therefore, more detailed information regarding the adverse effects reported in the 11 and 19 included RCTs evaluating fosaprepitant and olanzapine, respectively, was abstracted. These are presented in Appendix SA16 and SA17.
For fosaprepitant, the most commonly reported adverse effects and the incidence rates reported were: anorexia/decreased appetite (eight RCTs: 2–52%), constipation (10 RCTs: 3–40%), diarrhea (eight RCTs: 4–60%), dyspepsia/abdominal discomfort (seven RCTs: 0–17%), fatigue/asthenia (eight RCTs: 2–64%), headache (eight RCTs: 0–47%), and hiccups (six RCTs: 1–32%). Two patients in two separate RCTs experienced anaphylaxis or hypersensitivity. Four RCTs described infusion-related reactions; the incidence of these reactions ranged from 1 to 24%.
For olanzapine, rates of sedation or drowsiness were reported in 14 of the included RCTs and ranged from 0 to 100%. Few studies reported rates of dyslipidemia (two RCTs: 5 and 10%), extrapyramidal symptoms (four RCTs: 0–18%), hyperglycemia (five RCTs: 0–6%), or increased appetite (two RCTs: 2 and 13%). Descriptions of these adverse effects, when reported in included RCTs, are presented in Appendix SA17. No RCT described ECG changes. Two RCTs reported the rate of olanzapine dose reduction due to toxicity (0 and 20%).
4 RECOMMENDATIONS
Recommendations are summarized in Table 1 and Figure 1. The rationales for each recommendation are presented below and are summarized in Appendix SA18. Note that all references to aprepitant refer to oral administration. (Fos)aprepitant refers to intravenous (IV) fosaprepitant or oral aprepitant.
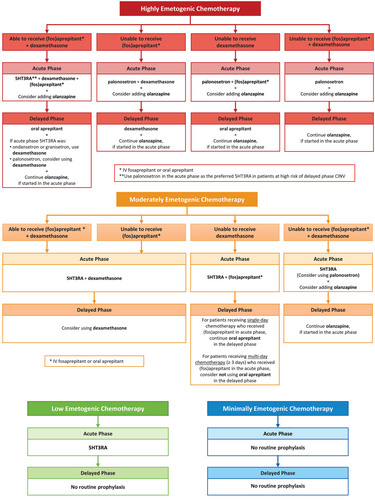
4.1 Health Question 1.1: In pediatric patients receiving HEC, what strategies are recommended to prevent acute phase CINV?
4.1.1 Recommendation 1.1a: Use a 5HT3RA + dexamethasone + (fos)aprepitant
4.1.1.1 Strong recommendation, high-quality evidence
Rationale: In making this recommendation, the panel considered a meta-analysis of 27 RCTs (four pediatric) that demonstrated benefit in giving triple-agent prophylaxis (5HT3RA + dexamethasone + NK1RA) versus dual-agent prophylaxis (5HT3RA + dexamethasone) for complete acute phase CIV control (RR 1.11, 95% CI: 1.07–1.14). Benefits in complete acute phase CIN and CINV control were also observed (RR 1.04, 95% CI: 1.01–1.08; RR 1.07, 95% CI: 1.01–1.13, respectively).
Granisetron, ondansetron, and palonosetron were the 5HT3RAs studied in the RCTs that were included in the above-mentioned meta-analyses demonstrating the benefit of triple-agent prophylaxis. Among five RCTs (one pediatric) that compared palonosetron versus granisetron/ondansetron in patients also receiving dexamethasone + NK1RA, no benefit of palonosetron was observed with respect to acute phase CIV control (RR 1.01, 95% CI: 0.97–1.05).
Synthesis of three RCTs in adults receiving HEC comparing oral aprepitant to IV fosaprepitant in combination with a 5HT3RA and dexamethasone showed comparable acute phase CIV control (RR 1.01, 95% CI: 0.99–1.03), suggesting either NK1RA may be used.
Thus, among patients receiving triple-agent prophylaxis, the choice of which 5HT3RA to use (granisetron, ondansetron, or palonosetron) and which NK1RA to use (IV fosaprepitant or oral aprepitant) should be based on institutional resources, feasibility, and patient preference. The choice of palonosetron over other 5HT3RAs may also be influenced by delayed phase CINV risk (see Recommendation 1.2a).
4.1.2 Recommendation 1.1b: Use palonosetron + dexamethasone in patients unable to receive (fos)aprepitant
4.1.2.1 Strong recommendation, moderate-quality evidence
Rationale: In the meta-analysis of seven RCTs (two pediatric) evaluating palonosetron + dexamethasone versus granisetron/ondansetron + dexamethasone in patients receiving HEC, palonosetron improved acute phase CIV control (RR 1.10, 95% CI: 1.02–1.18). A meta-analysis of the three RCTs (one pediatric) reporting acute phase CIN control demonstrated that palonosetron also benefits acute phase CIN control compared with granisetron/ondansetron (RR 1.53, 95% CI: 1.00–2.34). These analyses suggest that patients who are unable to receive fos(aprepitant) should receive palonosetron and dexamethasone.
4.1.3 Recommendation 1.1c: Use palonosetron + (fos)aprepitant in patients unable to receive dexamethasone
4.1.3.1 Strong recommendation, low-quality evidence
Rationale: Synthesis of three RCTs evaluating ondansetron + (fos)aprepitant versus ondansetron alone in patients receiving HEC (three pediatric) showed RR 1.20; 95% CI 1.00–1.44, suggesting benefit of (fos)aprepitant when added to a 5HT3RA regimen. Given the benefit of palonosetron versus granisetron/ondansetron when used in addition to dexamethasone (see Recommendation 1.1b) and the benefit of palonosetron versus granisetron/ondansetron when 5HT3RA monotherapy is used (see Recommendation 1.1d), the panel specifically recommended the 5HT3RA palonosetron plus fos(aprepitant) in patients unable to receive dexamethasone.
4.1.4 Recommendation 1.1d: Use palonosetron in patients unable to receive dexamethasone + (fos)aprepitant
4.1.4.1 Strong recommendation, moderate-quality evidence
Rationale: This recommendation is based on a meta-analysis of six RCTs (one pediatric) evaluating palonosetron versus granisetron/ondansetron single-agent prophylaxis that demonstrated improved acute phase CIV complete control with the use of palonosetron (RR 1.11, 95% CI: 1.02–1.20).
4.1.5 Recommendation 1.1e: Consider adding olanzapine to other CPG-consistent antiemetics
4.1.5.1 Conditional recommendation, moderate-quality evidence
Rationale: Meta-analyses of seven RCTs (one pediatric) demonstrated that when olanzapine versus no olanzapine was evaluated in combination with any concomitant antiemetics, olanzapine improved acute phase CIV control (RR 1.23, 95% CI: 1.05–1.44) and CIN control (RR 1.34, 95% CI: 1.10–1.62). Improved acute phase CIN control was seen in the subset of five RCTs (one pediatric) evaluating the addition of olanzapine versus no olanzapine to a 5HT3RA, dexamethasone, and an NK1RA. This is a conditional recommendation since the panel acknowledged uncertainty regarding olanzapine adverse effects (e.g., cardiotoxicity, metabolic toxicity, and neurotoxicity) in children and adolescents receiving chemotherapy.
4.2 Health Question 1.2: In pediatric patients receiving HEC, what strategies are recommended to prevent delayed phase CINV?
4.2.1 Recommendation 1.2a: Use palonosetron in the acute phase as the preferred 5HT3RA in patients at high risk of delayed phase CINV
4.2.1.1 Strong recommendation, moderate-quality evidence
Rationale: Synthesis of 10 RCTs (0 pediatric) that evaluated the influence of the acute phase 5HT3RA administered on delayed phase CIV control showed benefit of palonosetron versus granisetron/ondansetron (RR 1.26, 95% CI: 1.11–1.43) (Table 3). Benefit of palonosetron on delayed phase CIN control compared with granisetron/ondansetron (RR 1.68, 95% 1.16–2.43) was also seen on meta-analysis of five RCTs (0 pediatric). This is a strong recommendation due to the clear impact that palonosetron administration in the acute phase has on delayed phase CINV control. Given the uncertainty regarding the prevalence of delayed phase CINV in pediatric patients, this recommendation applies to patients at high risk of delayed phase CINV.
4.2.2 Recommendation 1.2b: Use oral aprepitant in the delayed phase, if (fos)aprepitant started in the acute phase
4.2.2.1 Strong recommendation, high-quality evidence
Rationale: Among 11 included RCTs (two pediatric) where patients were randomized to oral aprepitant versus no oral aprepitant for the acute and delayed phase (category 2), patients receiving an aprepitant-containing regimen had improved delayed phase CIV control (RR 1.44, 95% CI: 1.29–1.61), CIN control (RR 1.29; 95% CI: 1.12–1.48), and CINV control (RR 1.34, 95% CI: 1.14–1.56). Oral aprepitant was given for a total of three or 5 consecutive days in the included RCTs. No RCTs were identified that evaluated delayed phase IV fosaprepitant administration. In the context of the acute phase recommendations, the panel made a strong recommendation to use oral aprepitant in the delayed phase in addition to (fos)aprepitant in the acute phase based upon the consistent benefit of delayed phase oral aprepitant and direct pediatric evidence.
4.2.3 Recommendation 1.2c: Add dexamethasone in the delayed phase in patients who received granisetron or ondansetron in the acute phase
4.2.3.1 Strong recommendation, moderate-quality evidence
4.2.4 Recommendation 1.2d: Consider adding dexamethasone in the delayed phase in patients who received palonosetron in the acute phase
4.2.4.1 Conditional recommendation, moderate-quality evidence
Rationale: Among 11 RCTs where acute phase antiemetics were identical and there was randomization between dexamethasone versus no dexamethasone in the delayed phase (category 1), dexamethasone significantly improved delayed phase CIV control (RR 1.11, 95% CI: 1.02–1.20), delayed phase CIN control (RR 1.22, 95% CI: 1.10–1.35), and delayed phase CINV control (RR 1.38, 95% CI: 1.11–1.72).
In a stratified analysis of studies that randomized patients to dexamethasone versus no dexamethasone in the delayed phase (category 1) (Appendix SA10), the effect of dexamethasone on delayed phase CIV control was significantly different depending on the 5HTRA3 received (nonrandomly) in the acute phase (p int <.0001). Specifically, the benefit of dexamethasone compared with no dexamethasone in delayed phase CIV control among patients who received palonosetron in the acute phase was RR 1.04 (95% CI: 1.00–1.08) while among those who received granisetron/ondansetron in the acute phase, it was RR 1.31 (95% CI: 1.20–1.44). In a second stratified analysis (Appendix SA11), the effect of receiving dexamethasone (nonrandomly) in the delayed phase differed depending on receipt (randomized) of palonosetron in the acute phase versus granisetron/ondansetron (p int <.006) (category 3). Thus, the benefit of using dexamethasone in the delayed phase on delayed phase CIV control may be larger in patients receiving granisetron/ondansetron compared with palonosetron in the acute phase.
The panel made a strong recommendation to use dexamethasone in the delayed phase when granisetron/ondansetron had been administered in the acute phase. A conditional recommendation was made to use dexamethasone in the delayed phase when palonosetron had been administered in the acute phase since the potential benefit in delayed phase CINV control and the risks associated with dexamethasone use may be finely balanced.
4.2.5 Recommendation 1.2e: Use dexamethasone in the delayed phase in patients unable to receive oral aprepitant
4.2.5.1 Strong recommendation, moderate-quality evidence
Rationale: This recommendation is based on the meta-analysis of 11 RCTs described in recommendations 1.2c and 1.2d that demonstrated benefit of dexamethasone on delayed phase CINV control. Patients unable to receive an NK1RA are at higher risk of experiencing delayed phase CIV, CIN, and CINV. Thus, dexamethasone should be given in the delayed phase regardless of the 5HT3RA administered in the acute phase.
4.2.6 Recommendation 1.2f: Continue olanzapine in the delayed phase, if started in the acute phase
4.2.6.1 Strong recommendation, moderate-quality evidence
Rationale: The panel valued the results of the meta-analysis of six RCTs (one pediatric) that demonstrated improved delayed phase CIV control in patients who received olanzapine versus no olanzapine starting in the acute phase and continued in the delayed phase (RR 1.53, 95% CI: 1.25–1.89), regardless of the concomitant antiemetics. A benefit in delayed phase CIN control (RR 1.70, 95% CI: 1.45–1.99) was also noted. The panel reasoned that once a decision is taken to add olanzapine in the acute phase based on a risk:benefit assessment, the benefits of continuing olanzapine in the delayed phase should outweigh the risks for most patients. Thus, the panel made a strong recommendation for its continued use in the delayed phase.
4.2.7 Recommendation 1.2g: Do not use 5HT3RAs in the delayed phase
4.2.7.1 Strong recommendation, low-quality evidence
Rationale: Recognizing that 5HT3RA use during the delayed phase is a common practice in many pediatric oncology centers, the CPG panel evaluated their contribution to delayed phase CINV control in a meta-analysis involving four RCTs (0 pediatric) among patients receiving HEC. No benefit on delayed phase CIV control (RR 1.03, 95% CI: 0.97–1.10) or delayed phase CIN was seen (RR 0.98, 95% CI: 0.84–1.13). The recommendation not to use ondansetron or granisetron in the delayed phase after HEC administration was generalized to palonosetron.
4.3 Health Question 2.1: In pediatric patients receiving MEC, what strategies are recommended to prevent acute phase CINV?
4.3.1 Recommendation 2.1a: Use a 5HT3RA + dexamethasone
4.3.1.1 Strong recommendation, moderate-quality evidence
Rationale: This recommendation is based on a meta-analysis of three RCTs (0 pediatric), which demonstrated benefit in acute phase CIV control when a 5HT3RA and dexamethasone were given compared with a 5HT3RA alone (RR 1.29, 95% CI: 1.21–1.39). In addition, a meta-analysis of 11 RCTs (0 pediatric) observed no benefit of triple-agent (5HT3RA, dexamethasone, and (fos)aprepitant) compared with dual-agent (5HT3RA and dexamethasone) prophylaxis in acute phase CIV control (RR 1.03, 95% CI: 1.00–1.07) or acute phase CIN control (RR 1.02, 95% CI: 0.91–1.16).
With respect to 5HT3RA choice, a meta-analysis of three RCTs (one pediatric) showed no difference in acute phase CIV control with palonosetron + dexamethasone versus granisetron or ondansetron + dexamethasone (RR 0.94, 95% CI: 0.86–1.02). Hence, 5HT3RA choice (granisetron, ondansetron, or palonosetron) should be based on institutional resources, feasibility, and patient preference.
4.3.2 Recommendation 2.1b: Use a 5HT3RA + (fos)aprepitant in patients unable to receive dexamethasone
4.3.2.1 Strong recommendation, low-quality evidence
Rationale: Based on recommendation 2.1a, the panel believed single-agent 5HT3RA prophylaxis was likely to be inadequate for patients receiving MEC. While the meta-analysis of three RCTs (one pediatric) evaluating (fos)aprepitant + 5HT3RA versus 5HT3RA monotherapy did not show a significant difference in CIV control (RR 1.15, 95% CI: 0.96–1.37), the panel valued a pediatric RCT that demonstrated a 30% improvement in CIV control with the addition of oral aprepitant to ondansetron.21 In making this strong recommendation, the panel also generalized from the demonstrated efficacy of NK1RAs in pediatric patients receiving HEC and their favorable adverse effect profile.
4.3.3 Recommendation 2.1c: Use a 5HT3RA in patients unable to receive dexamethasone + (fos)aprepitant
4.3.3.1 Strong recommendation, low-quality evidence
4.3.4 Recommendation 2.1d: Consider using palonosetron as the preferred 5HT3RA in patients unable to receive dexamethasone + (fos)aprepitant
4.3.4.1 Conditional recommendation, low-quality evidence
Rationale: To formulate these recommendations, the panel relied on the individual study arm data of included RCTs that described CINV control in patients who received single-agent 5HT3RA prophylaxis. In these RCTs, complete CIV control was reported in 29–92% of patients receiving single-agent 5HT3RA prophylaxis. In recommending palonosetron as the preferred 5HT3RA, the panel drew on the evidence supporting recommendation 1.1d and the results of delayed phase meta-analyses and stratified analyses in patients receiving HEC showing that giving palonosetron in the acute phase leads to improved delayed phase CIV control. While use of a 5HT3RA is strongly recommended, use of palonosetron specifically is a conditional recommendation since the acute phase vomiting risk associated with MEC is wide (30–90%) and the risk of delayed phase vomiting is uncertain.
4.3.5 Recommendation 2.1e: Consider adding olanzapine to other CPG-consistent antiemetics in patients unable to receive dexamethasone + (fos)aprepitant
4.3.5.1 Conditional recommendation, low-quality evidence
Rationale: Synthesis was not possible to address olanzapine use in patients receiving MEC. This recommendation generalizes from the evidence supporting olanzapine use in patients receiving HEC (see recommendation 1.1e). It is a conditional recommendation based on the same safety concerns described previously, the wide range of emetic risk associated with MEC and the lack of direct pediatric evidence.
4.4 Health Question 2.2: In pediatric patients receiving MEC, what strategies are recommended to prevent delayed phase CINV?
4.4.1 Recommendation 2.2a: Consider using dexamethasone in the delayed phase
4.4.1.1 Conditional recommendation, low-quality evidence
Rationale: In making this recommendation, the panel considered the results of a meta-analysis of four RCTs (0 pediatric) where acute phase antiemetics were the same in each study arm and use of dexamethasone versus no dexamethasone in the delayed phase was randomized (category 1). This effect of delayed phase dexamethasone on CIN and CINV control was RR 1.13, 95% CI: 0.99–1.28 and RR 1.15, 95% CI: 0.91–1.44, respectively. In considering these results and the evidence supporting recommendations 1.2c and 1.2d, the panel made a conditional recommendation to use dexamethasone in the delayed phase.
4.4.2 Recommendation 2.2b: Continue oral aprepitant in the delayed phase in patients receiving single-day chemotherapy who received (fos)aprepitant in the acute phase
4.4.2.1 Strong recommendation, moderate-quality evidence
Rationale: A meta-analysis of eight RCTs (0 pediatric) (category 2) demonstrated a benefit in delayed phase CIV control when oral aprepitant was continued in the delayed phase once started in the acute phase versus no aprepitant (RR 1.20, 95% CI: 1.12–1.29). Seven of the eight included RCTs gave oral aprepitant on days 2 and 3 following single-day chemotherapy; one included RCT continued oral aprepitant until day 4 following 2-day chemotherapy. In making this strong recommendation, the panel considered the evidence supporting recommendation 2.1b, the preponderance of evidence in patients receiving single-day chemotherapy and the benefit of continuing oral aprepitant in the delayed phase for CIV control. The panel acknowledged that for patients receiving fosaprepitant in the acute phase, oral aprepitant administration in the delayed phase may vary depending on dosing approved by jurisdictional regulatory bodies (Appendix SA19).
4.4.3 Recommendation 2.2c: Consider not using oral aprepitant in the delayed phase in patients receiving multi-day chemotherapy (≥3 days) who received (fos)aprepitant in the acute phase
4.4.3.1 Conditional recommendation, low-quality evidence
Rationale: This recommendation was based on a meta-analysis of three RCTs (one pediatric; category 3) evaluating receipt of oral aprepitant only in the acute phase versus no aprepitant in patients receiving multi-day chemotherapy (≥3 days), regardless of concomitant antiemetics. Oral aprepitant receipt for three or 5 days of the acute phase did not improve delayed phase CIV control (RR 1.31, 95% CI: 0.97–1.78). However, CINV control based on timing of oral aprepitant administration (acute phase versus acute phase plus delayed phase) was not a primary endpoint of any included RCT. The panel balanced the uncertainty regarding the risk of delayed phase CINV in pediatric patients receiving MEC against the potential for improved delayed phase CIV and made a conditional recommendation to consider not giving oral aprepitant in the delayed phase in pediatric patients receiving MEC blocks 3 days or longer.
4.4.4 Recommendation 2.2d: Continue olanzapine in the delayed phase, if started in the acute phase
4.4.4.1 Strong recommendation, low-quality evidence
Rationale: This recommendation generalizes from the evidence and rationale supporting recommendation 1.2f.
4.5 Health Question 3.1: In pediatric patients receiving low emetogenic chemotherapy (LEC), what strategies are recommended to prevent acute phase CINV?
4.5.1 Recommendation 3.1a: Use a 5HT3RA
4.5.1.1 Strong recommendation, low-quality evidence
Rationale: Given the lack of direct evidence evaluating acute CIV, CIN, and CINV control in patients receiving LEC, the panel drew from evidence regarding CINV prophylaxis during HEC and MEC to make this recommendation. Since CIV risk associated with LEC in the absence of prophylaxis is 10–30%, the panel deemed that CINV prophylaxis was warranted. The panel made a strong recommendation to use a 5HT3RA for CINV prophylaxis in patients receiving LEC based on the extensive pediatric experience with 5HT3RAs, their proven benefit in preventing acute phase CINV in patients receiving HEC and MEC and their favorable adverse effect profile.
4.6 Health Question 3.2: In pediatric patients receiving LEC, what strategies are recommended to prevent delayed phase CINV?
4.6.1 Recommendation 3.2a: Do not use prophylaxis routinely in the delayed phase
4.6.1.1 Strong recommendation, very low-quality evidence
Rationale: We identified no RCTs to inform this question. The panel deemed that, given the low risk of vomiting in the acute phase for patients receiving LEC and the uncertainty regarding the risk of delayed phase CINV following LEC, routine delayed phase CINV prophylaxis is not warranted.
4.7 Health Question 4.1: In pediatric patients receiving minimally emetogenic chemotherapy (minEC), what strategies are recommended to prevent acute phase CINV?
4.7.1 Recommendation 4.1a: Do not use prophylaxis routinely in the acute phase
4.7.1.1 Strong recommendation, very low-quality evidence
Rationale: There were no RCTs identified that evaluated CIV, CIN, or CINV control in patients receiving minEC. Since acute phase CIV risk following minEC is less than 10% in the absence of prophylaxis, the panel deemed that routine acute phase prophylaxis is not warranted.
4.8 Health Question 4.2: In pediatric patients receiving minEC, what strategies are recommended to prevent delayed phase CINV?
4.8.1 Recommendation 4.2a: Do not use prophylaxis routinely in the delayed phase
4.8.1.1 Strong recommendation, very low-quality evidence
Rationale: No RCTs were identified that evaluated acute phase CINV prophylaxis in patients receiving minEC. Since the risk of acute phase CIV during minEC is low and the incidence of delayed phase CINV following minEC is unknown, the panel deemed that routine delayed phase CINV prophylaxis is not warranted.
5 DISCUSSION
We have developed a comprehensive CPG to provide recommendations for acute and delayed phase CINV prevention in pediatric patients. Recommendations were based on SRs that encompassed the experience of both pediatric and adult patients. This is the first CPG to make recommendations for delayed phase CINV prevention in pediatric patients.
Recommendations for CINV prophylaxis are provided for patients who are unable to receive dexamethasone or (fos)aprepitant. Cancer treatment or cellular therapy protocols or patient-specific concerns such as history of fungal infection or adverse effects may preclude dexamethasone use.22-24 Patient age, concerns regarding its effect on chemotherapy clearance, and unintended increases in chemotherapy dose intensity or increasing risk of chemotherapy toxicity may preclude (fos)aprepitant use.25 In most situations, the quality of evidence supporting alternate CINV prophylaxis regimens for patients who are unable to receive dexamethasone or (fos)aprepitant is moderate to low. Clinicians are encouraged to critically evaluate patients’ ability to receive these agents since their omission will likely increase risk of uncontrolled CINV.
Compared with the 2017 CPG on acute phase CINV prevention, fosaprepitant and olanzapine are newly recommended interventions. High-quality evidence supports the interchangeability of IV fosaprepitant and oral aprepitant. Preference will be driven by feasibility issues (e.g., IV access, ability to take oral medications, funding models). Conditional recommendations to consider olanzapine signal the need to carefully weigh the risk:benefit for individual patients in consideration of patient-related risk factors for CIV such as older age, long chemotherapy block duration, history of motion sickness, and inability to receive an NK1RA.26, 27 It is notable that the included pediatric RCT evaluating olanzapine excluded patients <5 years old.
Our chosen proxy for safety, rates of antiemetic discontinuation due to toxicity, was not helpful to identify antiemetic-related adverse effects. Nevertheless, 5HT3RAs, dexamethasone, and oral aprepitant are associated with a low incidence of adverse effects which are usually of minor clinical significance.28 The adverse effects reported in included RCTs of IV fosaprepitant are similar to those associated with oral aprepitant, with the exception of infusion-related reactions. While the safety profile of olanzapine is acceptable among pediatric patients with mental health conditions, some potential adverse effects may be serious (e.g., metabolic effects, QTc prolongation, and extra-pyramidal reactions)29, 30 and the risk:benefit assessment may not be favorable in all pediatric patients receiving chemotherapy. The prospective safety monitoring incorporated into RCTs evaluating olanzapine does not allow a full appreciation of its safety profile in pediatric cancer patients; thus, optimal monitoring strategies are unknown.
The strengths of this CPG relate to the rigorous methods applied to its development: comprehensive SRs; international, interprofessional, and patient advocate representation on the panel; and integration of the acute phase and delayed phase CINV recommendations. Our work is limited by the quality of the published evidence. Definitions of the acute phase and delayed phase and reporting completeness, especially in the delayed phase, lack consistency. Many included RCTs omitted CIN measurement and, when included, many did not use a validated tool. This was especially common in pediatric RCTs. No included pediatric RCT stratified enrollment based on known risk factors for acute phase CIV. Reporting of safety outcomes was inadequate in most studies.
Despite the number of studies included in our SRs, evidence gaps are plentiful and continue to be barriers to ensuring that pediatric patients experience complete CINV control. Among the most fundamental of the evidence gaps listed in Table 4 are the lack of RCTs examining nonpharmacological interventions, absence of strategies using pharmacogenomic data to inform initial CINV prophylaxis selection, scarcity of information regarding risk of delayed phase CINV associated with specific chemotherapies and lack of pediatric antiemetic dosing strategies applicable to multi-day chemotherapy blocks.
| What nonpharmacological interventions to prevent acute phase and delayed phase CINV are effective and safe? |
| What chemotherapy is associated with risk of delayed phase CINV? |
| What is the optimal dosing frequency of palonosetron for patients receiving multi-day chemotherapy? |
| What is the effective and safe oral palonosetron dose? |
| What is the optimal dose of dexamethasone? |
| What is the optimal duration of oral aprepitant in patients receiving multi-day chemotherapy? |
| What is the optimal fosaprepitant dose and dosing frequency? |
| Is it necessary to follow fosaprepitant with oral aprepitant? |
| What are the safety concerns with olanzapine use? |
| What adverse effect monitoring, if any, is required for patients receiving olanzapine? |
| What is the optimal olanzapine dose for prevention of acute and delayed CINV? |
| Can olanzapine be given safely to patients <5 years old? |
| What patient-related factors increase the risk for acute and delayed phase CINV? |
- CINV, chemotherapy-induced nausea and vomiting.
5.1 Implementation Considerations
Integration of these recommendations into clinical care will require intentional and systematic implementation. Obstacles may include intervention cost, lack of dosage forms appropriate for children in some jurisdictions, and clinician and patient resistance to deimplementation of 5HT3RAs in the delayed phase. Cost of recommended antiemetics was not weighed highly by the CPG panel since drug costs vary widely between jurisdictions and over time. As well, the cost of the intervention itself may be outweighed by reductions in costs of interventions to treat uncontrolled CINV (e.g., breakthrough antiemetics) or manage the effects of uncontrolled CINV (e.g., hospital admission, nutrition support). Pediatric-friendly formulations are marketed in most jurisdictions or extemporaneous formulations are available for all recommended antiemetics except for oral olanzapine and oral palonosetron.
The panel chose not to develop antiemetic dosing recommendations. To assist implementation, the pediatric doses of the antiemetics with approved pediatric labels in Canada, the United States, and European Union are presented in Appendix SA19. No optimal pediatric dexamethasone dose for acute phase CINV prophylaxis has been identified.31 While the oral olanzapine dose used in the single included pediatric RCT was 0.14 mg/kg/dose po daily (max: 10 mg),32 others have suggested doses of 0.05–0.2 mg/kg/dose (max: 5 or 10 mg).33, 34
6 CONCLUSION
Based on current literature, control of CINV during the acute and delayed phases in pediatric patients can be optimized. While antiemetic selection is initially based on chemotherapy emetogenicity, CINV prophylaxis should be adjusted from one chemotherapy block to the next based on each patient's history of CINV control. Research to address critical evidence gaps will ensure continued progress toward achieving complete CINV control for all pediatric patients.
ACKNOWLEDGMENTS
Funding was provided by the POGO. We would like to thank Elizabeth Uleryk for her help with conducting the literature searches. We would also like to thank Arden Parker, Nora Wahib, Patrick Cheung, and Thomas Wong for their help with data extraction for the SRs. Lillian Sung is supported by the Canada Research Chair in Pediatric Oncology Supportive Care.
CONFLICT OF INTEREST
L. L. D. has received a research grant from Heron Therapeutics for work outside of this CPG. A. O. owns Pfizer stock. No other author has relevant financial or nonfinancial interests to disclose.




