The relationship between cognitive and neuroimaging outcomes in children treated for acute lymphoblastic leukemia with chemotherapy only: A systematic review
Abstract
Cognitive late-effects have been identified in patients treated with chemotherapy-only protocols for childhood acute lymphoblastic leukemia (ALL), yet the underlying neuropathology is not well understood. This review synthesized recent findings from eight articles investigating the relationship between neurocognitive and neuroimaging outcomes for patients treated for ALL with chemotherapy-only protocols. Reported cognitive domains, imaging methods, and neuroanatomy examined were variable. Despite this, 62.5% (n = 5) of the reviewed studies found a significant relationship between cognitive and imaging outcomes. Greater understanding of the effects of treatment on neuroanatomy and cognitive outcomes is critical for proactively managing ALL cognitive late-effects. Research directions are suggested.
ABBREVIATIONS
-
- ALL
-
- acute lymphoblastic leukemia
-
- CNS
-
- central nervous system
-
- CRT
-
- cranial radiation therapy
-
- IQ
-
- intelligence quotient
-
- MRI
-
- magnetic resonance imaging
-
- MTX
-
- methotrexate
-
- PRISMA
-
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
1 INTRODUCTION
Pediatric acute lymphoblastic leukemia (ALL) is the most common childhood cancer, with approximately two per 100,000 new diagnoses worldwide per year.1 Central nervous system (CNS) directed prophylactic treatment, precise risk assessment, and improved supportive care have led to 5-year survival rates for standard-risk pediatric ALL exceeding 90%.2, 3 With improved survivorship, quality of life outcomes are increasingly important. By the year 2000, methotrexate (MTX) chemotherapy has largely replaced prophylactic cranial radiation therapy (CRT) in modern protocols and while MTX is considered less neurotoxic than CRT, reviews suggest that up to 50% of children develop cognitive deficits after being treated with chemotherapy-only protocols.4 Although outcomes following chemotherapy-only protocols are less severe, with groups performing up to 1 standard deviation higher on formal assessment, the profile of cognitive difficulties appears similar to children treated with CRT.5, 6 Specifically, intelligence quotient (IQ) outcomes are reduced in adults treated for childhood ALL many years after treatment,5, 7, 8 although core deficits are similar to those reported in studies including CRT, occurring predominantly in information-processing domains: working memory,9-11 processing speed,11-14 and attention.9, 10, 14-21
Due to inconsistent clinical neuropsychological follow-up and few longitudinal studies, it remains unclear when cognitive deficits arise and if they are progressive. Cross-sectional data suggest that children with ALL do not differ from their peers on measures of cognition at the commencement of treatment13, 19, 22 but display deficits in IQ and specific core processing skills 2–7 years posttreatment.8, 10 These core skills are important for the development of higher order executive functioning, such as planning, problem-solving, and decision-making,23 which, in turn, impact academic and vocational success and quality of life.
Despite these findings, methodological inconsistencies in study design and setting means it is difficult to accurately define risk for the development of cognitive late-effects after ALL treatment for individual patients. Several factors have been shown to increase risk including female sex,7, 20, 24 younger age at treatment (i.e., <5 years of age),20, 21, 24 treatment variables (cumulative MTX dose, treatment intensity, and routes of administration),20, 21 and genetic factors (single nucleotide polymorphisms in folate pathway genes).25 However, the variance attributed to these factors is typically small to moderate.20 Models incorporating combinations of these variables show an interaction effect but still fail to account for a large amount of variance,20 suggesting that additional factors are mediating the effects of treatment.
Understanding the neuroanatomical mechanism of change underpinning cognitive late-effects is particularly important within a pediatric context. Potential injury is occurring to a system in a state of maturation, with implications for ongoing development in widespread functional neural networks. ALL is most commonly diagnosed in children aged 2–5 years,1 a time when the brain is in a state of intense growth and refinement. Maturational processes such as myelination and the development of complex neuronal connections26 occur at a high rate during this time, a critical period unique to childhood and vital for healthy brain development. These processes operate in a protracted, overlapping fashion. Myelination tends to begin with proximal and then distal pathways, with intracortical regional connections and association networks being the final pathways to develop.27
Several potential mechanisms of damage may underlie cognitive change including direct injury to the cerebral parenchyma resulting in demyelination, secondary inflammation, and microvascular injury.28 Research focus has primarily been on the neurotoxic effects of MTX and more recently, corticosteroids, due to their documented CNS toxicity and ability to penetrate the blood–brain barrier.28, 5 MTX is an antifolate drug that has been associated with demyelinating white matter injury and vascular damage in the developing brain, and corticosteroids have anti-inflammatory properties that can reduce vascular permeability.28 However, given that treatment regimens include multiple potentially neurotoxic therapeutic agents,5 it is difficult to partial out the unique or combined effects of specific agents on the developing brain.
Neurological biomarkers present an alternative strategy to help identify children at risk of cognitive deficits and provide a portal to better target pharmacological and behavioral interventions already shown to be successful in similar groups such as traumatic brain injury and attention deficit/hyperactivity disorder.29, 30 The young age of most children treated for ALL, coupled with the typical cognitive profile, suggests deficits are likely underpinned by disruption to maturing neural networks.31, 32 As the prefrontal cortex and association fibers are the last areas to mature, they remain the most vulnerable to damage by external factors such as MTX and steroids.33
Neuroimaging methodologies used to explore neuropathology after treatment for pediatric ALL have, until recently, focused on structural techniques, which measure the amount and composition of brain tissue and tracts rather than functional changes that may occur in distributed neural networks that underpin cognitive functions identified as vulnerable in ALL. The development of these networks is characterized by increasing connectivity between brain regions, supported by processes such as synaptic development and myelination, which are at risk when neurotoxic agents are administered. Research groups have therefore started to employ functional imaging techniques, such as resting-state magnetic resonance imaging (MRI), to explore the impact of treatment on function and development of specific networks. Despite promising findings, most imaging studies have been conducted in isolation of neurocognitive studies; and it is the combination of these two approaches that potentially offers the greatest insight into the possible contribution of neurobiological insult to ALL cognitive late-effects.
Previous reviews have evaluated cognitive or imaging outcome literature in isolation; the current review focuses on the relationship between these two outcomes. The aim of this paper is to systematically review the literature and synthesize the findings relating to the relationship between cognitive and neuroimaging outcomes in children who have undergone chemotherapy-only treatment for pediatric ALL, to inform research and clinical endeavors.
2 METHODS
The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.34 A comprehensive literature search of six electronic databases was conducted in April 2015. Parameters included (i) English peer-reviewed journal articles that (ii) examined cognitive and neuroimaging outcomes in individuals treated with chemotherapy-only protocols for any subtype of pediatric ALL (i.e., precursor B-cell, mature B-cell, or T-cell ALL) (iii) between the ages of 0 and 18 years. Cognitive measures were defined as any formal tools used to measure cognitive performance (e.g., Wechsler Intelligence Scale for Children). Neuroimaging measures were any methods used to acquire and extract imaging data (e.g., MRI). From 2000, standard-risk ALL patients were typically treated without CRT.3, 5 To limit articles to those including modern treatment regimens, only articles published or accepted for publication in the year 2000 or later were included. Detailed search terms and strategy are listed in Supplementary Table S1.
Papers were excluded if (i) they did not explore the relationship between both outcomes of interest, (ii) they did not include objective, standardized, child-direct measures of cognition, or (iii) they included multiple diagnostic groups (e.g., ALL and acute myeloid leukemia) or treatment groups (e.g., CRT and chemotherapy-only) but did not stratify results by group. The returned articles were reviewed independently by two authors (SH and MC) for eligibility in a stepwise method: (i) title review, (ii) abstract review, and (iii) full-text review. Any discrepancies between authors regarding paper selection were resolved through discussion. For articles that were retained at step iii, full texts were evaluated against eligibility criteria (see Fig. 1). Interrater agreement regarding article inclusion was 100%.
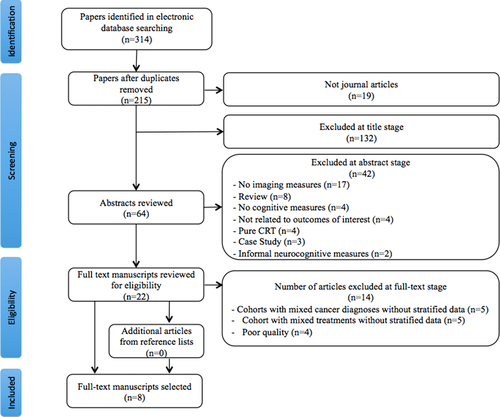
2.1 Quality assessment process
To ensure high-quality evidence and reduce the risk of methodological biases,35 the review authors independently rated each paper retained at step iii for quality. Twenty quality criteria were drawn from previous studies34, 36-38 and modified for the current review (Table 1). All studies comprehensively described the scientific background, defined outcomes and methods of assessment, and summarized results. However, participant numbers, reasons for nonparticipation, efforts to address bias, confounder-adjusted results, and effect sizes were poorly reported (see Table 1) Based on the number of criteria met, two studies were rated of high quality (≥15 criteria met) and four were classified as low quality (<11 criteria met). Low-quality studies were excluded from further analyses, leaving eight articles.
| Section | Criteria | n | % | |
|---|---|---|---|---|
| Introduction | 1 | Explain the scientific background and rational for the study | 8 | 100 |
| 2 | State specific objectives and hypotheses | 5 | 63 | |
| Methods | 3 | Describe the setting, locations, and relevant dates, including periods of treatment, recruitment and data collection | 5 | 63 |
| 4 | Give the eligibility criteria and the sources and methods of selection of participants (and controls if applicable) | 7 | 88 | |
| 5 | If included control group, gives matching criteria | 3/4 | 75 | |
| 6 | Included appropriate and representative methods of selection | 4 | 50 | |
| 7 | Sufficient original dataa | 7 | 88 | |
| 8 | Clearly defines all outcomes, treatment, predictors, potential confounders, and effect modifiers | 8 | 100 | |
| 9 | For each variable of interest, gives sources of data and details of methods of assessment (measurement). Uses valid and reliable measures | 8 | 100 | |
| 10 | Describe any efforts to address potential sources of bias (e.g., blinded to group, double marking, reports interrater reliability) | 3 | 38 | |
| 11 | Describe all statistical methods, including which groupings were chosen and why | 7 | 88 | |
| Results | 12 | Report numbers of individuals at each stage of study, e.g., numbers potentially eligible, recruited, etc., including reasons for nonparticipation at each stage | 2 | 25 |
| 13 | Gives characteristics of study participants (demographics, clinical/treatment details, social) | 6 | 75 | |
| 14 | Report numbers/summary measure of outcome in each outcome of interest category | 5 | 63 | |
| 15 | Gives unadjusted estimates and confounder-adjusted estimates and their precision/effect size. Make clear which confounders were adjusted for and why included | 2 | 25 | |
| 16 | Report category boundaries where continuous variables were categorized | 3/6 | 50 | |
| Discussion | 17 | Summarize key results with reference to study objectives | 8 | 100 |
| 18 | Discuss limitations of study | 7 | 88 | |
| 19 | Gives cautious overall interpretation of results, including generalizability | 4 | 50 | |
| Other | 20 | Gives source of funding and conflict of interest declaration | 4 | 50 |
2.2 Data extraction process
Information extracted. from the eight articles included sample characteristics for patient and control groups (sample size, sex, age at diagnosis, age at assessment, time since treatment), treatment variables (protocol, cumulative MTX), methodological factors (cognitive measures, scanning methods, neuroimaging measures), results (significant and nonsignificant relationship between outcomes of interest), and authors interpretation of results. The principle summary measure used was effect size, as measured by the strength of correlation between cognitive and imaging outcomes.
3 RESULTS
Results of the article selection process are presented in Figure 1. The reference lists of the eight articles were manually searched; however, it did not yield any additional references. The summary data extracted from the reviewed articles can be seen in Supplementary Table S2.
3.1 Sample characteristics
Patient sample sizes ranged from 10 to 97, with a mean of 37.25 and median of 28.5, indicating skew toward smaller sample sizes. Five studies (62.5%) included patient samples sizes less than 3012, 39-42 (Table 2). Four studies (50.0%) included control groups with the following variable characteristics: community-based,12, 41 siblings or close friends,39 relatives, or neighbors.40 Groups were commonly matched for socioeconomic status,12, 39-41 age,12, 40, 41 and sex.12, 40, 41 One study matched groups for handedness.40
| ALL, n (% male) | Control, n (% male) | |
|---|---|---|
| Ashford et al. 20109 | 97 (57) | – |
| Duffner et al. 201443 | 59 (54) | – |
| Edelmann et al. 201344 | 38 (67) | – |
| Genschaft et al. 201339 | 27 (48) | 27 (48) |
| Hill et al. 200440 | 10 (40) | 10 (40) |
| Kesler et al. 201012 | 28 (54) | 31 (52) |
| Kesler et al. 201441 | 15 (60) | 14 (43) |
| Montour-Preloux et al. 200542 | 24 (54) | – |
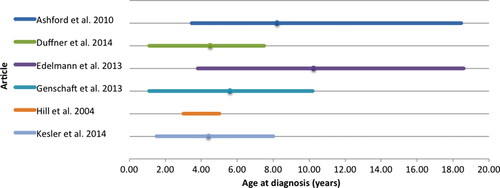
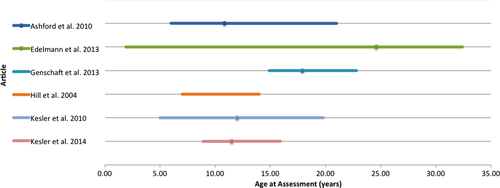
The pooled mean age at diagnosis was 6.31 years (range 1.1–18.6 years) (Figure 2). One study42 did not provide a range for age at diagnosis and another study12 did not provide range or mean age at diagnosis. Pooled mean age at assessment was 13.97 years (range 1.9–32.4 years) (Figure 3). One study40 did not provide a mean age at assessment and another study43 provided no data for range or mean age at assessment. Time since treatment ranged from active treatment to 18 years posttreatment remission. One study44 did not provide a mean time since treatment completion but did include mean time since diagnosis. Three studies did not report mean data for time since treatment; however, two of these included a minimum time off treatment,9, 40 and the other study provided a range for time off treatment.39
All studies included patients treated with intrathecal drugs. Four studies included patients treated with high-dose MTX.9, 12, 44, 41 In six studies, patients were treated with glucocorticosteroids, most commonly a combination of prednisone and dexamethasone,9, 12, 44, 39, 41 while leucovorin was used in four studies.12, 43, 41, 42
3.2 Methodological factors
All eight studies employed a cross-sectional design. Cognitive measures varied. Most commonly utilized were the Wechsler Intelligence Scales,9, 12, 43, 44, 41, 42 Woodcock–Johnson Tests of Cognitive Abilities,12, 43, 44, 41 and the Wide Range Assessment of Memory and Learning.12, 43, 40, 41 The most commonly assessed cognitive domains were IQ,9, 43, 44, 39, 41, 42 attention,9, 12, 43, 39 memory,9, 12, 43, 44, 39, 41, 42 and processing speed.12, 43, 41
Four studies used whole brain measures of neuropathology such as global gray and white matter volumes,12 or presence of white matter changes consistent with leukoencephalopathy.9, 43, 42 Three studies used volumetric measures to produce regional brain volumes. This included segmented regions of white and gray matter volumes,12 subcortical white and gray matter volumes,39 and volumes of the left and right hippocampus.40 Two studies investigated functional neuropathology in the form of hyper- or hypoconnectivity41 and neural activation.44
3.3 Relationship outcomes
Five studies identified a significant relationship between cognitive and neuropathology outcomes in patients.9, 43, 44, 39, 41 No relationships were found between whole brain measures of tissue volumes and cognitive outcomes; however, presence of leukoencephalopathy was found to negatively correlate with measures of attention9, 43 and working memory.9 The strength of these relationships was not reported.
Significant relationships were found between subcortical regions and cognitive outcomes (see Table 3). Decreased amygdala volume was associated with decreased ability to encode and maintain verbal information and sustained attention performance.39 Decreased hippocampal volume was associated with decreased ability to encode and maintain figural information.39 Reduced caudate volume was associated with decreased sustained attention performance.39 Effect sizes were either not reported or small.45
| Area | Structure | Imaging outcome | Cognitive outcome | r |
|---|---|---|---|---|
| Global | Whole brain | ↑Leukoencephalopathy | ↓Attention9, 43 | NR |
| ↓Working memory9 | NR | |||
| Subcortical | Amygdala | ↓Volume | ↓Verbal memory39 | 0.291 |
| ↓Attention39 | −0.29 | |||
| Hippocampus | ↓Figural memory39 | 0.282 | ||
| Caudate (L & R) | ↓Attention39 | −0.36 | ||
| −0.31 |
- ↑, increased; ↓, decreased; L, left; NR, not reported; r, correlation coefficient; R, right.
Two studies, both published in the last 3 years, investigated the relationship between functional neuropathology and cognitive outcomes (Table 4). A significant relationship was found between increased neural activation in the left inferior frontal gyrus and recognition of word pairs.44 Effect size was not reported and a control group was not included. Significant relationships were also found for reduced connectivity between brain regions and cognitive outcomes: decreased connectivity between the left hippocampus and left lingual gyrus and lower IQ41 and reduced connectivity between the left lingual gyrus and right amygdala and poorer performance on a color naming task.41 Medium effect sizes were found for hypoconnectivity outcomes.41, 45 No relationships were found between connectivity and cognitive outcomes for age- and sex-matched healthy controls.
| Area | Structure | Imaging outcome | Cognitive outcome | r |
|---|---|---|---|---|
| Frontal | Left inferior frontal gyrus | ↑Activity intensity (fMRI) | ↑Word pair recognition44 | NR |
| Intercortical | Temporal-occipital | ↓Connectivity (rsfMRI) | ↓IQ41 | 0.541 |
| ↓Color naming41 | 0.524 |
- ↑, increased; ↓, decreased; fMRI, functional magnetic resonance imaging; NR, not reported; r, correlation coefficient; rsfMRI, resting-state functional magnetic resonance imaging; IQ, intelligence quotient.
4 DISCUSSION
Despite the limited number of studies investigating the relationship between cognitive and neuroimaging outcomes after chemotherapy-only treatment for pediatric ALL, five of eight studies found a significant relationship between cognitive and neuropathology outcomes. However, specific results were variable, largely due to methodological variations and limitations across studies (e.g., cross-sectional, small samples, inability to consider risk factors), and effect sizes were infrequently reported. In regards to imaging outcomes, whole brain measures of pathology, such as global brain volumes, were not significantly related to cognition and the presence of leukoencephalopathy did not correlate well with cognitive outcomes.9, 43, 42 Studies reporting on specific brain regions identified reasonably consistent relationships between subcortical white and gray matter volumes and measures of memory and attention.39 The two studies that employed advanced functional imaging methods found significant relationships between frontal brain activity and intercortical white matter connectivity and word pair recognition, color naming, and IQ.44, 41 The results of these three more targeted studies suggest that a frontal–subcortical network, such as the frontal–striatal circuit, may be implicated during chemotherapy-only treatment. Results from a previous study by Lesnik et al.14 in the pediatric ALL population reported similar findings, that is, a frontal subsystem comprised of the prefrontal cortices and lobuli VI–VII accounted for a significant amount of variance in a composite measure of visual-spatial and visuomotor skills.
Interestingly, two reviewed studies by Kesler and colleagues12, 41 found abnormal neural structure and connectivity in those treated for pediatric ALL compared with typically developing peers. While abnormal neural activity in adults is thought to reflect an adaptation of the CNS in response to the environment,46 outcomes after damage to the CNS during childhood are variable. Anderson et al.’s47 model of early brain injury places alterations to brain activity on a continuum from plasticity (good recovery) to vulnerability (poor recovery). They argue that, as damage occurs to the underdeveloped brain, abnormal anatomy or functional reorganization may reflect relocation of cognitive processes to undamaged areas to facilitate cognitive development47; however, neuroplasticity does not necessarily constitute cognitive recovery. In the same model, the authors suggest that a complex interplay of injury, age, environmental, and intervention factors will determine where an individual's recovery trajectory will fall along the continuum. This model may help understand the neural differences identified in survivors of pediatric ALL and the factors that influence cognitive outcomes, emphasizing the importance of interpreting abnormal neuroanatomy and function within a developmental context. Longitudinal studies investigating the reproducibility of this atypical neural reorganization in survivors of pediatric ALL and the relationship to cognitive outcomes are warranted.
Limitations in the neuroimaging techniques used to date may contribute to the current lack of association between imaging and cognitive outcomes. In particular, volumetric investigations have provided little insight into the mechanism of damage. Increasing spatial resolution of anatomical scans and more sophisticated cortical parcellations may produce more sensitive volumetric outcome data. Future research utilizing advances in the acquisition and analysis of structural and functional connectivity imaging techniques (e.g., tract-based spatial statistics or resting-state MRI) may provide a greater understanding of the impact of treatment on white matter pathways and brain activation. This can be achieved with serial neuroimaging; however, given the lack of longitudinal data, identifying appropriate time points to scan patients should be guided by neurocognitive findings. Transient changes have been seen while on treatment and varied findings have been reported 2–3 years posttreatment. Therefore, a scan at diagnosis, completion of treatment, and 2 years posttreatment would allow for a detailed evaluation of treatment-related neuropathology. This is in line with the COG AALL06N1 study, which utilizes a similar follow-up schedule. Future studies must adjust for factors that may mediate the impact of treatment on neuroimaging outcomes such as age at diagnosis and time since treatment.
Use of insensitive neurocognitive measures may also contribute to the lack of associations detected between cognitive and neuroimaging outcomes. For example, studies often define attention as a single construct and use a single outcome measure; however, children who have had chemotherapy-only treatment exhibit specific deficits, such as poorer sustained attention, with intact response inhibition.21 Future research should employ more targeted assessment protocols focusing on subdomains of attention, working memory, and processing speed. A valuable cognitive measure in this population should be sensitive enough to delineate the components of commonly reported deficits such as attention (verbal, visual, sustained, selective and divided attention, inhibition, and hyperactivity), working memory (visual, verbal, and cognitive load), and information-processing speed, which should be differentiated from motor speed. When designing studies to assess cognitive outcomes, it is imperative tasks be assessed for sensitivity and construct validity.
In addition to studies taking a generalist cognitive approach, several methodological differences rendered results difficult to synthesize and likely underlie the variability in study findings. Most notably, treatment and data collection dates were difficult to extract. Treatment for pediatric ALL has undergone significant change in the time period covered by the reviewed studies; therefore, significant differences exist between the intensity of treatment protocols. For example, protocol AALL0232 includes patients who are randomized to receive high-dose MTX or capizzi arms. The capizzi regimen combines graded intensification of MTX in association with asparaginase, a drug linked with sagittal sinus thrombosis and stroke.48 In the same protocol, leucovorin (folinic acid) is administered to those receiving high-dose MTX to mitigate the side effects of MTX.49 Other significant differences between treatment protocols that likely impact on neurocognitive outcome include dose, timing and administration of MTX, administration of vincristine, and steroid type.44, 50, 51 In addition, treatment of B- and T-cell ALL has previously differed in steroid type, with T-cell patients receiving predominately dexamethasone, which has been linked to negative neurocognitive outcomes.50 Detailed reporting of treatment details is imperative, and new studies are needed to determine the outcomes of modern regimens.
Of concern, methodological issues reported in this paper mirror issues raised in a review carried out nearly 30 years ago.52 The focus for future studies must be on improving study designs to streamline this field of research. Detailed reporting of data collection and treatment details, exclusion/inclusion criteria, and effect sizes should be emphasized. Targeted research questions based on previous findings, incorporation of baseline imaging and cognitive assessments, longitudinal studies, and fast-tracked publications to allow for the clinical translation of research findings to appropriate treatment regimens are areas that would increase the quality of research. Mapping the trajectory of neuropathology and cognitive development over this time would also provide a scientific basis for the timely introduction of interventions already shown to be effective in this and other populations.53
Several limitations of the current review must be acknowledged. Firstly, given that the reviewed studies were cross-sectional, causation cannot be attributed to either outcome of interest. Secondly, effect sizes were typically small for those studies that found significant relationships; therefore, it is likely that other factors mediate the relationship between outcomes such as age at treatment or sex.7, 54 Given the small number of articles extracted and the variance in methodology, information relating to mediators could not be reliably interpreted in this review. Lastly, given that studies outcomes were heterogeneous and effect sizes were not consistently reported, findings could not be compared statistically as would be done in a meta-analysis. Results should be interpreted with these limitations in mind.
This review has synthesized recent literature to support future efforts to explore these relationships with targeted imaging and cognitive batteries. By designing studies with the recommendations from this review, future findings will more accurately categorize cognitive outcomes and the related underlying neuropathology. Ultimately, with this information, biomarkers could be identified, allowing for early identification of children at risk of neurocognitive dysfunction. This would allow targeted interventions to be implemented early, employing a proactive approach to cognitive morbidity and reducing the impact of brain pathology on functional outcomes and their ongoing impact on academic, social and vocational success, and quality of life. In summary, due to variable methodologies and research findings, limited conclusions can be made about the neurological damage underlying cognitive deficits reported in survivors of childhood ALL at this time.
ACKNOWLEDGMENTS
We would like to acknowledge the support and advice provided by Poh Chua regarding the electronic literature search process. We would like to acknowledge the financial support of the Leukaemia Foundation, The Royal Children's Hospital Foundation and the Victorian Government Infrastructure Funding. SH is supported by an MCRI Postgraduate Health Research Scholarship, MM and CD are supported by MCRI Career Development Awards and VA is supported by an NHMRC Senior Practitioner Fellowship.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.




