Relapsed or Refractory Lymphoblastic Lymphoma in Children: Results and Analysis of 23 Patients in the EORTC 58951 and the LMT96 Protocols
Conflict of interest: Nothing to declare.
Abstract
Background
The treatment of children with T-cell lymphoblastic lymphoma (T-LBL) and precursor B-cell lymphoblastic lymphoma (pB-LBL) has improved during the last decades. However, patients with relapsed or refractory lymphomas still have a poor prognosis.
Methods
We report the characteristics and evolution of T-LBL and pB-LBL relapses in two multicenter prospective studies (LMT 96, European Organization for Research and Treatment of Cancer 58951).
Results
From 1997 to 2008, 194 patients were included in these studies (157 T-LBL; 37 pB-LBL); among them, 23 patients underwent relapse or progression (18 T-LBL and 5 pB-LBL). The median age was 7.7 years (range 1.4–16.3). The survival rate at 8 years was 8.7% (21 deaths). The median time from diagnosis to relapse was 9 months [1–69] and 11 months [1–45] for T-LBL and pB-LBL, respectively. Twenty-two patients received a second-line treatment but remission was achieved in only seven patients. In 10 patients, intensification with hematopoietic stem cell transplantation (HSCT) was performed and four of them had a second relapse. Two patients still alive had T-LBL, experienced relapses 15 and 69 months after diagnosis, and received HSCT. Relapse during the intensive phase and second-line treatment without HSCT were identified as risk factors for bad prognosis (P = 0.01).
Conclusions
The results of second-line treatment, including intensive chemotherapy and HSCT, show that salvage treatment is still disappointing in controlling refractory forms. Early identification of patients at high risk of relapse is mandatory, allowing earlier intensification. Valid prognostic parameters, such as biological markers, are needed. International cooperation is warranted to collect more data on these rare diagnoses.
Abbreviations
-
- BM
-
- bone marrow
-
- CNS
-
- central nervous system
-
- CR
-
- complete response
-
- HSCT
-
- hematopoietic stem cell transplantation
-
- pB-LBL
-
- B-cell lymphoblastic lymphoma
-
- T-LBL
-
- T-cell lymphoblastic lymphoma
INTRODUCTION
The majority of lymphomas in children are non-Hodgkin lymphomas, and among them, 30% are lymphoblastic lymphomas (LBL). T-cell lymphoblastic lymphomas (T-LBL) are the most frequent (about 80%), and precursor B-cell lymphoblastic lymphoma (pB-LBL) represent 20%.1 Over the past years, LBL prognosis has greatly improved. With acute lymphoblastic leukemia (ALL) treatment strategy including intensive phase, central nervous system (CNS) prophylaxis, and maintenance, 85% overall survival (OS) can be achieved, as discussed in recent studies.2-5
However, children with refractory or recurrent LBL are generally thought to have a poor prognosis. Recent data indicate that the survival of children who relapse is very low: a relapse rate of 10.5% with a 14% survival rate was reported in the German cohort of BFM 90–95 LBL patients.3 The Franco-Belgian cohort European Organization for Research and Treatment of Cancer (EORTC) 58881 of T-LBL patients reported a relapse rate of 15.9%, among which 44% survived.2 Intensification of first-line treatments resulted in fewer relapses, but refractory disease after first-line intensive therapy seemed more difficult to cure. Those with chemosensitive disease are usually considered for intensification, with either autologous or allogeneic hematopoietic stem cell transplantation (HSCT).
In this study, we reviewed the clinical characteristics and treatment received by children with T-LBL and pB-LBL who relapsed after a first-line treatment according to the EORTC 58951 and the French Society of Cancer of the Child (SFCE) LMT 96. The aims of this retrospective study were to describe the clinical characteristics of lymphoma relapses in these two protocols and identify common prognostic factors in this population of aggressive and refractory lymphomas.
PATIENTS AND METHODS
Patients
From February 1997 to July 2008, a total of 194 assessable children and adolescents with newly diagnosed LBL were enrolled in the EORTC 58951 and the LMT 96 trials.4, 5 Both were approved by the institutional review boards of the participating institutions. In EORTC 58951, 89 patients were enrolled in 14 centers in France, Belgium, and Portugal (74 T-LBL and 15 pB-LBL). In LMT 96, 105 patients were enrolled in 18 French centers (83 T-LBL and 22 pB-LBL). Patients were treated according to their stage of disease, after obtaining informed consent of the patients and/or their parents. Data were extracted retrospectively from local center databases and medical charts. The following information was collected: age at diagnosis, sex, site, disease burden, pathology and cytogenetic, date and site of relapse, treatment, and outcome.
First-Line Treatment
As for the previous 58881 EORTC protocol,2 the 58951 EORTC protocol 6 was derived from the Berlin–Frankfurt–Munster (BFM) study.7 Neuromeningeal prophylaxis consisted of six triple intrathecal injections (ITs) (methotrexate [MTX]/cytarabine/corticosteroids) during induction–consolidation–intensification, four triple IT during the cycles of high-dose MTX IV, and six triple IT during maintenance treatment. No cranial irradiation was administered, even in patients with CNS involvement. Length of maintenance was 18 months.
The LMT 96 protocol of the SFCE was also derived from the BFM protocol, but the administration of some chemotherapy agents was modified.5 IV MTX was infused over 3 hr at a lower dose (3 g/m² instead of 5 g/m²) and with more frequent injections. Patients with CNS involvement received radiation. Stage IV patients had a longer maintenance treatment than patients with lower stages (24 and 18 months, respectively, for the total duration of treatment).
Initial Staging and Evaluation of Response
The initial stage was evaluated according to the St. Jude classification.8 Tumor response was assessed at day 8, at the end of the induction phase, and after each treatment phase. The evaluation of the residual mass for mediastinal lymphomas was made by chest radiography or scan. Complete response (CR) was defined as the disappearance of clinical symptoms and radiological findings.
Definition of Progression and Relapse
Progression was defined as the increase in tumor burden before reaching remission during chemotherapy, that is increased volume or persistence of more than 25% of the initial mass, increased marrow blasts of more than 25%, any Cerebrospinal fluid blasts, or the appearance of new LBL locations. Relapse was defined as the presence of lymphoma cells in a medullary or extramedullary site, after having previously achieved a CR. Relapses were documented by cytology and/or histology. Early relapse was defined as the occurrence of relapse during treatment or less than 6 months after discontinuation of maintenance therapy. Late relapse was defined as the occurrence of relapse 6 months or more after the end of maintenance therapy.
Outcome
OS and event-free survival (EFS) were evaluated at 8 years follow up. Death, relapse, treatment-related toxicity, and emergence of a second cancer were considered as events.
Statistical Analyses
OS was calculated from the date of diagnosis of relapse until the date of death. EFS was defined as the time from diagnosis of relapse to the first outcome event (failure, induction death, death during remission, and new relapse). Survival curves were constructed by the Kaplan–Meier method and analyzed by the log-rank test.9 Descriptive statistics were used to characterize the population. For continuous variables, means and standard deviation or median and ranges were reported when appropriated. Comparisons were made using the t-test. For categorical variables, proportions were reported. Differences in group proportions were compared using χ2 test. Statistical significance was established with a P value < 0.05. The analysis was performed using GraphPad Prism 5 ® (GraphPad Softwear, San Diego, CA).
RESULTS
Characteristics of Patients
Among the 194 patients enrolled in the two protocols, 157 had T-LBL and 37 had pB-LBL. Twenty-three patients (11.7%) had a relapse or progressive disease: 18 with T-LBL and five with pB-LBL. The median age at diagnosis was 7.7 years [1.4–16.3]. The main characteristics of patients are summarized in Table I.
| All LBL | T-LBL | B-LBL | |||||
|---|---|---|---|---|---|---|---|
| No. of patients | pOS after relapse (%) | P value | No. of patients | pOS after relapse (%) | P value | ||
| Number of patients | 23 | 18 | 5 | ||||
| Gender | |||||||
| Male | 11 | 7 | 9 | 9 | 2 | ||
| Female | 12 | 10 | 0.97 | 9 | 14 | 0.61 | 3 |
| Age at diagnosis (years) | |||||||
| <10 | 14 | 22 | 12 | 25 | 2 | ||
| ≥10 | 9 | 8 | 0.94 | 6 | 11 | 0.92 | 3 |
| Initial stage | |||||||
| III | 15 | 6 | 15 | 3 | 0 | ||
| IV | 8 | 12 | 0.06 | 3 | 33 | 0.47 | 5 |
| LDH level | |||||||
| <2N | 12 | 8 | 7 | 14 | 5 | ||
| >2N | 8 | 12 | 0.41 | 8 | 12 | 0.95 | 0 |
| BM relapse | |||||||
| Yes | 12 | 8 | 8 | 12 | 4 | ||
| No | 11 | 10 | 0.85 | 10 | 11 | 0.4 | 1 |
| Progression or relapse during intensive therapy | |||||||
| Yes | 13 | 0 | 11 | 0 | 2 | ||
| No | 10 | 20 | 0.025 | 7 | 29 | 0.06 | 3 |
| HSCT | |||||||
| Yes | 10 | 20 | 6 | 33 | 4 | ||
| No | 13 | 0 | 0.011 | 12 | 0 | 0.015 | 1 |
| Allogenic | 8 | 5 | 3 | ||||
| Autologous | 2 | 1 | 1 | ||||
| Median time to relapse (months) | |||||||
| From diagnosis | 10 [3.5–14.5] | 9 [1–69] | 11[1–45] | ||||
Among 18 patients with T-LBL, 16 had mediastinal presentation and one had CNS involvement. Median tumor size was 13.7 cm [7.5–20]. Two patients progressed and 16 presented with relapse.
Of the five patients with pB-LBL, all were stage IV at diagnosis, three had CNS involvement, and four had bone marrow (BM) involvement. Among them, three experienced relapse and two had progressive disease.
Genetic or cytogenetic data were available for only 10 patients (six T-LBL and four pB-LBL) and abnormal in three cases (compound heterozygous mutations of the PMS2 gene inherited from both parents in a T-LBL patient, 2p deletion in a T-LBL patient, and trisomy 8 in a pB-LBL patient).
Chronology and Site of Relapse
The sites and times of relapses are shown in Figure 1. Among T-LBL patients, relapses were mainly local (eight isolated local relapses, four combined local–BM or other site relapses, and two combined local–BM–CNS relapses, none of whom had CNS involvement at initial diagnosis). Only two patients had BM or combined BM relapses. No isolated CNS relapse was reported. Two patients had local progression during treatment.
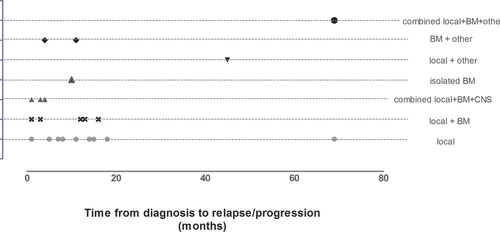
Among T-LBL patients, 16 had early relapse or progression: 11 patients during intensive treatment and five during maintenance therapy. Two patients relapsed more than 6 months after the end of maintenance treatment. The median time from diagnosis to relapse was 9 months [range 1–69].
For pB-LBL, two had early relapse (local–BM and local–BM–other site), two had early progression (combined local BM CNS and local BM), and one had late relapse (combined local BM other site). The median time between diagnosis and relapse was 11 months [range 1–45].
Second-Line Treatment
Both EORTC 58951 and LMT 96 protocols used a second-line treatment strategy, summarized in Table II, based on COOPRALL 97 for patients with LBL who experienced relapse or progression. The treatment consisted combined a course of VANDA (see Table II) with alternating blocks of chemotherapy (R1 and R2 in Table II) 10 followed by HSCT. The patient received allogeneic HSCT if an HLA identical donor was found, or autologous HSCT in the absence of a donor. All but one patient received the VANDA course; the patient who did not receive it had a local recurrence of the lymphoma with simultaneous diagnosis of a solid tumor (glioblastoma) and received palliative treatment.
| Medication | Route | Dosage | Day | |||||
|---|---|---|---|---|---|---|---|---|
| VANDA | ||||||||
| Dexamethasone | Per os | 20 mg/ m2/day | 1 | 2 | 3 | 4 | 5 | 1/2 dose |
| Aracytine | IV 3 hr | 2 × 2 g/m2/day | 1 | 2 | ||||
| Mitoxantrone | IV 2 hr | 8 mg/m2/day | 3 | 4 | ||||
| VP16 | IV 1 hr | 150 mg/m²/day | 3 | 4 | 5 | |||
| Peg-Asparaginase | IM | 2,500 UI/m² | 6 | |||||
| Cytarabine | IT | Depending on age | 5 | |||||
| Prednisolone | IT | Depending on age | 5 | |||||
| Bloc R2 | ||||||||
| Dexamethasone | Per os | 20 mg/m2/day | 1 | 2 | 3 | 4 | 5 | 1/2 dose |
| Thioguanine | Per os | 100 mg/m2/day | 1 | 2 | 3 | 4 | 5 | |
| Vindesine | IV | 3 mg/m2/day | 1 | |||||
| Methotrexate | IV 36 hr | 1 g/m2 | 1 | |||||
| Ifosfamide | IV 1 hr | 400 mg/m2/day | 1 | 2 | 3 | 4 | 5 | |
| Daunorubicine | IV 2 hr | 35 mg/m2 | 5 | |||||
| PEG-Asparaginase | IM | 2,500 UI/m2 | 6 | |||||
| Methotrexate | IT | Depending on age | 2 | 6 | ||||
| Cytarabine | IT | Depending on age | 2 | 6 | ||||
| Prednisolone | IT | Depending on age | 2 | 6 | ||||
| Bloc R1 | ||||||||
| Dexamethasone | Per os | 20 mg/m2/day | 1 | 2 | 3 | 4 | 5 | 1/2 dose |
| Mercaptopurine | Per os | 100 mg/m2/day | 1 | 2 | 3 | 4 | 5 | |
| Vincristine | IVD 2 mg) | 1.5 mg/m2/day | 1 | 6 | ||||
| Methotrexate | IV 36 hr | 1 g/m2 | 1 | |||||
| Cytarabine | IV 3 hr | 2 × 2 g/m2 | 5 | |||||
| PEG-Asparaginase | IM | 2,500 UI/m2 | 6 | |||||
| Methotrexate | IT | Depending on age | 2 | |||||
| Cytarabine | IT | Depending on age | 2 | |||||
| Prednisolone | IT | Depending on age | 2 | |||||
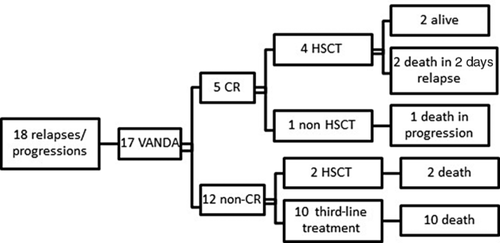
Evolution after Second-Line Treatment
T-LBL patients
Only five of 18 patients achieved a second CR after the second-line treatment: two isolated local relapses, one BM relapse, one combined local BM other, and one isolated local progression. Four of them underwent HSCT, and two patients are still alive. Of the two patients with progression, only one achieved CR, but was unable to receive an HSCT due to early second relapse, and the other underwent HSCT without achieving CR.
In patients treated with curative or palliative intent, third-line treatments were administered. One patient received protocols B1–B2 (from the FRALLE protocol.11) Several patients received chemotherapy according to the COOPRALL protocol (maintenance, second VANDA; etoposide-containing schemes, vindesine/mercaptopurine/MTX/corticosteroids). Other patients received various treatments as daunorubicin, MIDE (MTX, ifosfamide, etoposide, and dexamethasone) and local irradiation/vindesine/mercaptopurine, COPADEM, mitoxantrone/etoposide, aracytine/alemtuzumab, and vindesine/daunorubicin/vinblastine.
pB-LBL patients
Only one of three patients with relapse achieved a second CR and underwent HSCT. The others underwent HSCT as well, but without previous CR.
Among two patients with disease progression, one did not achieve a second CR but received HSCT. The other patient had a local BM CNS progression and received two courses of VANDA and two courses of COPADEM. He achieved CR and underwent HSCT, but died of conditioning toxicity. A third-line therapy administered before HSCT was attempted in one case using a “Capizzi” course (vincristine/MTX/asparaginase), but a CR was not achieved.
Allogeneic and Autologous Stem Cell Transplantations
Among the 23 patients with relapse or progressive disease, 10 HSCT (eight allogeneic and two autologous) were performed. HSCTs were performed soon after the diagnosis of relapse, with a median time of 2 months from relapse/progression to HSCT.2-6 Five HSCTs were done in CR, and five in progressive disease. Characteristics of patients, conditioning regimens, and types of HSCT are summarized in Table III.
| Patient | Diagnosis | Site of relapse | Time to relapse (months) | Status before HSCT | Conditioning regimen | Donor | Time to HSCT after relapse (m) | Complications | Time to 2 days relapse | Third- line treatment or post-HSCT failure | State | Time to death after relapse (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LMT05009 | pB-LBL | Combined local + BM | 11 | noCR | EDX, TBI, ATG | URD (UBMT) | 4 | Cardiac insufficiency | None | Palliative | Dead | 4 |
| LMT07019 | pB-LBL | Local + BM | 1 | P | EDX, TBI, ATG | URD (UBMT) | 3 | None | None | Palliative | Dead | 6 |
| LMT16005 | pB-LBL | Local + BM | 16 | CR | EDX, TBI, ATG | URD (UBMT) | 5 | GVHD II, CMV, EBV | None | Palliative | Dead | 13 |
| EORTC8 | T-LBL | BM + other | 2 | noCR | TBI, VP16 | MSD | 2 | GVHD II | 6 | Irradiation | Dead | 10 |
| EORTC43 | T-LBL | Isolated BM | 10 | CR | EDX, TBI | URD (UCBT) | 2 | CMV reactivation | 4 | ND | Dead | 4 |
| LMT13002 | T-LBL | Local | 1 | P | EDX, TBI | MSD | 3 | None | None | None | Dead | 5 |
| EORTC24 | T-LBL | Local | 15 | CR | EDX, TBI, ATG | URD (PBCT) | 4 | Isolated cutaneous GVHD | None | None | Alive | _ |
| LMT14011 | T-LBL | Local + BM + other | 69 | CR | TBI, VP16 | URD (UBMT) | 3 | Ophthalmic GVHD | None | None | Alive | _ |
| LMT07002 | pB-LBL | Local + other | 45 | noCR | BAM | Autologous HSCT | 6 | None | 10 | Palliative | Dead | 13 |
| LMT01001 | T-LBL | Local | 14 | CR | EDX, TBI | Autologous HSCT | 2 | None | 4 | Holoxan, VP 16 | Dead | 7 |
- Time to HSCT after relapse and time to death after relapse are in months. MSD, Match Sibling donor; PBCT, Peripheral blood cell transplantation; UBMT, Unrelated Bone Marrow transplantation; UCBT, Unrelated cord blood transplantation; URD, Unrelated donor transplantation.
Only two patients are alive; both were transplanted in CR. Four patients had a second relapse, all at a location different from that observed at diagnosis (two of two autologous and two of eight allogenic HSCT). Four died from early complications. For T-LBL, six HSCTs (five allogenic) were performed. Conditioning regimens were total body irradiation/cyclophosphamide (TBI/Cy) in four cases and TBI/etoposide in two cases. For pB-LBL, four HSCTs were performed: one autologous (busulfan/aracytine/melphalan) and three allogenic (TBI/Cy).
Outcome, Mortality, and Prognostic Factors
Overall, 21 of the 23 children died after relapse or progression (Fig. 2). The OS at 8 years for children with relapse or progression was 8.7% (Fig. 3A). The EFS was the same as OS. Only two T-LBL patients were still alive. They underwent relapses at 15 and 69 months, and had allogeneic HSCT from unrelated donors 4 and 3 months after the time of relapse.
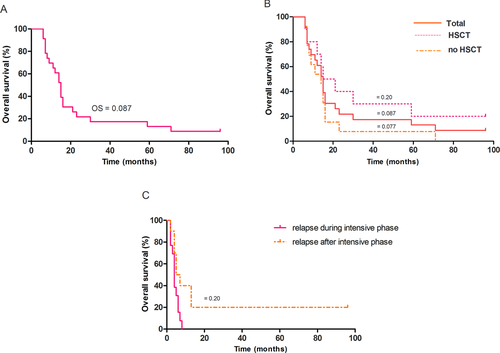
In univariate analysis (Table I), the absence of HSCT and relapse during the intensive phase (before 6 months) were identified as risk factors. In patients who had HSCT, OS was 20%, whereas all patients who did not undergo HSCT died (P = 0.011; Fig. 3B). All patients who had early relapses died, whereas OS of patients who relapsed after the intensive phase was 20% (P = 0.025; Fig. 3C).
DISCUSSION
Relapse of lymphoblastic lymphoma is now a rare event. Published data are very limited. Intensification of front-line treatment may have induced more resistant diseases upon relapse.
Relapse rates of T-LBL reported in the literature are low (8–16%), but the prognosis remains poor, with survival after relapse between 0% and 45% if patients undergo HSCT.7, 12 In recent protocols, even fewer relapses are observed. Uyttebroeck et al. 2 reported an incidence of relapse of 15.9% and 9.46% for T-LBL in the EORTC protocols 58881 and 58951, respectively. Data on relapsed pB-LBL are very scarce, but survival rates are reported to be very low, that is, under 20%.3, 4 In our study, we retrospectively analyzed 23 patients with relapse or progression of lymphoblastic lymphoma treated with two different protocols. We too report a very low survival rate (8.7%), and the main finding from the statistical analyses is the 0% survival of patients with refractory disease/relapse during the intensive therapy. This population represents an “ultrahigh risk” subgroup of patients.
The characteristics of the two subgroups of T and pre-B lymphomas were studied. As already reported elsewhere, the T-LBL patients relapsed earlier. Patte et al. and Reiter et al. reported early relapses of T-LBL (with medians of 13 and 12 months respectively), with very low survival rates.7, 12. Later relapses are described in the EORTC 58881, with a slightly higher survival rate.2 Our results are consistent with these observations: prognosis is poorer in early relapses.
In our study, pB-LBL patients underwent relapses later than T-LBL patients (three of five during maintenance therapy or later). These results are consistent with those observed in the literature.13, 14 Mann et al. reported three patients with late relapses at 1.6, 3, and 4 years, and only two survived.15
We report a majority of local relapses, and only a few BM relapses, as described in other studies: EORTC 58881, BFM 90–95, LSA2L2, and POG 8704 trials.14, 16 The rate of CNS relapses described in our study is low (only three patients), as reported in the previous EORTC 58881 protocol without CNS prophylactic irradiation.2 Reiter et al. did not report any de novo CNS relapse but they used cranial radiotherapy for patients with initial CNS involvement.7, 17
A study of all second-line treatments administered to the 23 patients showed that the achievement of a second CR remains difficult with the VANDA protocol actually being insufficient. In the German cohort, 18 of 28 patients with T-LBL and two of six patients with pB-LBL did not achieve a second CR. Five of nine patients who underwent HSCT in this study were not in CR before HSCT.3 In our study, the rare patients who survived were transplanted in CR. Several studies have reported similar results,18, 19 with the status before transplantation being an indicator of prognosis. Levine also reported a lower risk of relapse at 1 and 5 years after allogeneic HSCT but reported treatment-related toxicity that was significantly higher; the median age in this study was 27 years, which could explain the lower tolerance of treatment, as expected in adult patients.20 Only two autologous HSCT were performed and both patients underwent a second relapse. It seems that allogeneic HSCT after a second CR is the only potentially curative therapeutic option.
Identification of prognostic markers is needed. It will allow early adjustment of front-line treatment. Risk factors have been identified for ALL, but the situation is not as clear for lymphomas.7, 3, 15, 21, 22 Baleydier et al. reported that an immunogenetic classification of T-cell receptor (TCR) gene rearrangement could have a prognostic impact.23 Callens et al. have reported that NOTCH1/FBXW7 mutation, present in 55% of the EuroLB02 cohort analyzed in France, was associated with a better prognosis and thus would be a promising factor for early stratification.24 Other authors confirmed more recently in a larger cohort that NOTCH1 mutations were associated with a better prognosis and chromosome 6q alteration with a poor prognosis. This could be used as a stratification criterion in patients with T-LBL.25 Callens et al. and Burkhardt et al. reported that defects in genes coding for transcription-regulating factors have been identified as being associated with a poor prognosis: loss of heterozygosity on chromosome 6q14-24 and the absence of biallelic deletion of gamma TCR.26, 27 More recently, Trinquand et al. reported in adult population that the absence of NOTCH1 and/or FBXW7 mutations together with the presence of RAS or PTEN alterations was associated with poor prognosis.28 In children, Bandapalli et al. showed that NOTCH1 activation clinically antagonized the unfavorable effect of PTEN inactivation.29
As early response is a key risk factor in T-cell ALL, a better assessment of tumor burden and early response would be an interesting approach for LBL. Coustan-Smith et al. and Mussolin et al. reported the possible value of minimal disseminated disease in lymphomas.30, 31 Detection of disseminated specific markers in blood or BM could be a tool for disease follow-up.
New medical imaging techniques could be useful tools to assess early response and identify resistant disease. However, the data reported on positron emission tomography scan are mostly retrospective and nonstandardized, thus further prospective and studies are necessary to identify prognostic follow-up factors.32-35 The use of magnetic resonance imaging has also been recently studied for tumors, including non-Hodgkin lymphoma, but will need further investigation to confirm its utility in identifying resistant disease.36
New therapeutic alternatives have also been evaluated, but the results are limited; there have been reports of the use of antimetabolites of nucleoside analogues. Clorafabine has shown encouraging results in the treatment of childhood leukemia.37, 38 Nelarabine is currently under evaluation for T-cell ALL. It is a potential option for T-LB. It was associated with 13% and 48% rates of second CR in phase I and II studies, respectively, and 23% CR after a second relapse.39, 40 Commander et al. have described the use of nelarabine in relapsed ALL and in late local LBL relapses, but the prognosis remained poor (only partial responses, death in second relapse).41
In summary, the results of this study are consistent with the literature: the survival rate of refractory or relapsed lymphoblastic lymphomas remains low particularly for early relapses, the standard second-line treatments still have limited efficacy, and favorable results are obtained in the rare T-LBL cases where HSCT is possible. This study emphasizes that the early identification of aggressive forms of lymphoma is mandatory for early intensification. Recent data showed that prognostic biological features as immune-genetic parameters (PTEN, RAS, del 6q, NOTCH) are essential. Furthermore, new therapeutic agents are needed; chemotherapy, including the latest generation products, does not appear to be sufficient in controlling refractory forms. Large multicenter studies are essential to confirm the prognostic value of immune-genetic parameters in this rare population of patients.
ACKNOWLEDGMENTS
The authors want to acknowledge all the centers in France and Belgium for sharing data: C. Patte, Pediatric Department, Institut Gustave Roussy, Paris; S. Ducassou, Pediatric Hematology/Oncology Department, University Hospital of Bordeaux; C. Coze, Pediatric Hematology/Oncology Department, Hôpital d'Enfants de La Timone, Marseille; F. Mazingue, Pediatric Hematology/Oncology Department, Lille University Hospital; and O. Lejars, Pediatric Hematology/Oncology Department, University Hospital, Tours, France.




