Risk of recurrence and survival after relapse in patients with Ewing sarcoma†
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Abstract
Background
The prognosis in patients with relapsed Ewing sarcoma is unfavorable. Our investigation identifies factors predicting for the outcome following relapse.
Procedure
We analyzed type of relapse, time to relapse and overall survival after relapse (OSr) in 714 patients with first recurrence. All patients had been treated within the Cooperative Ewing Sarcoma Studies (CESS) 81 or 86, or the European Intergroup CESS (EICESS 92). OSr time was calculated from diagnosis of first relapse to last follow-up or death.
Results
Median follow-up time from diagnosis of primary disease was 2.2 years (mean = 4.0; range: 0.2–24.9). Relapse sites were local in 15%, combined local and systemic in 12%, and systemic in 73%. Among patients with a localized primary tumor, 20% relapsed locally, while 12% showed combined and 68% systemic relapse. When the primary disease was disseminated, 82% developed systemic, 13% combined, and 5% local relapse. Five-year OSr was 0.13 (SE = 0.01). Outcome following local relapse, with a 5-year survival rate of 0.24 (P < 0.001), was superior to outcome after systemic or combined recurrence. Five-year OSr was 0.07 (SE = 0.01) in patients who relapsed 0–2 years after the diagnosis of primary disease, as compared to a 5-year OSr of 0.29 (SE = 0.03) when relapse occurred later.
Conclusions
5-year OSr in Ewing sarcoma is poor (<0.2). Prognostically favorable factors are: late onset (>2 years) and strictly localized relapse. Pediatr Blood Cancer 2011; 57: 549–553. © 2011 Wiley-Liss, Inc.
INTRODUCTION
The outcome after an initial diagnosis of Ewing sarcoma in patients with localized disease has been significantly and steadily improved up to 75% over the last years 1-3. This success has been attributed to multimodal treatment including multiagent chemotherapy, surgery, and radiotherapy 1, 2, 4, 5.
By contrast, endeavors to improve survival in high-risk patients with primary disseminated disease have been without success so far. In these patients, 5-year overall survival remains at a low level of 13%–30% despite the introduction of high-dose chemotherapeutic approaches 6. Detailed analysis identified relevant prognostic factors in this group of patients: age, tumor volume, number, and site of metastases. In patients with disseminated disease the unfavorable prognosis may be improved by consistent local treatment 7, 8.
In conclusion, there is still a considerable rate of recurrent disease in patients with Ewing sarcoma. About 30%–40% of patients with a localized primary and 60%–80% of patients with primary disseminated disease sustain a relapse 9.
Survival following relapse is 20%, and despite the introduction of new therapeutic agents including high-dose chemotherapy, all attempts to improve the prognosis have not been successful 10-16. Recent analyses on Ewing sarcoma relapse focus on a putative association between outcome and treatment given 17-20. The present study analyzes a group of patients who received highly heterogeneous treatment and thus provides no valid information on this aspect. The value of various therapeutic regimens will not be subject of this paper.
We present our analysis on risk of recurrence and survival after relapse in a large cohort of 714 patients. All patients were initially treated within the GPOH CESS 81, CESS 86, or EICESS 92 trials. We aimed at a detailed analysis of clinically relevant factors in a large cohort of patients with relapsed Ewing sarcoma in order to discover relevant prognostic parameters. Our vision was to get solid data amenable to providing a relevant risk score as a useful guideline for future follow-up programs and clinical trials in relapsed patients. The analysis aims to identify the prognostic relevance of type of relapse and time to recurrence.
PATIENTS AND METHODS
Between 1980 and 1998, 1,549 patients with histologically proven Ewing sarcoma of bone or soft tissue were registered into three consecutive GPOH trials, i.e., CESS 81, CESS 86, and EICESS 92. The trials were approved by the appropriate ethics committees. All patients and/or their legal representatives gave informed consent to treatment as well as data storage and analysis according to the appropriate guidelines. Details of the treatment regimens including agents, dosage, routes, and schedules of chemotherapy administration, the dose and schedule of radiotherapy, and the extent of surgery given for local control were described elsewhere 1, 21-24. The present analysis includes data from the time of enrollment into the respective trial until July 2010 when the database was frozen.
Relapse was confirmed by imaging including technetium scintigraphy and/or positron emission scan and/or whole body MRI in all cases, and confirmation of relapse by biopsy was recommended in ambiguous cases. Patients with progression of disease under therapy were excluded from the analysis. Complete data sets were available in 714 patients with relapse (Fig. 1).
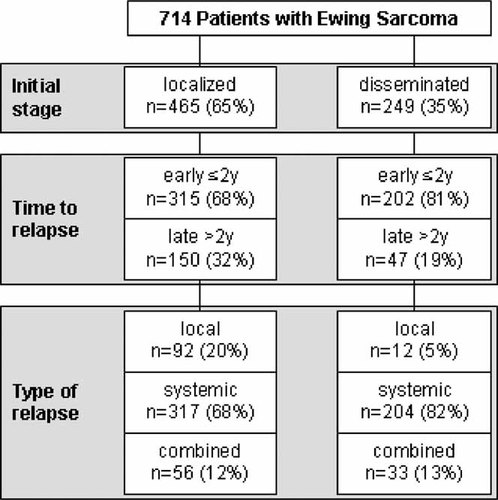
Cohort of relapsed patients according to initial stage of disease at diagnosis (localized or disseminated), time to relapse (before or after 2 years after initial diagnosis), and type of relapse (local, systemic, or combined relapse).
Relapse was classified in three groups: local (local recurrence alone), combined (local and synchronal distant recurrence), and systemic (distant recurrence only).
Statistical analyses of overall survival after relapse (OSr) were performed using the Kaplan–Meier method 25. OSr time was calculated from diagnosis of first relapse to last follow-up or death. Univariate comparisons between groups of patients and statistical significance were done by log-rank test. Multivariate test procedures applied Cox and logistic regression analyses 26-28. Frequencies were compared using the Chi-square or log-rank test, as appropriate.
RESULTS
Patient Characteristics
38.5% of the patients were female, and 61.5%, male. The median age at diagnosis was 15.8 years (range: 0.2–57.7) and the median follow-up time was 2.2 years (mean = 4.0; range: 0.2–24.9) from initial diagnosis. All patients received chemotherapy according to the appropriate trial, 80.7% were given radiotherapy, and 62.4% underwent surgery.
Type of Recurrence
We analyzed 714 patients with first recurrence of Ewing sarcoma; 465 patients (65%) had primary localized, 249 patients (35%), primary disseminated disease.
The predominant type of recurrence was systemic relapse (n = 521), which was seen in 73% of the total group, followed by local (n = 104; 15%) and combined relapse (n = 89; 12%). The site of systemic relapse was pulmonary in 35% of the cases, bone in 29% and multisystem or other in 36%. Systemic relapse occurred more often in patients with primary disseminated disease (82%) than in patients with primary localized disease (68%; P < 0.001). Strictly localized relapse was mainly observed in patients with primary localized disease and was rare in patients with primary disseminated disease (20% versus 5%; P < 0.001, Fig. 2).
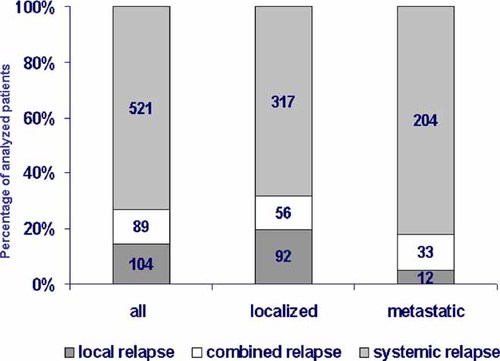
This figure shows the type of recurrence in 714 patients. The columns represent either the entire group pf patients or patients with localized disease at diagnosis of metastatic disease at diagnosis. Total number and percent of the type of relapse are given. The different type of relapse are illustrated in different colors (n = 714/%).
Time to Recurrence
Seventy-two percent of first relapses occurred within 2 years from initial diagnosis, 86% within 3 years, and 94% within 5 years. The median time to relapse was 501 days from the initial diagnosis. Patients with primary disseminated disease relapsed significantly earlier, with a median time to relapse of 434 days, than patients with localized disease where the median time to relapse was 563 days (P < 0.001). Systemic bone relapse and combined relapse were diagnosed earlier, after median intervals of 460 and 432 days from initial diagnosis, respectively, while other types of relapse were diagnosed significantly later: multisystem, 516 days; systemic lung, 533 days; local, 595 days (P < 0.001).
Survival After Relapse
The 1-year OSr was 0.43 (SE = 0.02), 5-year OSr, 0.13 (SE = 0.01), and 10-year OSr, 0.09 (SE = 0.01). Patients who relapsed early after the primary diagnosis had a significantly poorer outcome. Among patients who relapsed within the first 2 years after primary diagnosis, 1-year OSr was 0.28 (SE = 0.02), 2-year OSr, 0.12 (SE = 0.01) and 5-year OSr, 0.07 (SE = 0.01). By contrast, patients who were in remission for more than two years after the first diagnosis of Ewing sarcoma achieved 1-, 2-, and 5-year OSr rates of 0.82 (SE = 0.03), 0.54 (SE = 0.04), and 0.29 (SE = 0.03). A longer relapse-free interval from primary diagnosis (exceeding 3 years) had no additional impact on survival (1-year OSr: 0.81; 2-year OSr: 0.56; 5-year OSr: 0.30, Fig. 3).
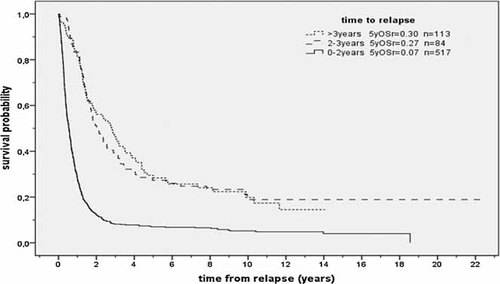
Survival after relapse according to time to relapse (n = 714; P < 0.001). OSr = overall survival. n = number of patients.
The type of relapse, i.e., local, systemic, or combined, had a significant impact on survival (P < 0.001). Patients with local relapse had a superior outcome with an OSr of 0.58 (SE = 0.05) at 1 year, 0.39 (SE = 0.05) at 2 years, and 0.24 (SE = 0.04) at 5 years, while systemic relapse was associated with an unfavorable OSr of 0.43 (SE = 0.01) at 1 year, 0.23 (SE = 0.02) at 2 years, and 0.12 (SE = 0.02) at 5 years. The least favorable outcome was observed in patients with combined relapse where OSr was 0.24 (SE = 0.05) at 1 year, 0.06 (SE = 0.03) at 2 years, and 0.04 (SE = 0.02) at 5 years. Considering that systemic and combined relapse both include dissemination to distant sites, we determined which patients with systemic relapse had a more favorable outcome than patients with combined relapse (P < 0.001, Fig. 4).
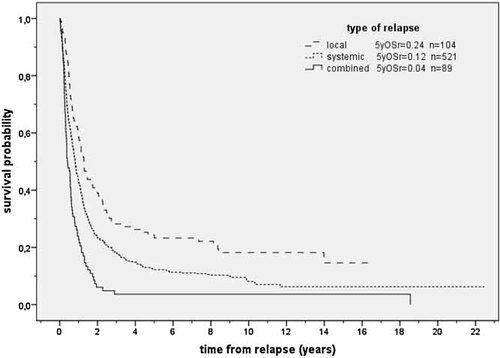
Survival after relapse according to type of relapse (n = 714; P < 0.001). OSr = overall survival. n = number of patients.
A detailed analysis of 521 patients with systemic relapse showed a significant relationship between site of systemic relapse and outcome. Relapse in the lung, as compared to other sites, was associated with a significantly better outcome; the proportion of pulmonary relapse thus determined outcome results for the entire group of patients with systemic relapse. By contrast, patients with bone or multisystem relapse showed an outcome comparable to patients with combined relapse. Thus, the site of systemic relapse did have a significant impact on survival (Fig. 5).
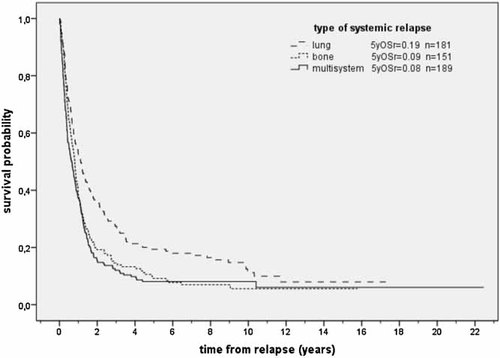
Survival according to type of systemic relapse (n = 521; P < 0.001). OSr = overall survival. n = number of patients.
The univariate results given above were confirmed by a multivariate Cox analysis which included the factors time to relapse (≤2 years; >2 years) and site of relapse (local, bone, lung, multisystem, and combined; Table I).
| RR | 95% CI | P | |
|---|---|---|---|
| Time to relapse | |||
| Early (< = 2y) | 2.95 | 2.44–3.57 | <0.001 |
| Type of relapse (P < 0.001) | |||
| Local | 1 | — | — |
| Lung | 1.30 | 0.99–1.69 | =0.056 |
| Bone | 1.70 | 1.30–2.24 | <0.001 |
| Multisystem | 2.08 | 1.60–2.71 | <0.001 |
| Combined | 2.62 | 1.93–3.56 | <0.001 |
We found early relapse within 2 years from initial diagnosis (risk ratio (RR): 2.95; 95% CI 2.44–3.57, P < 0.001) to predict for poor prognosis; we found that combined (RR: 2.62, 95% CI 1.93–3.56, P < 0.001), systemic bone (RR: 1.70; 95% CI 1.30–2.24, P < 0.001) and multisystem relapse (RR: 2.08, 95% CI 1.60–2.71, P < 0.001) were confirmed as prognostically poor.
DISCUSSION
Relapse in Ewing sarcoma is associated with a critical prognosis and therefore represents a challenge for health care providers. Even though significant advances have been made in the treatment of primary Ewing sarcoma, therapeutic approaches tend to fail when applied second line even when novel agents are included that were not used first line 14-16. Recent approaches focusing on novel molecular targets as monoclonal antibodies to the insulin-like growth factor receptor failed, at least when used in a monotherapeutic approach 11, 29. Therefore, in relapsed Ewing sarcoma ongoing efforts toward an improvement of the prognosis is urgently needed. In our investigation we focused on putative prognostic factors by analyzing clinical data of a large cohort of patients. We analyzed the data of 714 patients with Ewing sarcoma treated within the CESS 81, CESS 86, and EICESS 92 trials. We focused our analysis on the impact of time to relapse and type of relapse on the prognosis. The patients had not been included into a prospective clinical trial suitable to provide solid data on the value of therapeutic approaches and the cohort analyzed was highly heterogeneous regarding systemic treatment. Consequently, we did not include the analysis on chemotherapy for relapsed disease in the data presented in this manuscript.
The analysis regarding outcome in this high-risk group of patients not only identified several prognostic factors but also revealed a relationship between primary presentation and presentation at relapse. Our results support some previously published data, but also point to important additional novel aspects. Our data, in agreement with Leavey et al. 30 who analyzed 262 relapsed patients treated according to the Children's Oncology Group protocols between 1988 and 2000, confirm the importance of time to relapse as a prognostic factor. Furthermore, we report a similar 5-year OSr rate (13% versus 12%) and outcome of patients with late relapse more than 2 years after primary diagnosis (29% versus 30) or early relapse less than two years after primary diagnosis (7% versus 7%). In this cohort of patients we were able to conduct a detailed analysis on the type of relapse and the correlation between the time to relapse and the type of recurrence. The main findings of our analyses may be summarized focusing on the time to relapse, the type of relapse and the time to relapse and type of relapse combined. Over 80% of the recurrences occurred within 3 years from diagnosis. Our data showed a significantly poorer prognosis in patients with early compared to a later relapse. The prognostic cut-off in our analyses was 24 months after diagnosis. This is in accordance with data reported for smaller cohorts 9, 17, 18, 30, 31. The analysis of a large cohort such as the one reviewed here provided the opportunity to identify additional prognostic factors. Patients with Ewing sarcoma disseminated at the time of primary diagnosis tended to have systemic recurrences, while in patients with primary localized Ewing sarcoma local recurrence was more common. The reason for this difference in relapse pattern is not easily attributable to the treatment given. While localized relapse in patients with a single localized lesion at the time of diagnosis might be blamed on poor local control, the very low prevalence of local relapse in primary disseminated disease, where quite often no local control at all is achieved, seems to contradict this argument 8, 31. Whether or not biological factors play a role in these patients might be a question for ancillary studies. Distant bone and combined relapses occurred significantly earlier than local relapses. One might speculate that tumor-related and/or patient-related factors may render the Ewing sarcoma cells prone to spread to distant sites and to develop secondary systemic disease.
The multivariate analyses show that the site of the relapse is a prognostic factor itself: Local relapse is the type of relapse with a superior prognosis. This result is to some extent in contradiction to the report of Shankar et al. 17 who reviewed a smaller cohort of 64 relapsed patients and described that the site of relapse had no influence on the final outcome. The discrepancy might be due to the smaller patient number or to other factors like selection bias due to different first line treatment, or success of local treatment in relapse. Bielack et al. 32 have reported that in osteosarcoma obtaining a complete surgical remission is the most important prognostic factor for survival even in a second or higher relapse.
Combined relapse is associated with a poor prognosis 30. Reasons may be the early occurrence as an independent prognostic factor and the dissemination as a second factor. It is well known that patients with primary pulmonary metastases fare better than patients with extrapulmonary metastases 33-36. Our results suggest a better prognosis in patients diagnosed for pulmonary metastases only at the time of relapse compared to patients with extrapulmonary lesions.
Even though the present analysis is based on a large number of patients with Ewing sarcoma and a long observation period, retrospective analyses always have certain limitations. A selection bias, incomplete, or ambiguous data, patient-, disease-, or treatment-related factors not recorded, may taint such analyses among other factors.
In conclusion, both the time to relapse and the type of relapse are relevant predicting factors concerning the prognosis in a Ewing sarcoma relapse. Relapse occurring later than 2 years after primary diagnosis and local relapse in particular are favorable predictors.
The overall prognosis after relapse remains unfavorable. Besides the urgent need of the development of novel second line treatments, our data implicate that prevention from early and most unfavorable relapse may be feasible by modifying first line regimens. From our data it is most reasonable to expect relapse in the first 2 years after diagnosis. Thus, in patients with high-risk disease, prolonged treatment, and/or introduction of novel agents could be of benefit. The prognostic factors identified in our analysis may have an impact on the development of novel treatment strategies and serve as a basis for comparison.
Acknowledgements
This work was supported by Deutsche Krebshilfe: 50-2551-Jü3 and 50-2551-Jü4, DKH- 108128 and by Federal Ministry of Education and Research Germany, BMBF (TranSaRNet), Deutsches Zentrum für Luft- und Raumfahrt e.V 01GM0869, EuroBoNet, EU-Framework 6. The authors like to thank Regina Kloss and the Ewing trial staff Münster for their kind support.




