Outcome of children with aplastic anemia treated with immunosuppressive therapy
Abstract
Background
Immunosuppressive therapy (IST) is the alternative treatment in children with aplastic anemia (AA) who do not have an HLA-matched sibling. The aim of this study is to evaluate the outcome of children with AA treated with IST.
Methods
We retrospectively reviewed the hospital records of children with AA from 1984 to 2004, treated at our institution with antithymocyte globulin (ATG), cyclosporine (CS), and short course of prednisone.
Result
Forty-two patients were treated with IST (24 boys, 18 girls); of whom 26% received G-CSF. The median age at diagnosis was 8.5 years. Sixty-nine, 19, and 12% were diagnosed with severe, very severe, and moderate AA, respectively. Twenty-one percent had hepatitis-associated AA. Median follow-up time was 53.3 months. Sixty-two percent had complete response; 19% had partial response. Two patients relapsed and received a second course of ATG; both had a partial response. The actuarial 5 years survival rate was 67.5%. Two patients developed myelodysplastic syndrome (MDS); both received long-term G-CSF and had partial response after two courses of IST. Fifteen percent of survivors had significant hypertension which persisted after CS was discontinued.
Conclusions
This study shows promising response in children with AA treated with IST; however, the outcome was inferior to our institutional results with hematopoietic stem cell transplantation from a sibling donor. Hypertension and MDS are late complications. Longer follow-up, larger cohorts, and prospective studies are warranted to evaluate late complications and risk factors. Pediatr Blood Cancer 2008;50:52–57. © 2007 Wiley-Liss, Inc.
INTRODUCTION
Severe aplastic anemia (SAA) was previously considered a fatal hematologic disease; however, advances in supportive care and therapeutic options improved survival rate, quality of life, and long-term outcomes in this group of patient. In cases where a human leukocyte antigen (HLA)-identical related donor is available, hematopoietic stem cell transplantation (HSCT) is widely accepted as the treatment of choice for SAA 1. The current cure rate with such treatment is 90% 2. Immunosuppressive therapy (IST) is an alternative first line treatment in patients without an HLA-matched sibling donor. The combination of antithymocyte globulin (ATG) or anti-lymphocyte globulin (ALG), and cyclosporine (CS) results in approximately 75% response rate 3-5. The addition of hematopoietic cytokine such as granulocyte colony stimulation factor (G-CSF) and granulocyte-macrophage colony stimulation factor (GM-CSF) to the IST may shorten the time to neutrophil recovery and decrease infection rate, but was not shown to improve response rate6-8 and overall survival 9.
The long-term outcome of children treated with IST is still unclear. An increased risk of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in children with aplastic anemia (AA) treated with IST were reported 10. A relationship between long-term use of G-CSF and secondary MDS has been suggested by several groups but not others 7. Because the limited data on the outcome of children with AA treated with IST, we reviewed all cases with AA treated with IST at our institution. Herein, we report the clinical outcome, survival rate and follow-up, including development of MDS/AML.
METHODS
Inclusion Criteria
The study was approved by the institutional Research Ethics Board. We retrospectively reviewed the medical records of all newly diagnosed pediatric patients with AA treated at The Hospital for Sick Children between 1984 and 2004. Patients were excluded if they were diagnosed with an inherited marrow failure syndrome before treatment or if they had an HLA-matched related donor and underwent HSCT.
Disease Grading
Patients were classified according to published severity criteria 11, 12. AA was considered severe if the marrow cellularity was <25%, and at least 2 of the following criteria were met: neutrophil count <0.5 × 109/L, platelet count <20 × 109/L, or reticulocyte count <20 × 109/L. AA was considered very severe (VSAA) if the above criteria for SAA were fulfilled, and the neutrophil count was <0.2 × 109/L. Moderate AA was defined as hypocellular bone marrow with at least two of the following hematological values: neutrophil count <1 × 109/L, platelet count <50 × 109/L, or reticulocyte count <60 × 109/L. Hepatitis-associated AA was defined as AA which occurred either concurrent or within 6 months after presentation with an increase in serum alanine aminotransferase (ALT) level by at least five times the upper reference limit.
Treatment
All patients received horse-derived ATG (hATG) 160 mg/kg over 4 days. Intravenous methylprednisolone or oral prednisone at a dose of 2 mg/kg/day was administered for 4–7 days followed by a 2-week taper for prevention of serum sickness. CS was started either orally or intravenously on day 1 and was adjusted to maintain serum levels between 150 and 200 ng/ml. Patients who had infections or ANC <0.2 × 109/L received G-CSF at the dose 5–10 µg/kg/day intravenously or subcutaneously. Platelet transfusions were given for platelet counts <10–20 × 109/L or bleeding, and red blood cells transfusions for hemoglobin <70 g/L or symptomatic anemia. All blood products were screened for cytomegalovirus and irradiated, and single donor platelets units were used whenever available. A second course of hATG or rabbit-derived ATG (rATG) was administered if the patient had not responded after 3 months of initial treatment or relapsed after initial response.
Response Criteria
Complete response (CR) was defined as achieving normal levels of hemoglobin adjusted to age, platelet count >100 × 109/L and neutrophil count >1.5 × 109/L. Partial response (PR) was defined as transfusion independence, reticulocyte count >30 × 109/L, platelet count >30 × 109/L, and neutrophil count >0.5 × 109/L above baseline. Overall response was calculated as the sum of PR and CR. The diagnosis of MDS was established based on the criteria of Hasle et al. 13 using characteristic of blood and bone marrow morphology and cytogenetics. MDS were sub-classified according to the CCC 14 and WHO classification 13 system.
Data Analyzed
The following data were collected by chart review: age, absolute neutrophil count, hemoglobin, platelet count, reticulocyte count, fetal hemoglobin, mean corpuscular volume (MCV), times to achievement of CR, time to discontinuation of CS, and number of IST course.
The following investigation was done to exclude an inherited bone marrow failure syndrome: family history, medical history, physical examination, bone marrow cytogenetics, chromosome fragility studies with diepoxybutane and mitomicin-C, echocardiogram and ultrasound of the abdomen. Paroxysmal nocturnal hemoglobinuria was excluded by Ham's test or determination of CD55 and CD59 by flow cytometry. Additional tests included liver function test and serology for hepatitis A, B, C, Epstein–Barr virus, cytomegalovirus, and parvovirus.
Follow-up data was available either until the age of 18 years when the patient was referred to an adult hematology center, or until the patient deceased or December 30, 2005, whichever came first. In case of a good response and no evidence of recurrence, no surveillance bone marrow testing and screening for paroxysmal nocturnal hemoglobinuria were done. These tests were performed in case hematological deterioration was suspected from the results of the clinical history, physical examination, and complete blood count. Data with non-nominal distribution was expressed in median values. The actuarial survival rate was determined by Kaplan–Meier method.
RESULTS
Patient Characteristics
From 1984 to 2004, 42 patients were treated with IST at The Hospital for Sick Children. Patient characteristics are shown in Table I. Of the 42 patients who received IST, 33 patients had idiopathic AA and nine had hepatitis-associated AA. In the latter group (Table II), serological tests for hepatitis A, B, C, cytomegalovirus, Epstein–Barr virus, and parvovirus were negative. Polymerase chain reaction of sequences of herpes simplex virus 1, 2, varicellar zoster virus, human herpes virus 6, 7 were negative. Four patients in this group had liver biopsies which confirmed a diagnosis of hepatitis. In all these cases, the liver specimens showed massive hepatic necrosis without an identifiable cause. Three patients presented with fulminant hepatic failure and underwent liver transplantation. All of them received IST with tacrolimus and steroids as part of the liver transplantation treatment before they developed AA. The other six patients did not receive treatment for the hepatitis. Of the nine patients with hepatitis-associated AA, eight had SAA and one had VSAA. All 42 patients had negative tests for paroxysmal nocturnal hemoglobinuria, chromosome fragility studies with diepoxybutane and mitomicin-C and normal bone marrow karyotype at diagnosis.
| Patient characteristics | No. of patients (N = 42) |
|---|---|
| Male/female | 24/18 |
| Median age at diagnosis in years | 8.5 years (range 1.4–17.3) |
| Cause of aplastic anemia | |
| Idiopathic | 33 (79%) |
| Hepatitis associated | 9 (21%) |
| Severity of disease at diagnosis | |
| Very severe | 8 (19%) |
| Severe | 29 (69%) |
| Moderate | 5 (12%) |
| Number of IST courses received | |
| One (hATG) | 27 (64%) |
| Two (hATG) | 8 (19%) |
| Two (1 hATG + 1 rATG) | 7 (17%) |
| G-CSF administration | |
| Yes (range 5 days to 18 months) | 11 (26%) |
| No | 31 (74%) |
| Response to treatment | |
| Complete response | 26 (62%) |
| Partial response | 8 (19%) |
| No response | 8 (19%) |
| Median time to complete response | 6.5 months (range 1–32 months) |
| Median time to discontinue cyclosporine | 13 months (range 3–50 months) |
| Outcome | |
| Alive with transfusion independency | 32 (76%) |
| Alive with transfusion dependency | 1 (2%) |
| Dead | 9 (22%) |
| Median time of follow-up | 53.3 months (range 3–244 months) |
- G-CSF, granulocyte colony stimulating factor; IST, immunosuppressive therapy; hATG, horse-derived antithymocyte globulin; rATG, rabbit-derived antithymocyte globulin.
| Patient | 3 | 9 | 10 | 11 | 16 | 25 | 26 | 27 | 30 |
|---|---|---|---|---|---|---|---|---|---|
| Severity of AA | SAA | SAA | SAA | SAA | VSAA | SAA | SAA | SAA | SAA |
| Cause of hepatitis | Idio | Idio | Idio | Idio | Idio | Idio | Idio | Idio | Idio |
| Peak ALT (U/L) | 813 | 2,072 | 2,750 | 2,507 | 2,383 | 1,057 | 999 | 2,818 | 2,420 |
| Lag to AA (weeks) | CC | 2 | 4 | CC | CC | 4 | CC | 28 | 16 |
| Liver biopsy | No | Yes | Yes | Yes | No | No | No | Yes | No |
| Liver transplantation | No | Yes | Yes | No | No | No | No | Yes | No |
| No. of IST | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 1 |
| G-CSF use | Yes | Yes | Yes | Yes | No | No | No | No | No |
| Respond to IST | CR | PR | PR | NR | CR | CR | CR | CR | CR |
| Outcome | Alive | Dead | Dead | Dead | Alive | Alive | Alive | Alive | Alive |
| MDS transformation | No | Yes | Yes | No | No | No | No | No | No |
- SAA, severe aplastic anemia; VSAA, very severe aplastic anemia; Idio, idiopathic; CC, concurrent; CR, complete response; NR, no response; PR, partial response.
Response to Immunosuppressive Therapy
Twenty-seven patients (64%) received one course of hATG and CS. Fifteen patients (36%) received two courses of ATG either because of non-response (NR) at least 3 months after the first ATG course or because of relapsed. In eight cases, both ATG courses were of hATG, and in seven cases the second course was with rabbit ATG (rATG). One patient with moderate AA received one course of hATG and steroids without CS and had a CR. Of the 15 patients who received two courses of ATG, five, seven, and three patients had a CR, PR, and NR, respectively. Two patients, who received hATG, CS, and steroids and achieved CR, relapsed 11 and 13 months after CS was discontinued. Both were retreated with hATG and CS and achieved a PR.
Of the nine patients with hepatitis-associated AA, five received only one course of IST. Four of the later five had a CR and survived, and one did not respond and died of respiratory failure due to pulmonary fungal infection. Two of the nine patients with hepatitis-associated AA received a second course of IST, since they did not respond to the first course; both had a CR and survived (Table II). An additional two patients presented with fulminant hepatic failure, and underwent liver transplantation. Both received IST and G-CSF for their AA, had a PR, and developed MDS post IST.
Eleven patients (26%) required G-CSF for severe neutropenia and infections. The duration of G-CSF therapy ranged from 5 days to 18 months. The characteristics of the patients in this group are shown in Table III.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity | VS | S | S | VS | VS | S | VS | S | S | S | S |
| Cause of AA | Idio | Idio | Hepatitis | Idio | Idio | Idio | Idio | Idio | Hepatitis | Hepatitis | Hepatitis |
| No. of IST | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 |
| Duration of G-CSF therapy | 16 months | 2 months | 4 months | 5 days | 14 days | 10 days | 12 days | 4 months | 18 months | 14 months | 1 month |
| Response | CR | PR | CR | CR | NR | PR | CR | CR | PR | PR | NR |
| Outcome | Alive | Alive | Alive | Alive | Dead | Alive | Alive | Alive | Dead | Dead | Dead |
| MDS transformation | No | No | No | No | No | No | No | No | Yes | Yes | No |
- AA, aplastic anemia; CR, complete response; G-CSF, granulocyte-colony stimulating factor; Idio, idiopathic; IST, immunosuppressive therapy; MDS, myelodysplastic syndrome; NR, no response; PR, partial response; S, severe; VS, very severe.
Development of Myelodysplastic Syndrome and Leukemia
Two patients developed MDS, 21 and 19 months post IST. The first patient (patient no. 9, Table II) had SAA associated with fulminant hepatitis. He received two courses of ATG together with a liver transplantation and had a PR. He continued to receive CS and G-CSF. On a routine follow-up evaluation, his complete blood count showed circulating blasts. Bone marrow testing showed hypercellular specimen, mild dysplastic change in erythroid and megakaryocytic lineage, 7% myeloid blast and a clonal marrow cytogenetic abnormality consisting of monosomy 7. A diagnosis of MDS was made. The patient underwent HSCT from a matched unrelated donor. His HSCT was complicated by Epstein–Barr virus infection, graft versus of disease of gut and liver, gastrointestinal bleeding, and renal failure. He developed multiorgan failure and died 5 months post HSCT.
The second patient (patient no. 10, Table II) presented with fulminant hepatitis and underwent liver transplantation. One month after liver transplantation, the patient developed SAA. She received two courses of ATG, and was continuously treated with CS and G-CSF after achieving a PR. During follow-up, the patient developed pancytopenia again, and bone marrow testing showed hypocellular specimen, trilineage dysplasia, and a clonal marrow cytogenetic abnormality consisting of monosomy 7. A diagnosis of MDS was made. The patient underwent haploidentical HSCT from her mother. Her post HSCT course was complicated by disseminated adenovirus infection and severe GVHD of gut, and she died 2 months post HSCT with multiorgan failure.
Hypertension
Five of 33 surviving patients (15%) had significant hypertension after CS was discontinued (Table IV). All of them developed significant hypertension (blood pressure level above 95th percentile adjusted for age and height) during CS treatment, which persisted after the CS was discontinued. During follow-up, one patient had blood pressure levels at 95–99th percentile, and the other four had blood pressure levels above the 99th percentile. Three of the patients received one course of ATG, and two received two courses. Three patients received CS for 8 months; one for 9 months, and one for 37 months. The latter patient, who achieved PR, required continuous antihypertensive therapy with amlodipine and lisinopril to control her hypertension.
| Patient | 16 | 18 | 27 | 28 | 39 |
|---|---|---|---|---|---|
| Severity of AA | Severe | Severe | Severe | Severe | Severe |
| No. of IST | 1 | 1 | 2 | 2 | 1 |
| Duration of CS (months) | 8 | 9 | 8 | 37 | 8 |
| Respond to IST | CR | CR | CR | PR | CR |
| Severity of hypertension (percentile) | >99th | >99th | 95–99th | >99th | >99th |
| Duration of hypertension after CS was discontinued (months) | 49 | 83 | 39 | 207 | 19 |
| Antihypertensive medication | No | No | No | Yes | No |
- IST, immunosuppressive therapy; CS, cyclosporine; CR, complete response; PR, partial response.
Overall Survival
Median follow-up in our study was 53.3 months (range from 3 to 244 months). Nine patients (21%) have died; seven of them did not respond to the IST, and two had PR. Overall survival at 53.3 months was 79% (33 patients). One patient who had no response was still alive 40 months post diagnosis and receives supportive care. The actuarial 5 years survival rate was 67.5% (Fig. 1).
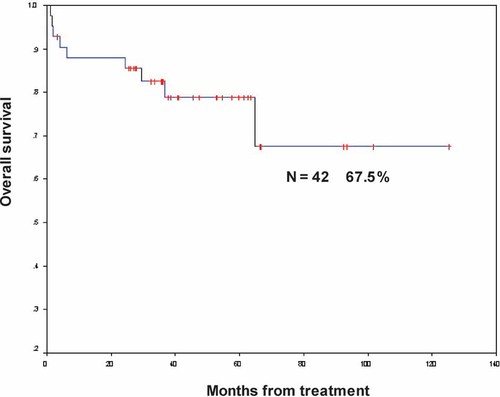
Actuarial overall survival rate in 42 children with aplastic anemia treated with immunosuppressive therapy.
DISCUSSION
The front line treatment of children with AA when an HLA-identical sibling donor for HSCT is unavailable is IST 2. The present study shows 62% CR and 19% PR to IST with ATG, CS, and steroids for children with AA. Similar IST regimens have shown to yield significant improved overall response rate of up to 65–75% at 6 months in comparison to 31% after ATG alone 3-5, 15. Furthermore, median time to response after combination therapy is shorter among patients receiving a combination of ATG and CS versus ATG alone 16. In our series, the median time to CR in patients who achieved such a response was 6.5 months. Fuhrer et al. 17 reported 86 pediatric cases with AA treated with IST and found a CR rates of 13, 39, and 55%, at 3, 6, and 12 months, respectively.
In most series, relapse after IST was frequent, occurring in up to 36% of patients treated with ATG and CS 3-5, 18. The incidence of relapse decreased from 30 to 10% when CS has been tapered slowly 19. In our single institution series, only two of the 42 treated patients (4.7%) relapsed after the first course of IST, possibly due to prolonged duration of CS therapy and slow tapering.
Patients who do not respond and patients who relapse after the first course of IST may benefit from a second treatment with the same or different ATG product. Responses to repeat ATG treatment are similar among primary refractory and relapsed patients 20. A second course of ATG for patients who did not respond to primary combination IST is usually given between 3 and 6 months from the initial course. Studies suggested comparable efficacy of ATG and ALG as secondary treatments; showing response in 22–50% of cases 18, 21. Interestingly, in the current series, 13 of the 15 patients (86.7%) who failed ATG and received a second course responded. Of these 15 patients, the response rate did not differ if the second course was hATG (87.5%) or rATG (71.7%) (P = 0.45).
Malignant transformation into MDS/AML is a major complication in long-term survivors after IST with a cumulative risk approximately 15% at 7–8 years 10, 22. It is unclear whether clonal disorders merely reflect the natural history of AA or whether they are also related to IST. In particular, it is hotly debated whether the use of G-CSF in combination with IST is a risk factor for the development of MDS in children 23, 24. Kojima et al. 10 found a 13.7% cumulative incidence of MDS at 8 years. NR to IST at 6 months and G-CSF therapy were risk factors in this study. This conclusion was not supported by data from other groups 7, 25, but warrants further laboratory investigations and prospective clinical analysis. It is possible that treatment with G-CSF is only associated with the occurrence of a severe stem cell defect rather than playing a causative role in malignant myeloid transformation. In the present study, 2 of 42 patients developed MDS post IST. Both received two courses of ATG, achieved PR, and required prolonged G-CSF therapy.
In summary, our study shows good overall response and overall survival at 5 years follow-up after IST for AA in children. High dose cyclophosphamide without HSCT has also shown promising results in SAA 26, 27. A second course of ATG may lead to CR in a substantial number of patients who do not respond to the first course. However, the results are still inferior to our institutional historical experience with HSCT as the first-line therapy in case an HLA-identical sibling is available 28, and does not eliminate the risk of MDS/AML. The role of G-CSF in transformation into MDS/AML remains unclear because of the small number of events and a lack of standardized criteria for administration of G-CSF. Longer follow-up, larger cohorts, and prospective studies are necessary to evaluate late complications after IST.




