Relationships between adiposity distribution and metabolic health in preconception women in South Africa
Abstract
Objective
Adipose tissue is a central regulator of metabolic health and a contributor to systemic inflammation. Patterns of adiposity deposition are important to understand for optimizing health. This study aimed to asses relationships between adiposity deposition and metabolic and inflammatory biomarkers in South African women prior to conception.
Methods
Non-pregnant, healthy women (n = 298) were recruited for this cross-sectional study via home visits. Body composition was measured by Dual X-ray Absorptiometry. Inflammation markers C-reactive protein (CRP), alpha1-acid glycoprotein (AGP), hemoglobin A1c (HbA1c), and blood pressure were scored according to risk. A summative metabolic health risk score was created for women with obesity. Generalized regression models assessed relationships between adiposity deposition and outcomes with adjustment for potential confounders.
Results
Obesity was present in 22% of women (mean age = 20.93 years). Fat mass index was associated with inflammation and metabolic health risk (β = 0.58; p < 0.01). Visceral fat, trunk:limb ratio, android:gynoid ratio, body mass index, weight, and waist circumference were positively associated with CRP, AGP, and metabolic health risk (p < 0.01). Weight was associated with Hba1c (β < 0.01; p < 0.05). Participants with obesity and low metabolic health risk had lower fat mass index and visceral fat than participants with obesity and higher metabolic health risk.
Conclusions
Black South African women accumulated excess adipose tissue in abdominal regions. While fat mass and body mass were associated with inflammation and metabolic health risk, women with obesity and with lower fat mass index and lower visceral adipose tissue were metabolically protected. Identification of women at risk for metabolic disease preconception could help ensure future healthy pregnancies and prevent transference of risk to offspring.
1 INTRODUCTION
Young South African women are at increased risk of developing obesity and related comorbidities, largely due to rapid urbanization resulting in physical inactivity1 and consumption of energy-dense convenient foods and beverages.2 Most of the current data examining obesity and adiposity distribution patterns are from white populations, yet there is evidence that Black women are more affected by obesity than their white counterparts.3, 4 When women with overweight or obesity become pregnant, they are at increased risk of developing gestational diabetes (GDM) and of having poorer pregnancy and delivery outcomes such as fetal defects and congenital anomalies, newborn macrosomia, neonatal hypoglycaemia, and/or stillbirth5; and they may not revert back to normal glucose tolerance postpartum.6 Furthermore, the offspring of women with GDM are metabolically programmed towards developing insulin resistance themselves.6 In addition, continued obesity and excessive gestational weight gain (GWG) during pregnancy are strongly predictive of fetal growth, birth weight and obesity risk in the offspring.7-9
Adipose tissue is a metabolically active organ—it is a major source of inflammatory cytokines and can interfere with insulin signaling by causing defects in insulin cascade, eventually resulting in insulin sensitivity or resistance.10 Furthermore, the location of adipose tissue deposition may determine metabolic activity and related effects, and risk for metabolic disease.11 Pregnancy itself is associated with changes in adiposity—resulting in temporarily reduced insulin sensitivity and low-grade inflammation.9, 10, 12 Thus, there is a dynamic interrelationship between adipose tissue deposition, insulin resistance, and inflammation—all three of which, when present during pregnancy, predict adverse outcomes for mother and infant.9, 10, 12 It is therefore essential to understand the interplay of these risk factors in younger women before they become pregnant to assist with developing interventions to minimize the transference of risk to the next generation.
Studies have shown that body composition differs between white and Black women in South Africa and elsewhere.3, 13 Specifically, Black women have less visceral adipose tissue (VAT), and more subcutaneous adipose tissue (SAT) when matched for body mass index (BMI).3, 13, 14 In addition, it appears that relationships between adipose tissue deposition and insulin sensitivity also differ between these races.3, 13-15 Typically, increased VAT is more strongly associated with insulin resistance compared to SAT; yet Black women are known to be more insulin resistant than white women at the same level of adiposity.3 There is evidence that lifestyle factors such as diet and physical activity may further impact these relationships, as may sociodemographic factors,3 all of which are impacted by impoverished communities becoming rapidly urbanized combined with poor healthcare systems.16 Social determinants of health combined with structural and institutional racial discrimination in South Africa are likely involved in these discrepancies; for example, prior to 1994, South Africa's healthcare system was racially divided into a highly resourced system for white individuals, and a systemically under resourced system for Black individuals.17 Post 1994, South African has a more coordinated system including public and private (paid) health care. Public healthcare provides most services for free, yet is plagued by problems such as “long waiting times, rushed appointments, old facilities, poor disease control and prevention practices, and poor quality of care, when compared to private health care,” and the healthcare systems in general are overburdened by both communicable and non-communicable diseases.17 Socioeconomic inequalities in health, as well as racial differences in health care, have been identified as key challenges to public health success in South Africa.17
Due to the lack of data on adiposity distribution in Black women, and specifically those in rapidly urbanizing countries such as South Africa; this study aimed to characterize a sample of young, Black South African women in the preconception period according to obesity, adipose tissue distribution, insulin sensitivity, and inflammation, as well as to determine the relationship between adiposity deposition and metabolic and inflammatory risk factors in this sample, while considering lifestyle and sociodemographic confounders. It was hypothesized that adiposity distribution patterns would differ in this sample in comparison to white women and that associations with metabolic risk factors may be weaker in these Black women.
2 METHODS
2.1 Study setting
The Healthy Life Trajectories Initiative (HeLTI) is an ongoing collaboration between South Africa, Canada, China, and India that was initiated in 2016 in response to the need for preconception interventions focused on optimizing growth and development trajectories in the next generation.18 The South African HeLTI site is in Soweto, Johannesburg. Soweto is a large urban area consisting of both formal and informal housing (shacks). In the most recent census from 2011, the population of Soweto was 1.27 million, with a population density of 6357 people per km.2, 19
2.2 Participants
The present study uses data from a subsample of participants in the Bukhali Young Women's Health Survey, which was the baseline data collection tool for the HeLTI trial study in Soweto. The subsample was part of a sub study examining iron deficiency in South African women and, thus, had additional biomarker analyses done.20 Non-pregnant, healthy self-identified Black women aged 18–25 years were recruited between June 2018 and June 2019 from randomly selected community clusters in Soweto via home visits conducted by trained fieldwork teams. Women with a medical history of type 1 diabetes, cancer, or epilepsy were not eligible. Participants self-reported their HIV status, and since the percentage who reported they were HIV+ was so small (5%), these women were excluded from the current analysis. All data collection was conducted at the SAMRC/Wits Developmental Pathways for Health Research Unit (DPHRU) at Chris Hani Baragwanath Academic Hospital in Soweto. Each participant completed an interviewer-administered survey and physical measurements. This study was approved by the Human Research Ethics Committee (Medical) at the University of the Witwatersrand (M171137, M1811111), and all participants provided written informed consent to participate.
2.3 Socio-demographics
An estimate of socioeconomic status (SES) was generated by summing the number of assets owned in the household from the following options: TV, car, washing machine, refrigerator, phone, radio, microwave, cell phone, DVD/Video, DSTV (cable channel), computer, Internet access, and medical aid to create a score out of 13.21 This was based on standard measures used in the Demographic and Health Surveys household questionnaire (available at: www.dhsprogram.com) and has been extensively utilized in this setting.22, 23
2.4 Body size and adiposity distribution
All measurements were made by trained research staff. Height was measured to the nearest 0.1 cm using a Holtain wall-mounted stadiometer and weight to the nearest 100 g using an SECA scale. Waist circumference was measured at the umbilical level using a soft tape measure, and a high waist circumference was defined as >89.45 cm. All measures were taken three times and the average was used, and all equipment was calibrated daily. Whole body composition was measured using a Hologic QDR 4500A DXA machine and analyzed using Apex software version 4.0.2 (Hologic Inc.). All scans were performed according to standard procedures by a trained technician. The machine was calibrated daily using a phantom spine. All standard DXA measurements were analyzed using Hologic APEX 3.1 software (Hologic). Whole body (excluding head) lean body mass (which includes muscle mass, skin, viscera, and salts and provides an estimate of lean muscle mass) and fat mass as estimated by the Hologic software were recorded. Fat mass index (FMI) was calculated as total fat mass (kg) divided by height (m) squared (kg/m2). FMI categories were determined using DXA reference values from NHANES.24 Abdominal VAT, SAT, and total adipose tissue estimates were calculated according to previously described methodology using Hologic software,25 and a ratio of VAT:SAT was determined. Other regions of interest were android fat (the area of the abdomen from the superior iliac crest and extended cranially for 20% of the distance to the base of the skull), gynoid fat (the legs from the greater femoral trochanter to mid-thigh), leg fat (the area of the entire leg), and trunk fat (includes the neck, chest, abdominal and pelvic areas), and trunk:limb ratio and android:gynoid ratio were calculated.
2.5 Biomarkers
The analysis of biomarkers used was previously described.20 Briefly, hemoglobin (Hb) concentrations were measured using a calibrated Hb 201+ HemoCue® system (HemoCue Johannesburg, South Africa). Hb data were adjusted for altitude according to WHO recommendations.26 The South African point-of-care cut-off to diagnose anemia was used, which is a finger-prick Hb <12 g/dl. Venous blood samples were drawn into lithium heparin tubes (BD, Plymouth, UK). Plasma was separated within 1 h. On a subset of participants, inflammation/infection markers C-reactive protein (CRP) and alpha-1-acid glycoprotein (AGP) were analyzed using the Q-Plex™ Human Micronutrient Array (7-plex; Quansys Bioscience, Logan, UT, USA).27 The presence of inflammation was defined by CRP > 5 mg/dl and/or AGP > 1 mg/ml. hemoglobin A1c (HbA1c) was tested and cut-offs of 6% and 6.4% were used to diagnose normal blood glucose control, prediabetes and diabetes, respectively.28 Blood pressure was measured on the left arm after the participant had been sitting for 10 min, and measures were repeated three times. The average value was recorded, and hypertension was defined as systolic blood pressure > 130 mmHg and/or diastolic blood pressure > 85 mmHg. Waist circumference was measured using a soft tape measured at the midpoint between the anterior superior iliac spine and the lower edge of the ribcage, and a cut-off of >89 cm was used to indicate high waist circumference for South African females.29 BMI was calculated by dividing weight by height squared. Participants were classified as having obesity (BMI ≥ 30 kg/m2) or not having obesity (BMI < 25 kg/m2).
2.6 Metabolic health
Metabolic health risk was estimated by creating a summative “metabolic health risk score” using the biomarkers that were available in this study. To create this summative score individuals with obesity individuals scored a “1” and individuals without obesity were scored “0.” Thereafter, individuals with obesity obtained an additional “1” for each of the following: Hb < 12 g/dl, CRP > 5 mg/dl, and/or AGP > 1 mg/ml; HbA1c > 6%; systolic blood pressure > 130 mmHg and/or diastolic blood pressure > 85 mmHg; and waist circumference > 89 cm. This score was based on traditional Metabolic Syndrome criteria,30 however, was adapted to focus on obesity, and excluded triglycerides and cholesterol markers while including additional metabolic-related markers (inflammation and anemia). Overall metabolic health scores could therefore range from 0 to 6, with higher scores indicating higher metabolic health risk. Metabolic health risk was then categorized for individuals with obesity as low if two or fewer risk factors were present, and high if more than two risk factors were present in addition to obesity.
2.7 Physical activity
Physical activity was assessed using the Global Physical Activity Questionnaire. Minutes per week spent in moderate-to-vigorous physical activity (MVPA) in various domains (work, transport, leisure) were calculated and summed. Compliance with WHO physical activity guidelines31 was assessed by classifying participants who met 150 min of moderate or vigorous activity per week and/or 75 min of vigorous activity per week as “Active,” and those who did not meet these guidelines as “Inactive.” Total time spent sitting was also assessed and summed from three domains (work, transport, leisure). While there is no current consensus on sedentary time guidelines for adults, with most countries advising minimizing sitting time,32 participants were classified as meeting sedentary guidelines or exceeding sedentary guidelines based on evidence for increased risk of all-cause mortality when exceeding 8 h per day.33 Participants were also asked about their daily TV time and screen time.
2.8 Diet diversity and diet quality
Diet diversity was assessed as described previously34 by asking whether the participants consumed foods from the following 14 groups on the day before the interview (grains, orange vegetables, white roots and tubers, dark green leafy vegetables, orange fruit, other fruit, other vegetables, organ meat, other meat or poultry, eggs, fish or seafood, beans or peas, nuts or seeds, milk or milk products). If the woman did not consume any items from each food group the day before, the response was coded “0” and if she did consume an item from the food group it was coded “1.” A diet diversity score was created by summing the responses, so a maximum score of 14 was possible indicating maximum diet diversity.
Diet quality was assessed by asking participants about their frequency of consumption per month of the 14 foods above, and additionally about processed meat, fried snacks, savory snacks, bakery items, sweets, and fizzy drinks using a food frequency questionnaire. The possible responses if women reported they did consume these items were “every day,” “2–4 times per week,” “5–6 times per week,” “once per week,” “less than once per week,” and “never.” A diet quality score was created based on these responses using a method adapted from that published by Imamura et al.35 For “healthy” foods, more frequent intakes scored more highly, while for “unhealthy” foods, more frequent intakes scored low. The maximum diet quality score of 126 would be achieved if a woman consumed all of the “healthy” foods on a daily basis and none of the “unhealthy” foods. If a woman consumed all of the “unhealthy” foods on a daily basis and consumed the healthy foods less than once per week, she would have a score of 0.
2.9 Data analysis
All data were analyzed in STATA/SE V16.1. Initially, data were summarized and presented as median (range) for continuous data, or n (%) for categorical data. Most of the variables were not normally distributed, and even after transformation model residuals were not linear. Therefore, generalized models were utilized for the analyses. Histograms were plotted for each outcome variable to determine the family and link to be used in these models. Thereafter, a series of multiple generalized regressions were run with each adiposity indicator as the exposure and each individual metabolic health variable and then overall metabolic health score as the outcomes. A final series of models were run with FMI, VAT:SAT ratio, android:gynoid ratio, and waist circumference all in the same model with each metabolic health indicator as the outcomes. In all cases, the models accounted for age, SES, physical activity and dietary scores. In all cases, predictors were entered into the model simultaneously and left in regardless of p-value. Students t-test was used to compare differences between higher and lower metabolic health risk in individuals with obesity. Statistical significance was established at p < 0.05.
3 RESULTS
3.1 Cohort demographic and clinical characteristics
While 503 women initially took part in the iron deficiency sub study, the current sample includes only those who were HIV negative and that had all key metabolic health variables of interest (CRP, AGP, HbA1c, hemoglobin, blood pressure, waist circumference, BMI), resulting in 298 women being included in the analysis (see Figure 1). Women were on average 20.93 years old (SD = 2.11 years) and spent on average (median (range)) 7.00 (0.00; 69.00) min in moderate-to-vigorous activity per day and 7.00 (0.33; 84.00) min per day sitting. Women scored on average 47.58/128 (SD = 6.15) on the diet quality score, where a higher score indicates a higher-quality diet, and scored on average 5.63/14 (SD = 2.49) for dietary diversity where a higher score indicates greater diversity in their diet. Access to household assets ranged between 1 and 13 out of a possible 13, with an average of 8.52 (SD = 1.90).
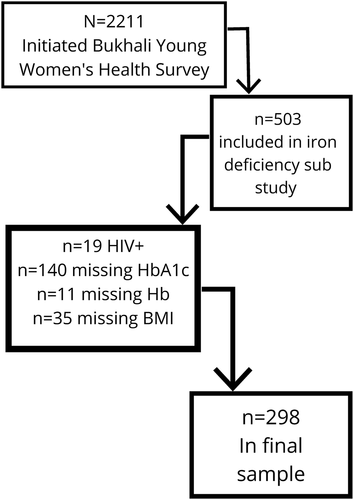
Flow diagram of participant data availability for inclusion in this analysis. Participants were excluded as per the order of variables presented above, and so each consequent n is of those who were included at that point, rather than from the total sample
Participants metabolic health data are presented in Table 1. Most participants had a normal BMI, while 21% presented with overweight and 22% with obesity. Of those who presented with obesity, 3% had morbid obesity. Inflammation was present in 25% of the sample, and 37% of women were anemic. Most women had a normal HbA1c, with only 8% presenting with prediabetes, and 4% presenting with diabetes first detected through the study. Overall, the metabolic health scores showed that only five women had obesity with no other metabolic complications. 13 percent (n = 39) of women had obesity and had three or more metabolic complications.
| Median (range) | N (%) | |
|---|---|---|
| CRP (mg/dl) | 1.27 (0.04; 128.78) | - |
| AGP (mg/ml) | 0.86 (0.01; 2.20) | - |
| Inflammation (yes) | - | 151 (35) |
| Hemoglobin (g/dl) | 12.78 (7.70; 17.05) | - |
| Anemia (yes) | - | 161 (39) |
| Systolic blood pressure (mmHg) | 107.00 (83.50; 174.00) | - |
| Diastolic blood pressure (mmHg) | 75.50 (58.00; 115.30) | - |
| Hypertension (yes) | - | 45 (11) |
| HbA1c (%) | 5.30 (4.00; 8.00) | - |
| Normal HbA1c | - | 272 (87) |
| Prediabetes | - | 2 (7) |
| Diabetes | - | 12 (4) |
| BMI (kg/m2) | 25.57 (15.17; 52.30) | - |
| Underweight | - | 27 (9) |
| Normal weight | - | 144 (48) |
| Overweight | - | 63 (21) |
| Obese | - | 64 (22) |
| Waist circumference (cm) | 74.63 (41.90; 129.23) | - |
| High waist circumference (yes) | - | 75 (18) |
| Metabolic health risk score (score/6) | 0.61 (0.00; 5.00) | - |
| Obesity with low metabolic health risk | - | 5 (2) |
| Obesity with high metabolic health risk | - | 56 (19) |
| No obesity | - | 234 (79) |
- Abbreviations: AGP, alpha1-acid glycoprotein; BMI, body mass index; CRP, C-reactive protein; HbA1c, hemoglobin A1c.
Table 2 shows participant adiposity distribution. In all cases, the range of values was large. The average VAT:SAT ratio was low (median; range) (0.19; 0.04–0.43), indicating increased SAT deposition relative to VAT deposition. Data from the android:gynoid ratio and trunk:limb ratio showed a higher average distribution of adiposity in the android and trunk areas, indicating preferential adiposity storage in the abdominal region, which is also evident from the fact that nearly 50% of women had a high waist circumference (76.89 cm; 41.90–129.23 cm).
| Median (range) | |
|---|---|
| Android fat (g) | 1503 (929; 2340) |
| Gynoid fat (g) | 4691 (3612; 6209) |
| Android:gynoid ratio | 0.84 (0.53; 1.26) |
| Trunk:limb ratio | 0.68 (0.43; 1.48) |
| VAT (cm2) | 57 (37; 91) |
| SAT (cm2) | 295 (195; 439) |
| VAT:SAT ratio | 0.19 (0.04; 0.43) |
| FMI (kg/m2) | 9 (7; 13) |
- Abbreviations: FMI, fat mass index; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
3.2 Regression results
Table 3 shows the relationships between each adiposity distribution variable with each metabolic health risk indicator, adjusted for covariates. Higher FMI was associated with higher CRP (β = 0.39) and AGP (β = 0.02), and with increased metabolic health risk score (β = 0.58; p < 0.01 in all cases). Similarly, visceral fat, trunk:limb ratio, android:gynoid ratio, BMI, weight, and waist circumference were all positively associated with CRP, AGP, and metabolic health risk score (all p < 0.01). Only weight was positively associated with Hba1c (β < 0.01; p < 0.05). Trunk:limb ratio showed a trend towards being associated with Hb, yet this did not reach significance (β = 1.49, p < 0.10). VAT:SAT ratio was only associated with metabolic health risk score (p < 0.01). None of the adiposity indicators were associated with hypertension in this sample. When examining the standardized coefficients for these regressions (Table S1), VAT:SAT ratio was the strongest predictor of CRP and of metabolic health risk score, while android:gynoid ratio was the strongest predictor of AGP.
| Variables | CRP | Hba1c | AGP | Hemoglobin | Hypertension | Metabolic health risk score |
|---|---|---|---|---|---|---|
| FMI | 0.387*** (0.0726) | 0.0110 (0.00763) | 0.0165*** (0.00388) | 0.0194 (0.0272) | −0.0167 (0.0731) | 0.582*** (0.0715) |
| VFAT area | 0.0391*** (0.00777) | 0.00108 (0.000820) | 0.00153*** (0.000421) | 0.00277 (0.00291) | −0.00204 (0.00805) | 0.0508*** (0.00615) |
| Trunk:limb ratio | 7.490*** (2.764) | 0.152 (0.249) | 0.438*** (0.128) | 1.486* (0.878) | 1.964 (2.724) | 7.225*** (1.336) |
| Android:gynoid ratio | 10.50*** (1.718) | 0.155 (0.249) | 0.486*** (0.127) | 0.914 (0.880) | 2.484 (2.642) | 16.01*** (2.306) |
| BMI (kg/m2) | 0.253*** (0.0479) | 0.00855 (0.00528) | 0.0107*** (0.00270) | 0.0138 (0.0188) | −0.00161 (0.0494) | 0.432*** (0.0535) |
| Weight (kg) | 0.0996*** (0.0170) | 0.00439** (0.00202) | 0.00368*** (0.00105) | 0.00308 (0.00724) | 0.00640 (0.0191) | 0.163*** (0.0195) |
| Waist circumference (cm) | 0.0929*** (0.0136) | 0.00418 (0.00258) | 0.00523*** (0.00132) | 0.0104 (0.00918) | 0.00340 (0.0256) | 0.169*** (0.0203) |
| VAT:SAT ratio | 9.471* (5.320) | 0.110 (0.680) | 0.417 (0.357) | 3.868 (2.398) | −4.210 (6.429) | 12.75*** (3.237) |
- Note: Standard errors are in parentheses. All models are corrected for age, SES, diet quality and diet diversity, MVPA, and sitting time.
- Abbreviations: AGP, alpha1-acid glycoprotein; BMI, body mass index; CRP, C-reactive protein; FMI, fat mass index; MVPA, moderate-to-vigorous physical activity; SAT, subcutaneous adipose tissue; SES, socioeconomic status; VAT, visceral adipose tissue.
- ***p < 0.01, **p < 0.05, *p < 0.1.
To determine the independent effect of adiposity distribution, the final regression models included FMI, VAT:SAT ratio, android:gynoid ratio, and waist circumference in the same models with each metabolic health outcome variable (data not shown). When combining these adiposity distribution variables, only FMI remained positively associated with CRP (β = 0.47, p < 0.01) and with overall metabolic health risk score (β = 0.45, p < 0.01).
3.3 Metabolic health risk categories
Figure 2 shows obesity phenotypes presented according to metabolic health risk categories in individuals with obesity. Significant differences existed for FMI, and VAT, whereby participants with obesity and lower metabolic health risk had lower FMI and lower VAT. The distributions of data between the two groups are also visible in Figure 2, largely showing less variance in adiposity deposition in individuals with obesity and higher metabolic health risk.
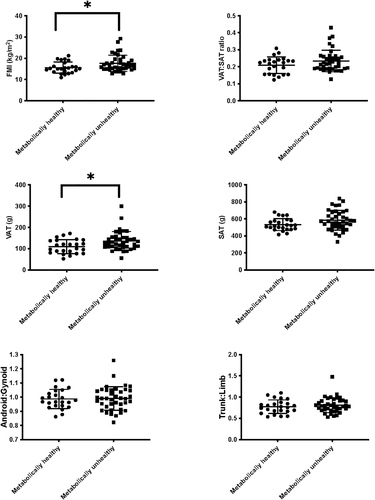
Adipose tissue deposition between metabolically healthy versus metabolically unhealthy individuals with obesity. *p < 0.05
4 DISCUSSION
Within the context of rising obesity prevalence in most low- and middle-income countries, young, Black South African women in the preconception or peri-conception period are at risk for poor pregnancy and offspring outcomes due to metabolic disease. Since there are limited data in Black South African women in comparison to white women, and women from higher-income countries, this study aimed to characterize adiposity distribution and to determine the relationship between adipose tissue deposition and metabolic and inflammatory risk factors in a sample of healthy Black South African women preconception. Women in this study preferentially stored adiposity in the abdominal region and had increased SAT deposition relative to VAT deposition. All adiposity indicators were positively associated with inflammation and with overall metabolic health risk; however, only body mass was associated with HbA1c and none of the adiposity indicators were related to hypertension or hemoglobin levels.
The VAT:SAT ratio and android:gynoid ratio were the strongest correlates of inflammation and metabolic health risk, indicating that fat accumulated in the abdominal region, and a higher ratio of visceral fat to subcutaneous fat was the most strongly associated with poor health indicators. Adipose tissue distribution is a well-known predictor of metabolic health risk, and adipose tissue accumulation in the abdominal region has been associated with obesity related comorbidities.11 Conversely, lower body adipose tissue accumulation has been shown to be protective against metabolic disease, in part due to the lower lipid turnover rate of lower-body fat.11 While most studies have found VAT accumulation key in metabolic disease, and SAT accumulation to be protective,11, 36 most of the data informing those studies are from white women. Our study confirmed the positive association between VAT and overall metabolic health risk in young Black women, yet higher SAT in relation to VAT did not seem to have any protective effect in this population.
There is evidence that VAT versus SAT deposition and contribution to metabolic health risk may differ in Black women.3, 13, 14 Typically, increased VAT has been more strongly associated with insulin resistance compared to SAT, yet in this study neither VAT nor SAT was associated with surrogate markers of insulin resistance. This is similar to findings from a previous study in South Africa, which showed that in Black women there was no relationship between VAT and insulin sensitivity, whereas in white women this relationship existed.3 Previous studies have shown that Black women are more insulin resistant than white women, and that they generally have less VAT and more SAT than white women.3 While women in the present study were generally accumulating more SAT in relation to VAT; further analyses showed that VAT:SAT ratio increased with body mass, which indicates that as body mass increased toward obesity, more adipose tissue was deposited in visceral compartments rather than subcutaneous compartments. Since body mass trended towards being associated with insulin resistance, it may be that in this population, increased body size and absolute mass (resulting in preferential VAT deposition), rather than location of adipose tissue is related to glucose metabolism. It may also be that in this relatively young population, with mostly normal HbA1c values, the differential effects of adiposity distribution on insulin sensitivity are not yet evident.
Body mass, BMI, and waist circumference, which are commonly used to estimate body composition, were significantly predictive of metabolic health risk outcomes; but associations were not as strong as with adipose tissue location. When considering all adiposity indicators in the same model, FMI was the only indicator that remained associated with inflammation and metabolic health risk. Therefore, as expected overall adiposity accumulation and deposition are harmful in this population, but adiposity deposition sites of specific concern for metabolic health risk and inflammation cannot be definitively isolated in this sample in the same way as has been shown in white populations. While less than half of this sample had overweight or obesity, of those who had obesity only 15% had high metabolic health risk (>2 metabolic health risk indicators). Those who had high metabolic health risk had significantly higher FMI as well as VAT deposition. This, too, is concurrent with prior studies showing that metabolically unhealthy obesity presents with higher VAT and decreased adipose tissue function (lower adipose tissue density and greater lipid content) leading to inflammation and insulin sensitivity.11 There is controversy around the benefit of classifying individuals with obesity as metabolically healthy or unhealthy, given that metabolic health presents a spectrum of risk, with obesity being detrimental to health regardless. However, it is clear that mortality risk is higher in metabolically unhealthy individuals, and it is thus important to prevent individuals with obesity from transitioning in that direction.37 Understanding differences in adiposity deposition between these two groups may help to identify individuals with obesity who are at risk of becoming metabolically unhealthy, so as to focus interventions on those at extreme risk.
While not related to adipose tissue deposition in the present study, anemia has previously been associated with obesity and fat mass.38 The prevalence of anemia was high in our cohort and is likely indicative of poor dietary diversity and socioeconomic circumstances38 (also confirmed in our models, which were adjusted for diet and SES given they were both significant confounders). Hypertension was also not associated with any of the adiposity indicators. A study on older participants in Soweto, South Africa (for females, the average age was 49 years), did find an association between BMI, waist circumference, and fat mass with hypertension.39 However, they also found that the strongest positive association was between lean mass-to-fat mass ratio and hypertension, although a strong rationale for this was not provided.39 We tested the potential association between lean body mass and hypertension in our sample, both with and without FMI in the models, and did not find any significant relationships (data not shown). Again, the young age of the present sample decreases hypertension risk, which likely limits the detection of any relationships with body composition.
Considering this sample of young women is likely to become pregnant given their age range and demographics, the high prevalence of overweight and obesity is of concern and is likely to impact pregnancy health, as well as the health of their offspring.7, 40, 41 Our results provide targeted phenotypic characteristics that could be considered when attempting to identify women at the highest risk of metabolic disease and chronic inflammation. Given the interlinked relationship between adiposity, inflammation, hypertension, insulin resistance, anemia, and overall metabolic health risk, any one of these factors in isolation puts women and future offspring at risk—and the presence of multiple risk factors even more so.
This study has several limitations, including the use of DXA-derived adiposity indicators. While DXA is commonly used to approximate adipose tissue compartments; these estimates are calculated based studies conducted in white participants and may lead to some uncertainty in this population.42 However, these imaging techniques remain superior to using BMI and waist circumference alone. Our sample size when considering all relevant outcomes was significantly reduced due to missing blood samples. Furthermore, the cross-sectional design of this study means that we were unable to determine the trajectories of metabolic health risk during- and post-partum. Although we were able to quantify the quality and diversity of participants' diets, we were not able to quantify total caloric intake, which is likely to also be associated with weight gain and adipose tissue deposition. Notwithstanding these limitations, this study has provided insight into the complex relationships between adiposity, metabolic health risk, and inflammation in a crucial period of life, thus providing considerations for primary care as well as intervention studies.
5 CONCLUSIONS
In conclusion, we have shown that Black South African women accumulate excess adipose tissue in abdominal regions and are thus at increased risk of developing chronic inflammation and metabolic disease. Higher FMI and VAT were linked to women with obesity having higher metabolic health risk, while women with obesity with lower FMI and VAT were metabolically protected. These findings inform the screening and identification of women at risk of poor metabolic health prior to conception.
ACKNOWLEDGMENTS
The authors would like to thank the HeLTI project staff for their role in collecting the data, as well as the women who participated in this study for their time and willingness to contribute to science and public health knowledge. This study was supported by the South African Medical Research Council and the Canadian Institutes of Health Research. Research reported in this publication was supported by the National Institute of Child Health and Human Development—AIDS Division (NICHD) and the Fogarty International Center and National Heart Lung and Blood Institute, National Institute of Mental Health, and National Institute of Neurological Disorders and Stroke of the National Institutes of Health under the Award Number D43 TW009337. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation and data collection were performed by Alessandra Prioreschi. Data analysis was performed by Alessandra Prioreschi, assisted by John R. Koethe and Shane A. Norris. Funding and resources were provided by Shane A. Norris. The first draft of the manuscript was written by Alessandra Prioreschi and all authors read and commented on all versions of the manuscript. All authors read and approved the final manuscript.