Lifestyle modification for weight loss: Effects on cardiorespiratory capacity in patients with class II and class III obesity
Abstract
Background
The prevalence of obesity has increased worldwide. Obesity affects the lungs and airways, limits peak oxygen uptake, and hampers physical performance; however, objective data are scarce. Does lifestyle modification for weight loss (LM) have an impact on cardiorespiratory capacity (CRC) in patients with class II and class III obesity?
Method
This was a single-center prospective 2-year follow-up pilot study. Four separated stays in the inpatient specialized medical center Muritunet with an integrated approach to LM, including an individual plan on diet and physical activity (PA) goals. Furthermore, it included lectures and counseling on human anatomy and physiology, nutrition, physical exercise, and motivation, as well as daily PA. Cardiopulmonary and blood chemistry tests were conducted.
Results
Seventy-seven participants were included; however, 47% (n = 36) dropped out during follow-up. Forty-one participants completed the study. At baseline (BL), the mean age was 45.4 (SD 10.2, range 23–62) years, with a mean body mass index (BMI) of 41.3 (SD 5.4) kg/m2, and 85% (n = 35) had one or more comorbidities, such as obstructive pulmonary disease (n = 15, 37%), obstructive sleep apnea (n = 19, 46%), type 2 diabetes (n = 20, 49%), and hypertension (n = 17, 41%). The mean functional residual capacity increased, significantly the second year (p = 0,037). CRC increased significantly the first year (p = 0.032). Weight and BMI declined, reaching statistical significance at 2 years for both males and females (p = 0.033 and p = 0.003, respectively). At BL, the participants reported lower health-related quality of life compared to the general Norwegian population. Across time the physical component summary score (quality of life) for both males and females (p = 0.011 and p = 0.049, respectively) increased significantly.
Conclusion
Lifestyle modification for weight loss improves CRC in patients with class II and class III obesity.
1 INTRODUCTION
Obesity is a multifactorial disease.1, 2 Treatment must be individualized, and the approach combined, targeting different areas of the disease. A recently published meta-analysis addressed different physical training programs combined with or without additional lifestyle modifications in patients with class II and class III obesity. The data included in the meta-analysis (8 of 26 extracted studies) revealed an improvement in walking speed and maximal oxygen uptake in the patients, but results should be comprehended with caution due to heterogeneity among the pooled studies.2 Lifestyle modification for weight loss (LM) refers to setting up an individual plan on diet and physical activity (PA goals. Furthermore, it typically includes interdisciplinary talks on human anatomy and physiology, nutrition, physical exercise, and motivation, as well as daily PA. Obesity class II and III lead to an increase in the all-cause mortality and are an independent probability in the development of noncommunicable diseases such as type 2 diabetes mellitus (T2DM), hypertension (HTN), and cardiovascular disease (CVD), as well as obstructive pulmonary disease (OPD) and obstructive sleep apnea (OSA).3
Cardiorespiratory capacity (CRC) is a predictor of CVD, and an improvement reduces mortality regardless of body mass index (BMI).4 Weight and CRC are strongly associated. PA led to energy expenditure (EE), approximately 5 kcal is liberated pr. liter oxygen consumed, and EE led to weight loss. PA and EE are important measurements objectively quantifying achievements in people treated for obesity.5 CRC (the primary variable is VO2max) or aerobic fitness is a measurement of how well the respiratory and circulatory systems supply O2 and remove CO2 from working skeletal muscles. Obesity class II and III are often associated with poor physical performance and impaired CRC due to structural and functional changes such as remodeling of the heart, restrictive pulmonary ventilation, and challenges to physical activities due to excess body mass.6 On the other hand people with obesity do not always have reduced CRC, but still they have attenuated risk for all-cause mortality known as the obesity paradox.7 In general the literature report inconclusive data due heterogeneity in study design and differences in how data are reported. Just recently the literature report VO2peak reference values in people with obesity tested on a bicycle, that differ to data obtained in studies performed on a treadmill.8 Patients with class II and class III obesity breathe at a lower lung volume due to a restriction of the diaphragmatic movements causing alveolar and airway closure at the lung base. This contributes to an altered breathing pattern and changes in ventilation and diffusion of gases in the pulmonary alveoli.9 A patient with class II or class III obesity often senses dyspnea and wheezing during normal breaths, which indicates possible OPD. Obesity-related hypoventilation during sleep typically causes profound changes in arterial oxygen (PO2) and carbon dioxide (PCO2) tension. Furthermore, there is an inverse relationship between CRC and BMI with an exponential fall in the functional residual capacity (FRC), expiratory reserve volume (ERV), and oxygen consumption (VO2) with increasing BMI.10 This relationship may be seen regardless of fitness level.11 The respiratory system in people with obesity is characterized by a stiffening tissue, presumed to be due to a combined effect on the pulmonary and thoracic tissue compliance. Reductions in compliance may be the result of increased pulmonary blood volume, closure of dependent airways resulting in small areas of atelectasis, or increased alveolar surface tension due to the reduction in FRC.9
In addition, adipose tissue is hormonally and inflammatory active, as demonstrated by leptin and high-sensitivity C-reactive protein (hs-CRP), a biomarker of inflammation.12 The regular hormone leptin, a neuropeptide, has a rather strict role as a communicator of nutritional status, including food intake, hunger, satiety, energy storage, and consumption between the body and brain. Furthermore, leptin has effects on sleep and respiration and is typically paradoxically increased in patients with class II and class III obesity and even higher if OSA or OPD is present.13, 14 An association between hypoxia and inflammation, as seen in sleep-related hypoventilation and OPD, may also exist.12
To most people LM is the tool used in effort to manage a multifactorial chronic disease. LM-programs involve a combination of interventions including diet, PA, and behavioral issues, and most people with obesity are left with LM as their only treatment option.15, 16 Today different lifestyle modification programs to treat people with obesity exists. They are different with regards to location (at home or in an institution), duration (weeks, months, or years), number of session (low, moderate, and high) and how they are followed-up (phone, video-chat, community nurse, etc.) after the intensive part of the LM-program has ended.17, 18 However, LM is still arduous to achieve and is a complex treatment and specially to accomplish regular PA and to avoid regaining weight when returning to home are complicated. In summary, the most effective LM approach for patients with class II and class III obesity is yet to be uncovered.
In this prospective pilot study involving patients with class II and class III obesity, the hypothesis was LM improves CRC.
2 MATERIALS AND METHODS
2.1 Trial design
The present study was a single-center, non-randomized, prospective pilot study with four inpatient stays. The baseline (BL) stay was 4 weeks. The following stays were scheduled as follows: 6 months, 1 year, and 2 years after the BL stay, each of 2 weeks duration. No planned contact with participants between stays. The primary outcome of the study was the effects on CRC at 1 and 2 years reported as VO2peak (oxygen consumption at anaerobic threshold) and FRC (parameter of dynamic lung volume). The secondary outcomes were changes in the health-related quality of life (HRQoL). Exploratory outcomes leptin and hs-CRP as biomarkers related to adipose tissue and respiratory effects (Figure 1).
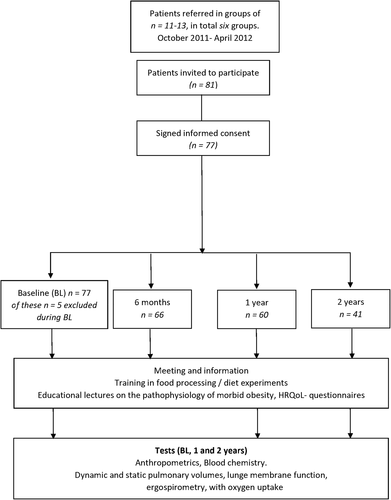
Trial design
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and the Regional Committees for Medical and Health Research Ethics of North Norway (2011/1482), and all procedures involving human subjects were evaluated. The participants provided informed written consent prior to enrollment.
2.2 Participants
Participants were referred to Muritunet, because of unhealthy weight, between October 2011 and April 2012 and were followed for 2 years. Muritunet is 72-bed-capacity inpatient specialized medical center for the rehabilitation of people with chronic illnesses. At Muritunet, they were screened for general eligibility and compliance with the inclusion and exclusion criteria and invited to participate in the study.
Inclusion criteria were age 18–67 years, BMI ≥35 kg/m2, and one or more of weight-related comorbidities such as T2DM, CVD, HTN, OPD, and OSA. Exclusion criteria were either extensive pulmonary/cardiac dysfunction, musculoskeletal disease which precluded physical training, psychological/social dysfunction unfit for group interaction, pregnancy, alcohol and drug abuse, or noncompliance to the study protocol.
2.3 Study protocol
The participants were clinically examined, and anthropometric measurements (height, weight, and waist circumference) were taken. They also did repetitive cardiopulmonary (see assessments) and blood chemistry tests and answered questionnaires on HRQoL (Figure 1).
2.4 Lifestyle modification
During the first stay each, participant developed an individual plan with diet and PA goals for the entire rehabilitation period. At every follow-up stay, talks on human anatomy and physiology, nutrition, physical exercise, and motivation were given. The four stays in the inpatient program lasted in total 10 weeks. The BL visit was 4 weeks. The following visit were 6 months, 1 year, and 2 years after the BL visit, each of 2 weeks duration. The participants were given lectures as one-on-one talks, in total 20 h, with a dietician, physiotherapist, sport educator, nurse, and physician. Furthermore, the participants had lectures and PA in groups-sessions, in total 193 h, that is, 15 h of motivation and goal promoting work, 30 h education and practice on nutrition, 10 h of lectures on obesity, and 138 h of PA, that is, approximately 14 h weekly.
The dietician lectured on different issues such as the essence of food processing, low caloric diet, repetitive small meals, and advice according to the Norwegian nutritional guidelines.19
PA focused on weight-bearing activities such as walking, jogging, ball games including exercises in a swimming pool and resistance training. Once or twice a week, the participants hiked in the rough terrain of the surrounding mountains. PA achievement was measured during each stay, on a treadmill as describe below. A daily afternoon debrief was held, focusing on individual achievements/learning aspects. A daily caloric reduction of 500–1000 kcal was the target and a weight reduction of 5%–10% in the first year (Norwegian guidelines20, 21).
2.5 Assessments
Cardiopulmonary tests were all instructed and observed by an experienced physiotherapist. The tests were performed in the morning between 9 AM and 10 AM. Pulmonary dynamic volumes were measured using flow/volume spirometry, including lung membrane function using carbon monoxide (CO) single breath diffusion capacity.9 Pulmonary static lung volumes were measured using the helium rebreathing technique (calibrated daily using a 1 L syringe), Jaeger Master Lab (Erich Jaeger). The patients sat in a vertical position with a nose clip.
CRC was tested on a treadmill (Woodway PPS 55 Med) using a modified Bruce protocol designed for people in poor physical condition.22 CRC's primary variable is VO2max, the maximum rate of oxygen consumption during exercise of increasing intensity. The test end point in this study was when the participant peaked the level of individual exhaustion and achieved anaerobic threshold, reported as VO2peak (ml·kg−1·min−1.5 In this study VO2peak (defined as the start of the plateau phase of the oxygen uptake curve) equals VO2max. VO2peak (ml·kg−1·min−1) was reported relative to body weight. When the participants reported a history of unusual daytime fatigue, snoring with episodes of apnea at night and morning headache, screening for OSA was done and the Epworth Sleepiness Scale23 was filled in. Furthermore, two consecutive overnight oximetry tests (PalmSAT® 2500 series; Nonin Medical B.V.) were performed.
Recorded dynamic lung volumes were forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and FEV1/FVC ratio. Recorded static lung volumes (Figure 2) were total lung capacity, vital capacity, inspiratory capacity, FRC, ERV, and residual volume. All values were adjusted for weight, height, and sex and reported in liters (l) except for the FEV1/FVC ratio, which was reported as percentage. The reference values used were those recommended by the European Respiratory Society.24 Reference values regarding ERV are not offered by ERS; therefore, the Jaeger Master Lab reference values were used. OPD was defined as FEV1/FVC <70%. OSA was defined as an apnea–hypopnea index of ≥5.

The subdivisions of lung volume as recorded by a flow volume spirometer. The term capacity is applied to a subdivision composed of two or more volumes
2.6 Analysis of blood samples
After an overnight fast, a venous blood sample from the antecubital vein was taken at 9 AM by the same technician. The sample was immediately processed and cooled down and later transported in a cooler to the Department of Medical Biochemistry at Ålesund Hospital, Ålesund, Norway, where it was analyzed or stored at −80 C° for later analyses. Frozen samples were shipped to laboratories in southern Norway on dry ice for analysis of leptin and hs-CRP.
2.7 HRQoL Short Form-36
HRQoL Short Form-36 (version1.1) was used in the study, a self-administered questionnaire containing 36 multi-items covering eight domains, measuring HRQoL among adults (age > 18 years). The questionnaire has high validity and reliability and is regarded as the gold standard of QoL questionnaire. In this study, a version translated into Norwegian, and validated in Norway's general population was applied.25 The eight domains (with the corresponding number of items) were physical functioning (PF = 10 items), role limitations due to physical problems (RP = 4 items), bodily pain (BP = 2 items), general health (GH = 5 items), vitality (VT = 4 items), social functioning (SF = 2 items), role limitations due to emotional problems (RE = 3 items), and mental health (MH = 5 items). Each score in the dimension was translated into a scale from 0 to 100. Higher scores indicated better HRQoL. As recommended, the subscales were summed up in two broader summary scales: PF, RP, BP, and GH in the Physical Component Summary (PCS) and RE, SF, MH, and VT in the Mental Component Summary (MCS).26 All HRQoL data in this study are reported longitudinally.
2.8 Statistical analysis
Due to its design as a pilot study, no power calculation were done. Descriptive statistics is reported as mean and standard deviation for continuous variables. For categorical variables, frequencies and relative frequencies were used. Univariate comparisons of outcomes between different time points were analyzed using paired sample t-tests. The impact of LM on changes in various measures of cardiopulmonary function, HRQoL, and selected biochemical variables was explored by estimating mixed linear models, with random intercepts for individuals, with the outcome measure as the dependent variable and time (BL and follow-up times) as independent variables. Missing data were imputed using multiple imputation by chained equations. Analyses of respiratory function were not stratified by sex due to nonexisting normality data. All other analyses were stratified according to sex due to considerable effect differences. p-values <0.05 were considered statistically significant. All analyses were performed using STATA v 15.1 (StataCorp, 2017) and SPSS v25.
3 RESULTS
Seventy-seven participants (F = 57, 74%) were initially included in the study. However, five of these were excluded early during the BL visit; two withdrew, one had an injury (not study related), and two were excluded because of technical issues. During the follow-up stays, 31 patients were lost to follow-up and were consequently excluded, yielding a dropout rate of 47% (n = 36). No significant differences between those who dropped out of the study and those completing were seen. Among those who dropped out of the study there was a slightly higher proportion of women (p = 0.190). Furthermore, they tended to be somewhat younger (p = 0.199) and had higher BL BMI (p = 0.080).
Forty-one participants completed the 2-year visit (F = 25, 61%) and were included in the final analysis. Of the 41 participants, 85% (n = 35) had one or more of the following comorbidities: OPD (n = 15, 37%), OSA (n = 19, 46%), T2DM (n = 20, 49%), and HTN (n = 17, 41%). Furthermore, the mean age was 45.4 (SD 10.2, range 23–62) years. Mean BMI (kg/m2) at BL, 1 year, and 2 years were 41.3 (SD 5.4), 38.7 (SD 6.0), and 39.6 (SD 6.4), respectively. Mean weight (kg) at BL, 1 year, and 2 years were 122.8 (SD 25.6), 115.4 (SD 25.8), and 117.6 (SD 26.6), with an average weight reduction of six percent (p < 0.001) the first year. The average score of weight (kg) and BMI (kg/m2) were separately reported at every time point for males and females, complemented with the regression coefficient of time from mixed model analyses (Table 1), which displayed a significant decrease in average weight and body mass for both males and females during the study.
| Baseline | 1 year | 2 years | Regression coefficient (+95% CI) | p-value | |
|---|---|---|---|---|---|
| Weight kg | |||||
| Males (SD) | 135.6 (21.6) | 130.1 (20.0) | 128.7 (20.6) | −2.34 (−4.49 to −0.19) | 0.033* |
| Females (SD) | 114.6 (25.0) | 106.5 (25.1) | 110.4 (27.9) | −1.79 (−2.98 to −0.61) | 0.003* |
| BMI (>35 kg/m2) | |||||
| Males (SD) | 41.8 (4.7) | 39.8 (5.2) | 39.7 (4.6) | −0.73 (−1.29 to −0.17) | 0.011* |
| Females (SD) | 40.9 (5.9) | 38.1 (6.5) | 39.5 (7.3) | −0.60 (−1.07 to −0.13) | 0.011* |
| Demographicsa | |||||
| Males n (%) | 16 (39) | ||||
| Females n (%) | 25 (61) | ||||
| Caucasian n (%) | 41 (100) | ||||
| Legal status | |||||
| Single n (%) | 17 (41.5) | ||||
| Married n (%) | 20 (48.8) | ||||
| Divorced n (%) | 3 (7.3) | ||||
| Education | |||||
| Primary school (10 years) n (%) | 8 (19.5) | ||||
| Secondary school (13 years) n (%) | 22 (53.7) | ||||
| Collage/University (1–4 years) n (%) | 9 (22.0) | ||||
| University (>4 years) n (%) | 2 (4.9) | ||||
| Employment | |||||
| Employed n (%) | 27 (65.9) | ||||
| Unemployed n (%) | 13 (31.7) | ||||
| Missing data n (%) | 1 (2.4) | ||||
| Occupation | |||||
| Academic n (%) | 7 (17) | ||||
| Office n (%) | 5 (12.2) | ||||
| Sales n (%) | 4 (9.7) | ||||
| Craftsman n (%) | 11 (26.8) | ||||
| Missing data n (%) | 14 (34.3) | ||||
| Comorbiditiesa | |||||
| Obstructive pulmonary disease n (%) | 15 (37) | ||||
| Obstructive sleep apnea n (%) | 19 (46) | ||||
| Diabetes mellitus type II n (%) | 20 (49) | ||||
| Hypertonia n (%) | 17 (41) |
- Note: Demographic and comorbidities data at baseline. Analysis of anthropometric data across time. Data are presented as estimated means (SD) and regression coefficient + 95% CI.
- a Frequency.
- *significant p-value.
In Table 2, the average scores or proportions at every time point for various respiratory outcome measures again complemented with the regression coefficient of time from the mixed model analyses. There was an increase in the mean FRC percentage (p = 0.037) during the study, significant between the first and second year. At BL, 1 year, and 2 years stays, CRC reported as mean VO2peak (ml·kg−1·min−1) were 23.3 (SD 3.6), 25.6 (SD 4.8), and 23.3 (SD 5.3), respectively, and significantly increased in the first year compared to BL (p = 0.032).
| Baseline | 1 year | 2 years | Regression coefficient (+ 95% CI) | p-value | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Cardiorespiratory capacity | |||||
| VO2 peak (ml·kg−1·min−1) | 23.3 (3.6) | 25.6 (4.8) | 23.3 (4.7) | 0.16 (−0.40 to 0.72) | 0.57 |
| Gas diffusing capacity | |||||
| DLCO % | 93.5 (12.8) | 97.1 (15.9) | 96.3 (14.6) | 1.03 (0.05 to 2.00) | 0.039* |
| DLCO (l) | 8.9 (2.3) | 9.4 (2.6) | 9.3 (2.5) | 0.15 (0.12 to 0.28) | 0.033* |
| DLCO/VA % | 104.4 (14.6) | 107.8 (15.3) | 108.3 (15.7) | 1.35 (0.44 to 2.26) | 0.004* |
| DLCO/VA (l) | 1.7 (0.24) | 1.7 (0.24) | 1.7 (0.25) | 0.01 (−0.01 to 0.03) | 0.274 |
| Dynamic pulmonary volumes | |||||
| FVC (% pred.) | 99.9 (13.1) | 102.2 (13.9) | 100.9 (15.0) | 0.42 (−0.32 to 1.16) | 0.265 |
| FVC (l) | 3.9 (1.0) | 4.0 (1.1) | 3.9 (1.1) | 0.02 (−0.04 to 0.09) | 0.484 |
| FEV1 (% pred.) | 93.5 (16.3) | 95.3 (17.6) | 92.9 (18.5) | −0.06 (−1.01 to 0.90) | 0.904 |
| FEV1 (l) | 3.0 (0.9) | 3.2 (1.0) | 3.0 (0.9) | 0.01 (−0.05 to 0.06) | 0.816 |
| FEV1/FVC % | 77.2 (9.4) | 76.6 (8.7) | 75.6 (9.6) | −0.50 (−1.04 to 0.04) | 0.072 |
| Static pulmonary volumes | |||||
| TLC (% pred.) | 92.7 (10.3) | 93.6 (8.6) | 95.4 (8.9) | 0.85 (0.07 to 1.62) | 0.032* |
| TLC (l) | 5.6 (1.2) | 5.6 (1.4) | 5.8 (1.3) | 0.05 (−0.02 to 0.11) | 0.183 |
| VC (% pred.) | 98.0 (12.8) | 102.4 (12.4) | 98.0 (12.3) | 0.32 (−1.03 to 1.67) | 0.640 |
| VC (l) | 4.1 (1.1) | 4.1 (1.3) | 4.0 (1.0) | −0.01 (−0.09 to 0.07) | 0.802 |
| IC (% pred.) | 108.4 (19.1) | 108.6 (16.6) | 107.7 (21.3) | −0.18 (−2.30 to 1.93) | 0.866 |
| IC (l) | 3.1 (0.9) | 3.1 (1.1) | 3.1 (0.9) | −0.01 (−0.09 to 0.07) | 0.852 |
| FRC (% pred.) | 79.8 (14.5) | 81.0 (13.0) | 84.1 (16.9) | 1.31 (−0.47 to 3.08) | 0.147 |
| FRC (l) | 2.4 (0.6) | 2.5 (0.6) | 2.6 (0.8) | 0.05 (−0.01 to 0.11) | 0.120 |
| ERV (% pred.) | 78.3 (37.9) | 85.4 (41.0) | 75.0 (33.3) | −0.43 (−6.52 to 5.66) | 0.889 |
| ERV (l) | 0.95 (0.4) | 0.98 (0.4) | 0.84 (0.4) | −0.03 (−0.07 to 0.02) | 0.207 |
| RV (% pred.) | 82.0 (22.8) | 77.9 (16.8) | 89.5 (15.5) | 1.84 (−0.46 to 4.14) | 0.116 |
| RV (l) | 1.5 (0.5) | 1.5 (0.5) | 1.7 (0.5) | 0.05 (0.00 to 0.51) | 0.049* |
- Note: Analysis of cardiorespiratory capacity, gas diffusing capacity, dynamic/static pulmonary volume parameters across time. Data are presented as estimated means (SD) and regression coefficient + 95% CI.
- Abbreviations: % pred., % predicted = respiratory parameters adjusted for weight, height, and sex; DLCO, diffusing capacity for carbon monoxide; DLCO/VA, DLCO/alveolar volume; ERV, expiratory reserve volume; FEV1, forced expiratory volume in 1 s; FRC, functional residual capacity; FVC, forced vital capacity; IC, inspiratory capacity; l, liters; RV, residual volume; TLC, total lung capacity; VC, vital capacity; VO2 peak, cardiorespiratory capacity.
- *significant p-value.
Both males and females exhibited a significant increase across time in the PCS (p = 0.011 and p = 0.049, respectively) (Table 3). For both sexes, there was a peak in PCS at the 1-year stay, followed by a decline at 2 years of stay. Both males and females reported lower pain and better physical functioning across time, whereas only males reported better general health (p = 0.006). As for the MCS score, there were clear differences between males and females. Across time, MCS significantly increased for males (p = 0.036), whereas no such increase was found for females (p = 0.271). Furthermore, males reported less fatigue (p = 0.005) and better social functioning (p = 0.002) across time, whereas no such findings were seen in females. Neither males nor females reported any effects on limitations due to emotional problems and emotional well-being across time.
| Baseline | 6 months | 1 year | 2 years | Regression coefficient (+ 95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| PCSa | ||||||
| Males | 57.9 (20.9) | 64.1 (22.9) | 69.2 (19.0) | 67.6 (22.3) | 3.43 (0.78 to 6.07) | 0.011* |
| Females | 57.9 (17.9) | 66.5 (19.3) | 68.7 (16.8) | 63.5 (17.7) | 1.90 (0.01 to 3.80) | 0.049* |
| Physical functioning | ||||||
| Males | 73.1 (22.9) | 77.7 (22.3) | 83.6 (14.3) | 80.0 (20.2) | 2.65 (0.25 to 5.05) | 0.030* |
| Females | 68.8 (18.8) | 78.8 (18.8) | 75.8 (22.1) | 76.3 (16.4) | 1.94 (−0.17 to 4.05) | 0.071 |
| Role physical | ||||||
| Males | 36.4 (32.9) | 37.5 (33.7) | 45.2 (34.4) | 44.2 (34.8) | 3.11 (−2.34 to 8.57) | 0.263 |
| Females | 35.4 (32.3) | 43.4 (31.1) | 50.7 (26.1) | 41.4 (32.2) | 2.53 (−1.56 to 6.62) | 0.226 |
| Body pain | ||||||
| Males | 58.4 (32.2) | 62.3 (33.4) | 65.1 (29.1) | 68.9 (27.9) | 3.42 (0.15 to 6.69) | 0.040* |
| Females | 52.2 (29.1) | 59.7 (30.5) | 64.3 (27.2) | 57.8 (31.4) | 2.14 (−0.78 to 5.06) | 0.150 |
| General health | ||||||
| Males | 38.1 (16.9) | 54.7 (21.4) | 55.6 (23.3) | 53.8 (24.6) | 4.78 (1.34 to 8.21) | 0.006* |
| Females | 49.2 (18.7) | 57.6 (20.5) | 65.9 (15.7) | 49.2 (17.9) | 0.83 (−1.81 to 3.46) | 0.539 |
| MCSb | ||||||
| Males | 66.8 (14.7) | 65.6 (19.1) | 69.9 (15.6) | 73.7 (13.6) | 2.51 (0.16 to 4.86) | 0.036* |
| Females | 62.2 (17.2) | 69.9 (16.5) | 71.4 (14.3) | 65.9 (19.8) | 1.28 (−1.00 to 3.55) | 0.271 |
| Vitality | ||||||
| Males | 46.3 (17.7) | 49.3 (22.7) | 53.6 (21.8) | 57.8 (22.4) | 3.90 (1.15 to 6.64) | 0.005* |
| Females | 37.4 (18.5) | 47.1 (20.2) | 50.0 (18.1) | 42.4 (22.0) | 1.80 (−0.83 to 4.42) | 0.180 |
| Social functioning | ||||||
| Males | 71.1 (18.7) | 68.6 (20.6) | 78.7 (17.5) | 82.8 (15.1) | 4.52 (1.67 to 7.37) | 0.002* |
| Females | 78.5 (20.6) | 83.7 (21.2) | 80.6 (20.8) | 81.5 (28.4) | 0.58 (−2.67 to 3.84) | 0.725 |
| Role emotional | ||||||
| Males | 81.3 (24.2) | 83.0 (25.2) | 87.6 (24.6) | 85.4 (24.2) | 1.71 (−3.71 to 7.14) | 0.536 |
| Females | 69.3 (40.7) | 80.8 (28.5) | 80.3 (38.0) | 73.3 (41.9) | 1.16 (−4.39 to 6.70) | 0.683 |
| Mental health | ||||||
| Males | 72.8 (15.2) | 66.9 (22.9) | 68.8 (15.1) | 75.9 (13.4) | 1.15 (−1.42 to 3.71) | 0.381 |
| Females | 71.1 (16.2) | 76.0 (14.3) | 78.5 (12.1) | 74.1 (16.3) | 1.11 (−0.85 to 3.06) | 0.266 |
- Note: Analysis of data on health-related quality of life assessed by the SF-36 questionnaire across time. Data are presented as estimated means (SD) and regression coefficient + 95% CI. SF-36 = Health-related quality of life questionnaire, range 0–100 (higher score indicates better).
- Abbreviations: MCS, mental component summary score refers to: vitality, social functioning, role emotional, and mental health; PSC, physical component summary score refers to: physical functioning, role physical, body pain, general health.
- *significant p-value.
- a PSC = Physical component summary score refers to: physical functioning, role physical, body pain, general health.
- b MCS = Mental component summary score refers to: vitality, social functioning, role emotional, and mental health.
The participants had a significant increase in mean leptin levels across time, both males and females (p = 0.002 and p = 0.001, respectively). Furthermore, a decrease across time in hs-CRP outside the normal range were found, significant for males and females (p = 0.005 and p = 0.001, respectively) (Table 4).
| Baseline | 1 year | 2 years | Regression coefficient (+ 95% CI) | p-value | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Leptin | |||||
| Males (N < 465 pmol/l) | 1497 (696) | 1788 (883) | 2053 (1080) | 180.5 (65.2 to 295.8) | 0.002* |
| Females (N < 930 pmol/l) | 2293 (1131) | 2728 (1250) | 3967 (2007) | 509.8 (328.4 to 691.1) | 0.001* |
| High-sensitive CRP | |||||
| Males | 7.1 (5.5) | 5.2 (5.5) | 3.3 (2.2) | −1.23 (−2.09 to −0.37) | 0.005* |
| Females | 10.8 (9.0) | 8.4 (9.9) | 5.6 (5.7) | −1.64 (−2.63 to −0.65) | 0.001* |
- Note: Analysis of hormone and inflammatory parameters across time. Data are presented as estimated means (SD) and regression coefficient + 95% CI.
- Abbreviation: CRP, C-reactive protein.
- *significant p-value.
4 DISCUSSION
The study was a pilot study to investigate whether a systematic modification of lifestyle in patients with class II and class III obesity had an impact on CRC, when targeting a weight reduction of 5%–10% in the first year. A weight reduction of 6% the first year were reported. Furthermore, FRC increased gradually, but significant during the study period, and VO2peak increased significantly in the first year but declined in the second year. The patients reported a significant improvement on quality of life, with a peak after 1 year. A limitation to the study was the dropout rate of 47% across time, further discussed below.
Improvement in CRC is a health achievement. Results from this study indicate that lifestyle modification (PA including aerobic/resistance exercise, diet modification) improve CRC (VO2peak and FRC), comparable to a recently presented meta-analysis indicating aerobic/resistance training, and diet modification, to led to improved cardiovascular/muscular endurance in patients with class II and class III obesity.2 Future research should emphasize the importance of physical fitness, not only weight reduction, in people with obesity.2
PA leads to EE and weight loss, approximately 5 kcal is liberated pr. liter oxygen consumed.5 Any improvement in cardiorespiratory health is an important health achievement, even if the cardiorespiratory response is modest, as in this study.27 All VO2peak estimates were well below the average in a nonobese population. The reported improvement of 2.3 (mL·kg−1·min−1) in CRC after 1 year, reflects the positive health achievement as a feasible goal, an important knowledge for both the patient and health personnel working with obese patients.28, 29 The participants in this study had chronic respiratory problems, such as OPD, at a higher rate compared to the nonobese Norwegian population, in line with the literature reporting on class II and class III obesity.30 It is also discussed in the literature if OPD in obese patients is a distinct asthmatic phenotype.31
The participants in this study reached the goal, a modest weight reduction the first year, but with a tendency to regain weight the following year. However, people with obesity are sensitive to a modest weight reduction, also revealed in data from this study.32 Causes of weight gain are typically multifactorial based on both physiological and psychological factors, as we report (HRQoL score) in our population.29
Obesity with or without related comorbidities adversely affects PA and quality of life.33 Data in this study reveal that obesity was associated with a decreased physical and emotional well-being at all stays. The participants had low scores in quality of life compared to the normal population in Norway. Although an improvement after the first year were seen, the HRQoL score declined in the second year. SF-36 and its subscale scores should, however, be interpreted with care. Component summary scales of the SF-36 explain the variance better and are more reliable in class II and class III obesity.26 The positive change in HRQoL was more evident, and significant in PCS, especially for males, which was weaker in the MCS. The findings in this study support the majority of published studies in which weight loss is related to HRQoL.34
The mechanical component of obesity also affects CRC. A significant improvement in FRC, a parameter influenced by chest wall and diaphragmatic compliance, were seen in this study. Improvement in FRC led to an improvement in the sensation of dyspnea that patients with class II and class III obesity experience with even very minimal physical exercise. The modest, but significant change in FRC in this study, is an important information that might help patients with class II and class III obesity to overcome the anxiety of dyspnea they experience even during minor physical exercise. Furthermore, the data show an almost 50% occurrence of OSA among the participants who completed the study (Table 1), compared to only 4%–6% in the nonobese Norwegian population. People with obesity class II and III are at risk of developing OSA with sleep-disordered breathing and daytime hypercapnia.14
In addition to mechanical factors, inflammatory and hormonal mechanisms caused by an extensive amount of fat tissue are factors that complicated the challenges of obesity. Explorative data in this study display a declining trend in hs-CRP, possibly caused by a reduction in the adipose tissue load. Pro-inflammatory activity may have an effect on CRC, one of the several factors explaining the cardiorespiratory challenge in patients with class II and class III obesity.12 Furthermore, the neuropeptide leptin may also be an important factor in obese individuals. The data in this study exhibit an increase in leptin levels during the 2-year follow-up, that is, an inverse reaction to weight loss. Most studies have focused on obesity class I where weight loss led to a decline in leptin levels. The population in this study was different and involved people with patients with class II and class III obesity. A possible rationale for the increased leptin in this study, especially during the second year follow-up, is the hypothalamic resistance favoring a continuous secretion of leptin, which also stimulates the hunger response and drive to eat.35 The combined actions of mechanical obstruction, low-grade inflammation, and hyperleptinemia are all factors that may explain the higher incidence of pulmonary obstruction as well as the OSA seen in our data.
4.1 Limitations
This small-scale study had several limitations due to its design as a pilot study, no power calculation, female/male ratio 3:2 and no control group. A small sample size increased the risk of having both type I and type II errors, that is, finding results where none exists and not finding results where they actually exist. It is still worth mentioning that smaller studies like this are valuable, both to examine the feasibility of an intervention and to provide results that could be subject to further testing and may also generate other testable hypotheses. In any study, a study dropout is often a critical issue. In this study, we report a dropout rate of 47% during the 2-year follow-up. The dropouts could be individuals who least benefited from the intervention, for example, due to lack of motivation. If this was the case, there is a risk of overestimating the effect of the intervention. In this study, especially in the first year, a positive trend in almost all outcome parameters was seen. However, with 1 year between the third and the final stay, a substantial number of participants were lost to follow-up, which affected the 2-year data. More stringent measures should have been in place, including more frequent alternative follow-up visits as reported in other LM studies on obesity.17, 18 Furthermore, several technical difficulties were faced leading to missing data since the study population has demanding chronic diseases and several day-to-day health issues causing inability to participate.
5 CONCLUSION
A systematic LM improved CRC in patients with class II and class III obesity, even with minor, but clinically relevant, and significant weight decline. Knowledge that even a minor improvement in CRC leads to health achievement, should be considered as a motivating factor to the patient as well as the health provider.
ACKNOWLEDGMENTS
The authors thank all the involved personnel at Muritunet A/S, Centre for Achievement and Rehabilitation, Valldal, Norway; Lutz Schwettmann of the Department of Medical Biochemistry, Ålesund Hospital, Norway, for the kind assistance during the blood chemistry analysis; and Solveig Roth Hoff of the Department of Radiology, Ålesund Hospital, Norway for the valuable comments on the final manuscript. The Norwegian Extra Foundation for Health and Rehabilitation has supported this project with a grant (ID 2010.3.0149).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: Finn Wammer, Sveinung Vemøy, and Andrea Harberberger. Analysis and interpretation of data: Finn Wammer and Dag Arne Lihaug Hoff. Drafting of the manuscript: Finn Wammer and Dag Arne Lihaug Hoff. Critical revision of the manuscript for important intellectual content: Finn Wammer, Anita Dyb Linge, and Dag Arne Lihaug Hoff. Statistical analysis: Tor Åge Myklebust. Obtaining funding: Finn Wammer and Sveinung Vemøy. All authors have read and approved the final manuscript.




