L-arginine as a potential GLP-1-mediated immunomodulator of Th17-related cytokines in people with obesity and asthma
All authors have read and approved the submission of the manuscript.
Abstract
Obesity is considered as a risk factor for COVID-19 with insulin resistance and increased production of inflammatory cytokines as likely mechanisms. Glucagon-like peptide-1 (GLP-1) agonists and inhaled nitric oxide are proposed therapeutic approaches to treat COVID-19 because of their broad anti-inflammatory effects. One approach that might augment GLP-1 levels would be dietary supplementation with L-arginine. Beyond cytokines, multiple studies have started to investigate the relationship between new-onset diabetes and COVID-19. In a posthoc analysis of a randomized, placebo-controlled human clinical trial of L-arginine supplementation in people with asthma and predominantly with obesity, the results showed that 12 weeks of continuous L-arginine supplementation significantly decreased the level of IL-21 (p = 0.02) and increased the level of insulin (p = 0.02). A high arginine level and arginine/ADMA ratio were significantly associated with lower CCL-20 and TNF-α levels. The study also showed that L-arginine supplementation reduces cytokine levels and improves insulin deficiency or resistance, both are two big risk factors for COVID-19 severity and mortality. Given its safety profile and ease of accessibility, L-arginine is an attractive potential therapeutic option that allows for a cost-effective way to improve outcomes in patients. An expedition of further investigation or clinical trials to test these hypotheses is needed.
1 INTRODUCTION
The high mortality and morbidity of coronavirus disease in 2019 (COVID-19) has stimulated worldwide research to look into novel approaches to block the severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) or dampen the associated cytokine driven hyper-inflammatory response. Obesity is a risk factor for COVID-19 and increased insulin resistance, activation of the renin-angiotensin system, and increased inflammatory cytokines production may all be factors in host susceptibility.1, 2 Glucagon-like peptide-1 (GLP-1) agonists have been proposed as potential therapies due to their strong anti-inflammatory effect in the lung, through the stimulation of the eNOS signaling cascade and the inactivation of the NF-kB pathway.3 Inhaled nitric oxide has also been similarly proposed as an approach to treat COVID-19 because of its effects on the vascular endothelium and potential direct antiviral activity.4 Nitric oxide also functions as an immunomodulator by modifying cytokine release from alveolar macrophages.5 To our knowledge, there is one clinical trial evaluating the effectiveness of GLP-1 agonist (semaglutide) on reducing complications of COVID-19 infection (ClinicalTrials.gov Identifier: NCT04615871) and many ongoing clinical trials evaluating the utility of inhaled nitric oxide to treat COVID-19 patients (ClinicalTrials.gov Identifier: NCT04476992, NCT04383002, NCT04338828, and NCT04397692).
Augmenting and sustaining nitric oxide levels and their effects in humans are challenging. Inhaled nitric oxide is not widely available outside of the intensive care unit and its costs remain prohibitively high. An indirect approach to augmenting nitric oxide levels is dietary supplementation with L-arginine, the substrate for nitric oxide, and a more cost-effective way to increase in vivo nitric oxide levels in patients with COVID-19. L-arginine supplementation can also increase the production of GLP-1 in both mice6 and humans7 and may work in a synergistic manner to reduce Th17-related cytokine production through nitric oxide. Th17 related cytokines are some of the candidates of interest in the cytokine storm and hyperinflammation of COVID-195. Th17 inflammatory responses play a critical role in the pathogenesis of COVID-19 induced pneumonia8 and the number of CCR6+ Th17 cells was found to be extraordinarily high in the peripheral blood of patients with severe COVID-19.9
Findings of a randomized, placebo-controlled trial of L-arginine supplementation in severe asthmatics assigned prospectively to low and high exhaled nitric oxide groups10 were published recently. A predominant number of people in the cohort had obesity (BMI: 33.7 ± 8.6 [mean ± SD]) which allowed for secondary analysis of L-arginine, nitric oxide, and Th17-related cytokines. These factors are of interest in COVID-19. Multiple studies have also started to investigate the relationship between new-onset diabetes and COVID-19. A recent editorial proposed a potential diabetogenic effect of COVID-19, beyond the stress response that is secondary to the clinical illness.11 Two recent epidemiological studies also shown increases in COVID-19 related mortality in patients with diabetes.12, 13 The mechanism remains unclear, but diabetes-related alterations in the angiotensin-converting enzyme 2 (ACE2) receptor may be causative.14 SARS CoV-2 can bind to the ACE2 receptor, expressed in several tissues, including the pancreas,15 and possibly lead to a new onset or worsening of pre-existing diabetes in patients with COVID-19. A recent clinical trial, DARE-19 (ClinicalTrials.gov Identifier: NCT 04350593), proposed the use of Dapagliflozin (a medication used to treat type 2 diabetes) for treating COVID-19, given its anti-inflammatory and insulin sensitivity-improving effects.
L-arginine has been shown to improve the integrity of pancreatic β-cells and islets in the presence of proinflammatory cytokines16 and increase insulin secretion and/or sensitivity in human and animal models.17-19 Activation of the GLP-1 receptor (GLP1R) also stimulates insulin secretion from pancreatic β-cells.20 Interestingly, oral L-arginine supplementation may have acute effects on insulin release mediated through GLP-16 as shown in mice. L-arginine supplementation has also been shown to increase postprandial circulating GLP-1 levels in humans.7 L-arginine supplementation therefore may be beneficial in COVID-19 patients due to its anti-inflammatory effects (directly or indirectly through GLP-1) and improvement of insulin secretion and sensitivity.
Aside from the beneficial effects of L-arginine supplementation, accumulation of asymmetric dimethylarginine (ADMA), a byproduct of protein modification that is closely related to L-arginine, can lead to impaired nitric oxide production.21-23 The arginine/ADMA ratio may represent arginine bioavailability and has been used as a marker for severity in cardiovascular diseases24 and asthma.25 In this study, a posthoc analysis of the previously published L-arginine supplementation in severe asthmatics clinical trial, outline the relationship between dietary L-arginine supplementation, GLP-1, insulin, nitric oxide levels, and Th17-related cytokines.
The hypotheses in the study are (1) L-arginine supplementation would reduce Th17 related cytokines and increase insulin levels, and (2) increased in vivo arginine levels, or arginine/ADMA ratios are associated with lower Th17-related cytokines and increased insulin levels.
2 METHODS
Study participants were drawn from the L-arginine interventional trial at the University of California-Davis (ClinicalTrials.gov Identifier: NCT01841281). Adult patients with severe asthma were included in the study. Briefly, subjects were on either controller treatment with high-dose inhaled corticosteroids or treatment with oral steroids more than 50% of the year. The detailed inclusion/exclusion criteria can be found in our previous publication.10 After informed consent, participants were randomized into the 30-weeks cross-over study trial. Each participant received Treatment A (L-arginine or placebo) for 12 weeks, then a 6-week washout period (not received either L-arginine or placebo), and finally another 12 weeks of Treatment B (e.g., if treatment A is L-arginine then Treatment B is placebo and vice versa). Subjects received placebo or L-arginine dosed orally at 0.05 mg/kg (ideal body weight) twice daily. With the cross-over study design, each subject acted as the subject's control, and a smaller number of participants are required. Each subject had six visits (every 6 weeks) during the study period, during which the subject consented to a research blood draw (not fasting and not at a specific time). Stored frozen plasma was subjected to untargeted metabolomics by HILIC-QExactive HF mass spectrometry and GC-TOF MS performed as previously described. GLP-1, insulin, and cytokine levels were measured at visits 1, 3, 4, and 6 (start and end of each intervention period), and the concentrations of Th17-related cytokines, TNF-α, GLP-1, and insulin were determined using a Bio-Plex Pro Human TH17 Cytokine Assay (Bio-Rad) and Human Diabetes Bioplex Kit (Bio-Rad) according to the manufacturer's protocol.
The analysis examined insulin and cytokine level changes correlated with L-arginine supplementation. GLP-1, insulin, and cytokine level changes between the final visit and the first visit of each intervention period were used as outcomes. For example, cytokine level (Visit 3) minus cytokine level (Visit 1) or cytokine level (Visit 6) minus cytokine level (Visit 4). A generalized linear mixed model was used for analysis, accounting for correlated repeated measurements in our crossover study design. Then, the association between the baseline (Visit 1) arginine-related metabolites and GLP-1, insulin, or cytokines were tested using a multivariate regression model adjusted for age, gender, race, and body mass index (BMI).
3 RESULTS
A cohort of 19 participants that completed a total of six visits, were compliant with the L-arginine supplement, and underwent both metabolomic and cytokine measurements, were included in the final analysis. There were a total of 76 measurements of GLP-1, insulin, and cytokine levels (four measurements for each subject) and 19 baseline metabolomic measurements. The average age of all participants was 54.3 ± 12.1 years, they were female predominant (74%) and with obesity (74%, BMI: 33.7 ± 8.6).
3.1 The treatment effect of L-arginine supplement on cytokine, GLP-1, or insulin concentration
The results showed that 12 weeks of chronic L-arginine supplementation significantly decreased the concentration of IL-21 by 18.99 pg/ml (p = 0.02) and increased the concentration of insulin by 585.76 pg/ml (p = 0.02) after 12 weeks of L-arginine supplement. The L-arginine supplement also decreased other cytokines and increased GLP-1 although did not reach the statistical significance (Table 1). Figure 1 showed boxplots of concentration change of cytokines, GLP-1, and insulin for each phase (placebo or treatment phase). Each dot represented an individual participant.
| Cytokines, GLP-1, insulin | Absolute concentration change after 12 weeks of L-arginine supplement (pg/ml) | p |
|---|---|---|
| IL-17F | −0.69 | 0.17 |
| IFN-γ | −19.24 | 0.18 |
| CCL20 | −11.18 | 0.24 |
| IL-17A | −17.34 | 0.11 |
| IL-22 | −0.70 | 0.15 |
| IL-21 | −18.99 | 0.02 |
| TNF-α | −3.86 | 0.18 |
| GLP-1 | 2.53 | 0.60 |
| Insulin | 585.76 | 0.02 |
- Bold: statistically significant.
- Abbreviation: GLP-1, glucagon-like peptide-1.
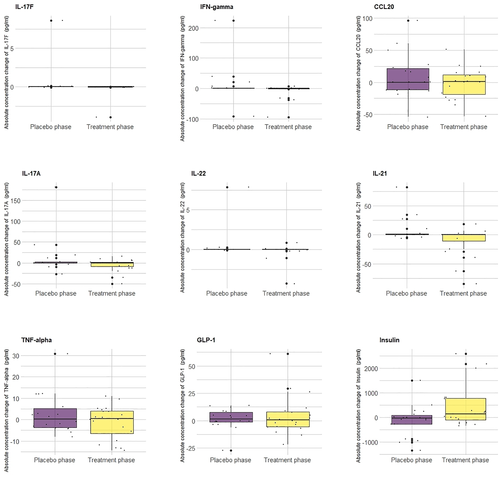
Boxplots of concentration change of cytokines, glucagon-like peptide-1 (GLP-1), and insulin for each phase (placebo or treatment phase). Each dot represented each individuals
3.2 Association between baseline arginine related metabolites levels and cytokine, GLP-1, or insulin level
Table 2 shows the results of the association between the baseline arginine-related metabolites levels and cytokine, insulin, or GLP-1 level. A higher arginine level was associated with lower CCL-20 (p = 0.03) and lower TNF-α (p = 0.03). A higher arginine/ADMA ratio was also found to be associated with lower CCL-20 (p = 0.004) and lower TNF-α (p = 0.03). A higher urea level was associated with higher GLP-1 (p = 0.0008). The arginine/ADMA ratio was also found to trend with lower IL-21 (p = 0.07). The scatter plots that showed the significant associations between arginine and arginine/ADMA ratio and cytokines (CCL-20, TNF-α) with the simple regression line (without covariates adjustment) are shown in Figure 2.
| Cytokines, GLP-1, insulin | Arginine | ADMA | Citrulline | Ornithine | Urea | Arginine/ADMA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inta | p | Int | p | Int | p | Int | p | Int | p | Int | p | |
| IL-17F | −3.6e−09 | 0.40 | 6.5e-07 | 0.76 | −3.5e-08 | 0.32 | −1.6e-06 | 0.47 | 1.9e-07 | 0.51 | 2.4e-05 | 0.96 |
| IFN-γ | −1.8e−06 | 0.18 | 1.0e−03 | 0.11 | −1.1e−05 | 0.30 | −8.5e−04 | 0.22 | 5.4e−05 | 0.55 | −2.6e−01 | 0.10 |
| CCL20 | −2.2e−06 | 0.03 | 6.1e−04 | 0.23 | 5.1e−07 | 0.95 | −2.4e−04 | 0.66 | −9.4e−05 | 0.16 | −3.0e−01 | 0.004 |
| IL-17A | −9.3e−07 | 0.10 | 3.2e−04 | 0.26 | −2.2e−09 | 0.65 | −2.8e−04 | 0.35 | 2.9e−05 | 0.45 | −1.1e−01 | 0.12 |
| IL-22 | −1.1e−08 | 0.43 | 3.5e−06 | 0.61 | −6.9e−08 | 0.53 | −4.9e−06 | 0.48 | 3.0e−07 | 0.74 | −4.2e−04 | 0.80 |
| IL-21 | −1.8e−06 | 0.16 | 8.4e−04 | 0.17 | −2.9e−06 | 0.78 | −6.5e−04 | 0.31 | 3.0e−05 | 0.72 | −2.6e−01 | 0.07 |
| TNF-α | −5.6e−07 | 0.03 | 1.8e−04 | 0.17 | −2.3e−07 | 0.92 | −7.0e−05 | 0.62 | 3.1e−06 | 0.87 | −6.5e−02 | 0.03 |
| GLP-1 | 1.3e−07 | 0.35 | −6.43e−05 | 0.34 | −1.7e−06 | 0.12 | −6.4e−05 | 0.36 | 2.5e−05 | <0.001 | 2.2e−02 | 0.40 |
| Insulin | 8.75e−06 | 0.89 | 3.2e−03 | 0.68 | 4.5e−05 | 0.56 | 4.9e−03 | 0.44 | −2.6e−04 | 0.67 | 4.0e−01 | 0.68 |
- Note: The bold values indicate p <0.05.
- Abbreviations: BMI, body mass index, GLP-1, glucagon-like peptide-1.2
- a Int: Intensity; The level is the intensity, not the absolute concentration.
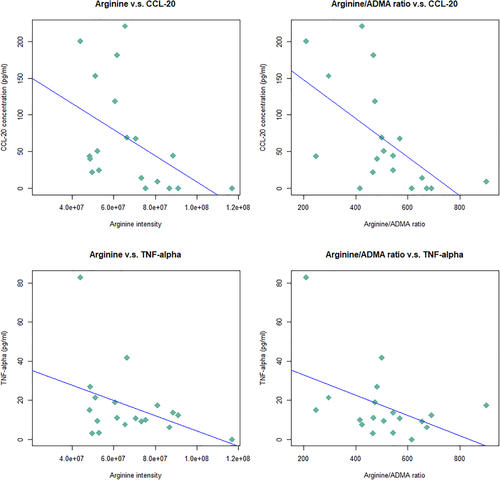
Scatter plots of the associations between baseline arginine and arginine/ADMA ratio and the cytokines (CCL-20, TNF-α) with the simple regression line (without covariates adjustment)
A post hoc power analysis was performed for those statistically significant findings. For the analysis of treatment effects and IL-21 or insulin (statistically significant). The powers were 0.90 and 0.99 respectively using Diggle et al.'s method.26 The power for the correlation between cytokines and metabolites is raged from 0.77 to 0.98 using Cohen et al.'s27 method.
4 DISCUSSION
To the best of our knowledge, this is the first study to elucidate how L-arginine can serve as an immunomodulator and decrease Th17-related cytokines. It is also one of the few studies that demonstrates L-arginine supplementation increases insulin levels in people with asthma and obesity. The overlapping hypotheses derived from the shared biological pathways associated with L-arginine, GLP-1, and nitric oxide make it feasible to consider several approaches to decrease production of Th17-related cytokines. The additional effects of L-arginine on insulin concentration are outlined in Figure 3. L-arginine may suppress Th17-related cytokines through an increase of nitric oxide while synergetically increasing GLP-1, which also results in increased nitric oxide. This relationship may also have a direct anti-inflammatory effect through the NF-kB pathway. Nitric oxide has been shown to suppress the proliferation and function of human Th17 cells.28 By suppressing the COVID-19 responsive cytokine IL-21,5 the B cell differentiation and cytotoxic program of natural killer cells are reduced29 and this may reduce the severity of acute respiratory distress syndrome. Higher arginine levels and arginine/ADMA ratios are both associated with lower CCL-20 and TNF-α. CCL-20 is a ligand for CCR6, a part of the chemotaxis system which is induced in response to SARS-CoV infection,5 and enhances Th17-mediated inflammation through immune cell trafficking.29 Anti-TNF-α therapy has been proposed as another potential therapy due to TNF-α′s action as an amplifier of inflammation and key cytokine of acute inflammation.30 Nitric oxide can directly inhibit TNF-α secretion5 whereas GLP-1 indirectly inhibits TNF-α secretion through increasing nitric oxide levels and inhibition of the NF-kB pathway.31 Arginine supplementation inhibits TNF-α synthesis through the induction of arginase activity,32 supporting our hypothesis. Anti-TNF agents have been proposed as a therapy for COVID-19 since TNF is a major cytokine that causes hyperinflammation. Suppression of TNF may reduce the inflammation-driven capillary leak found in some COVID-19 patients.30
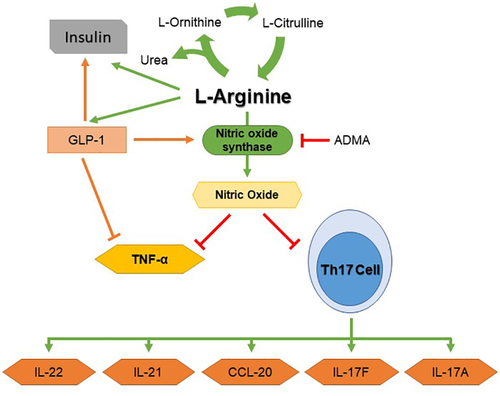
The biological pathway between arginine, glucagon-like peptide-1 (GLP-1), insulin, nitric oxide, and TH17-related cytokines
This study also supported that L-arginine supplementation may increase insulin secretion, observed in a previous rat model.18 The effect of increased insulin levels may be directly linked to L-arginine supplementation or indirectly working through synergetic effects with GLP-1 since GLP-1 can also increase insulin secretion in pancreas β cells.20
There are several limitations worth noting. First, although it is a randomized clinical trial, L-arginine supplementation was implemented over 12 weeks and may not directly be applied to the acute setting without a dosage adjustment. Second, the clinic participants were enrolled before the COVID-19 pandemic and further clinical trials are needed to specifically evaluate the effects of arginine supplementation in patients with COVID-19. Finally, these findings are generated in patients with severe asthma and should be applied with caution for patients without asthma.
This study showed that L-arginine supplementation can reduce the cytokines and improve insulin deficiency or resistance which are two big risk factors for COVID-19 severity and mortality. Given its safety profile and ease of accessibility, L-arginine is an attractive potential therapeutic option that allows for a cost-effective way to improve outcomes in patients. An expedition of further investigation or clinical trials to test these hypotheses is needed.
ACKNOWLEDGMENTS
This work was supported by NIH: 5R01HL105573-03 and TRDRP 28IR-0051.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Shu-Yi Liao, Angela Linderholm, Megan R Showalter, Ching-Hsien Chen, Oliver Fiehn., and Nicholas J. Kenyon wrote the manuscript. Shu-Yi Liao developed the analysis plan and performed the data analysis. Angela Linderholm and Nicholas J. Kenyon designed the study and wrote the grant. Angela Linderholm and Megan R Showalter performed sample handling and metabolomic profiling. Nicholas J. Kenyon and Oliver Fiehn supervised the research.