GLP-1 therapy increases visceral adipose tissue metabolic activity: lessons from a randomized controlled trial in obstructive sleep apnea
Silke Ryan and Donal O'Shea share joint senior authorship.
Abstract
Objective
Glucagon-like peptide-1 (GLP-1) analogues are currently the most widely used pharmacotherapies for weight loss. Their primary mechanism of action is attributed to reduction in energy intake. Data from murine studies also support an additional impact of those agents on energy homeostasis through upregulation of visceral adipose tissue (VAT) metabolic activity, but this remains uncertain in humans.
Methods
Here, we present data from a proof-of-concept study on 30 individuals with obstructive sleep apnea and obesity who were randomized to a GLP-1 therapy-based weight loss regimen, continuous positive airway pressure, or a combination of both for 24 weeks. At baseline and study completion, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT) was performed to evaluate VAT metabolic activity, expressed as VAT target to background ratio.
Results
Treatment with GLP-1, but not with continuous positive airway pressure, was associated with a significant increase in VAT target to background ratio. There was a strong correlation between the increase in VAT metabolic activity and the degree of weight loss.
Conclusions
These data support the hypothesis that upregulation of VAT metabolic activity by GLP-1 contributes to its weight loss action in humans, and this subject warrants further detailed investigation.
Study Importance
What is already known?
- Glucagon-like peptide-1 (GLP-1) analogues are powerful weight loss treatments
- GLP-1 analogues have a major impact on energy intake through delaying gastric emptying and promoting satiety.
What does this study add?
- GLP-1 treatment increases visceral adipose tissue (VAT) metabolic activity in humans.
- Low VAT metabolic activity at baseline is a predictor of good response to treatment with GLP-1.
How might these results change the direction of research or the focus of clinical practice?
- More work on the mechanism of GLP-1 regulation of VAT metabolic activity will inform our understanding of the how these newer gut hormone-based agents impact weight change.
INTRODUCTION
Persistent metabolic adaptation is a barrier to sustained weight loss. Glucagon-like peptide-1 (GLP-1)-based medicines are a class of pharmaceuticals designed to mimic the action of a naturally occurring hormone in the body, which plays an important role in the regulation of satiety and metabolism [(1)]. These medications are delivering significantly better weight loss outcomes than other pharmacotherapies, with recent studies having shown body weight reduction of up to 30% [(2, 3)]. In humans, the primary mechanisms of action of GLP-1 analogues are considered to be the promotion of early satiety and delay of gastric emptying. We have previously demonstrated increased visceral adipose tissue (VAT) thermogenesis and subsequent energy expenditure in mice in response to GLP-1 analogues [(4)], but this potential mechanism remains largely unexplored in humans. Here, we present data derived from a randomized controlled trial in individuals with obesity and obstructive sleep apnea (OSA) that supports a role for increased VAT metabolic activity in mediating the weight effects of GLP-1 [(5)]. The primary aim of this study was to investigate the potential benefit of GLP-1 analogue treatment on metabolic function and early cardiovascular disease in OSA. We have previously shown that VAT inflammation mediated by intermittent hypoxia is a hallmark feature of OSA and plays a key role in the cardiometabolic diseases seen in OSA [(6, 7)]. In this study, we focused specifically on measuring VAT metabolic activity via 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT) imaging. GLP-1 analogue treatment over 24 weeks increased VAT metabolic activity, and there was a correlation with the degree of weight loss. This supports the hypothesis that increased energy expenditure contributes to the weight loss effects of GLP-1 analogue therapy.
METHODS
These are data derived from a recently reported proof-of-concept randomized controlled trial [(5)]. Briefly, 30 individuals without diabetes and with moderate-to-severe OSA (apnea/hypopnea index [AHI] > 15 events/h) and body mass index (BMI) between 30 and 40 kg/m2 were randomized to GLP-1 analogue treatment (liraglutide with incremental increase to 3 mg daily), continuous positive airway pressure (CPAP) therapy, or a combination of both for 24 weeks. The primary aim of the study was to investigate the effect of GLP-1-mediated weight loss on metabolic status and early markers of cardiovascular disease in OSA. At baseline and study completion, 18F-FDG PET-CT scanning was performed on a Siemens Biograph mCT PET/CT system (Siemens Healthineers, Erlangen, Germany). PET image analysis to measure adipose tissue metabolic activity was performed in consensus by two radiology experts who were blinded to all clinical information, using dedicated PET-CT image viewing software (SyngoVia, Siemens Healthineers), based on a previously validated methodology [(8)]. Briefly, three circular regions of interest were placed on the peripheral adipose tissue and VAT at the level of L3 through L4, with adipose tissue maximum standardized uptake values (SUVmax) calculated as the mean of these individual measurements for the peripheral adipose tissue and VAT depots. Target to background ratio (TBR) for each individual was calculated by dividing adipose tissue SUVmax by mediastinal blood pool activity (average of three measurements of blood pool SUVmax made at the superior vena cava on sequential sections). The study was approved by the Ethics Committee of St. Vincent's University Hospital and registered with ClinicalTrials.gov (NCT04186494). Participants provided written informed consent. Comparisons among groups were performed via one-way ANOVA with post hoc Bonferroni analysis or χ2 test for categorical variables. Within-group changes were evaluated by paired t tests. Univariate Pearson correlation analysis was performed to assess for bivariate correlations, and an ANCOVA model was used to assess the relationship between baseline PET data and weight change. Statistical analysis was carried out using IBM SPSS Statistics version 27 (IBM Corp., Armonk, New York).
RESULTS
The characteristics of the study population have been described previously [(5)], and key parameters are summarized in Table 1. As expected for a clinical OSA population, individuals were predominantly male and of middle age, and most had severe OSA. There were no differences in demographic, anthropometric, and sleep study parameters among groups. Mean (SD) CPAP adherence in the CPAP and combination groups was 5.8 (1.4) h per night and 4.7 (1.8) h per night, respectively, and adherence to liraglutide was also good, as reported by the individuals and by monitoring the contents of the prefilled pens. As previously reported and as expected, CPAP alone or in combination resulted in effective treatment of OSA (AHI 3 [3] and 5 [5] events/h, respectively). Liraglutide was also accompanied by significant improvement in AHI, but to a lesser degree than in the other two groups, and remained high (42 [16] events/h).
| Total | Liraglutide (n = 10) | CPAP (n = 11) | Combination (n = 9) | p value | |
|---|---|---|---|---|---|
| Male | 24 (80%) | 8 (80%) | 10 (91%) | 6 (67%) | 0.403 |
| Age (y) | 50 ± 7 | 50 ± 10 | 49 ± 8 | 51 ± 4 | 0.919 |
| BMI (kg/m2) | 34.9 ± 3.2 | 35.1 ± 3.0 | 35.0 ± 3.5 | 34.4 ± 3.2 | 0.878 |
| Waist circumference (cm) | 118 ± 9 | 117 ± 9 | 120 ± 9 | 117 ± 11 | 0.697 |
| HbA1c (mmol/mol) | 38.4 ± 3.2 | 38.3 ± 3.9 | 37.7 ± 2.6 | 39.2 ± 3.2 | 0.606 |
| Fasting glucose (mmol/L) | 5.5 ± 0.5 | 5.4 ± 0.4 | 5.3 ± 0.5 | 5.7 ± 0.6 | 0.177 |
| AHI | 50 ± 19 | 54 ± 21 | 48 ± 20 | 48 ± 17 | 0.757 |
| ODI | 43 ± 18 | 45 ± 22 | 40 ± 17 | 43 ± 17 | 0.79 |
- Note: Data are expressed as mean ± SD or n (%).
- Abbreviations: AHI, apnea/hypopnea index; CPAP, continuous positive airway pressure; HbA1c, glycosylated hemoglobin; ODI, oxygen desaturation index.
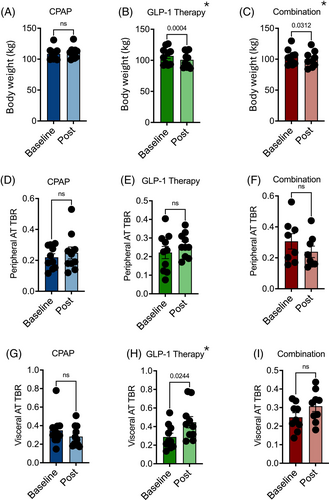
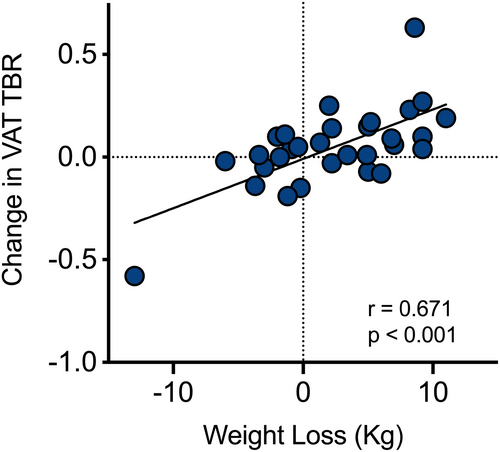
Significant weight loss was achieved with liraglutide alone and in combination with CPAP (6.17 [3.57] and 3.67 [4.22] kg, respectively), but not with CPAP as single modality (Figure 1A–C). Looking specifically at the metabolic activity in different depots of the adipose tissue via 18F-FDG PET-CT, we observed no difference in peripheral adipose tissue TBR with any of the trial interventions (Figure 1D–F). However, VAT metabolic activity significantly increased with GLP-1 analogue treatment. Combination treatment also led to a smaller, but nonsignificant, increase in VAT TBR (p = 0.09), whereas VAT TBR remained unaffected by CPAP (Figure 1G–I). Evaluating the whole cohort, there was a significant correlation between the magnitude of VAT TBR increase and the amount of weight loss (Figure 2). VAT baseline metabolic activity was a significant predictor of subsequent weight loss, independent of the treatment modality (β = −17.6; p = 0.004).
DISCUSSION
Here, we demonstrate that upregulation of metabolic activity within VAT occurs in patients treated with GLP-1 analogues. The fact that the degree of upregulation correlates positively with the degree of weight loss supports a contributory role of this pathway in the weight loss effects of GLP-1. Furthermore, lower baseline levels of VAT metabolic activity were associated with greater weight loss in response to GLP-1 therapy, suggesting that individuals with lower VAT metabolic activity may benefit most from the dual impact of GLP-1 on reduction of energy intake and increased metabolic activity.
Body weight homeostasis is complex, and the body's defense against weight loss is robust. Historically, the focus of weight management has been on the reduction of energy intake via changes in diet; however, this approach is limited in its efficacy for the majority of patients [(9, 10)]. Surgical and pharmacological approaches targeting restriction of energy intake have been far more successful; however, the mechanisms are complex and incompletely understood, and they likely extend beyond reduced energy intake alone [(11)]. Obesity is characterized by metabolically dysfunctional VAT [(12)], and intermittent hypoxia in OSA leads to similar morphologic and functional changes even in the absence of obesity, with adipose tissue macrophage polarization toward an M1-proinflammatory phenotype, which likely plays a key role in the development of insulin resistance in OSA [(6, 7)]. Increasing evidence has pointed to a role for brown adipose tissue in energy expenditure, and we have previously shown, in mice, that maximal weight loss by GLP-1 analogues is dependent on activation of adipose-resident invariant natural killer T (iNKT) cells, with subsequent browning of white fat [(4)]. However, this pathway remains poorly explored in humans. The use of 18F-FDG PET-CT scanning allows further exploration of this and facilitates accurate quantification of metabolic activity in various fat depots [(13, 14)], and an increase in uptake likely reflects browning of adipose tissue [(15)]. To the best of our knowledge, this is the first study demonstrating an increase in VAT metabolic activity with GLP-1 in humans. However, because this was not the primary focus of this study, and the study protocol did not employ a control group with a different weight loss strategy, we cannot conclude for certain that the effect was solely due to GLP-1 action. Furthermore, we cannot automatically extrapolate the results to individuals without OSA. However, because the effect of GLP-1 treatment on OSA severity was substantially less than that with CPAP, it is unlikely that the result is specific to this patient group. Further targeted studies comparing GLP-1 with other weight-loss modalities need to be undertaken to define the relative contribution of this pathway to the overall reduction in body weight. Such studies will improve our understanding of energy homeostasis and move us away from the narrative that the treatment of obesity is eat less, move more.
ACKNOWLEDGMENTS
We are grateful to all of the patients and their families for participating in this study and to all staff members of the Sleep Laboratory at St. Vincent's University Hospital for their help and support. Open access funding provided by IReL.
FUNDING INFORMATION
This study was funded by the Health Research Board of Ireland (Silke Ryan).





