Intensive Weight Loss Intervention and Cancer Risk in Adults with Type 2 Diabetes: Analysis of the Look AHEAD Randomized Clinical Trial
See Commentary, pg. 1575.
Abstract
Objective
This study was designed to determine whether intensive lifestyle intervention (ILI) aimed at weight loss lowers cancer incidence and mortality.
Methods
Data from the Look AHEAD trial were examined to investigate whether participants randomized to ILI designed for weight loss would have reduced overall cancer incidence, obesity-related cancer incidence, and cancer mortality, as compared with the diabetes support and education (DSE) comparison group. This analysis included 4,859 participants without a cancer diagnosis at baseline except for nonmelanoma skin cancer.
Results
After a median follow-up of 11 years, 684 participants (332 in ILI and 352 in DSE) were diagnosed with cancer. The incidence rates of obesity-related cancers were 6.1 and 7.3 per 1,000 person-years in ILI and DSE, respectively, with a hazard ratio (HR) of 0.84 (95% CI: 0.68-1.04). There was no significant difference between the two groups in total cancer incidence (HR, 0.93; 95% CI: 0.80-1.08), incidence of nonobesity-related cancers (HR, 1.02; 95% CI: 0.83-1.27), or total cancer mortality (HR, 0.92; 95% CI: 0.68-1.25).
Conclusions
An ILI aimed at weight loss lowered incidence of obesity-related cancers by 16% in adults with overweight or obesity and type 2 diabetes. The study sample size likely lacked power to determine effect sizes of this magnitude and smaller.
Study Importance
What is already known?
- ► Observational studies show clear associations between obesity and risk of some cancers, but there have been no clinical trials to date that have evaluated the effects of intensive lifestyle intervention for weight loss on the risk of incident cancer or cancer mortality.
What does this study add?
- ► Data from the Look AHEAD Trial indicated an intensive lifestyle intervention aimed at weight loss lowered incidence of obesity-related cancers by 16% in adults with overweight or obesity and type 2 diabetes. The 95% CI did cross 1.0, but that was likely because of the insufficient sample size.
How might these results change the direction of research?
- ► Future trials need to be adequately powered to test weight loss effects on risk of specific obesity-related individual cancers.
Introduction
Obesity is associated with the risk of several types of cancer ((1)). Based on meta-analyses or pooled analyses, relative risks range from 1.2 to 1.5 for overweight and 1.5 to 1.8 for obesity with respect to cancers of the colon ((2, 3)), gastric cardia ((4)), liver ((5)), gallbladder ((6)), pancreas ((7)), and kidney ((8)). The relative risks for esophageal adenocarcinoma and endometrial cancer are even higher, up to more than fourfold in those with BMI of 40 kg/m2 or more ((9, 10)). Many plausible mechanisms link obesity to cancer, including hormonal factors, circulating growth factors, and inflammation ((11)). Although excess weight is linked to increased cancer risk, evidence is limited on whether that excess cancer risk can be reversed through intentional weight loss. In the Nurses’ Health Study cohort, substantial and sustained weight loss over several years was associated with lower postmenopausal breast cancer incidence ((12)). The Women’s Health Initiative observational cohort found that intentional weight loss in postmenopausal women with obesity was associated with a lower risk of obesity-related cancer and most strongly with a lower endometrial cancer risk ((13)). Data from bariatric surgery generally showed reduced cancer risks in women but not in men ((14)). In the nonrandomized Swedish Obese Subjects study, women who underwent bariatric surgery experienced reduced cancer incidence compared with those who did not ((15)). Reduced cancer risks were also observed among female bariatric surgery patients in Utah ((16)) and in the Kaiser Permanente cohort ((17)). Still, there have been no clinical trials to date that have evaluated the effects of intensive lifestyle intervention (ILI) for weight loss on the risk of incident cancer or cancer mortality.
The Look AHEAD (Action for Health in Diabetes) trial was a multicenter randomized controlled trial of a lifestyle intervention designed to induce weight loss to reduce the risk of cardiovascular disease (CVD) among individuals with overweight or obesity and type 2 diabetes. Because patients with diabetes have elevated risk for several types of cancer ((18-20)), the Look AHEAD trial provided a unique opportunity to study cancer outcomes in a high risk population. As one of the prespecified outcomes, we investigated whether participants randomly assigned to ILI had reduced cancer incidence and/or cancer mortality compared with the control group that received diabetes support and education (DSE).
Methods
Study population
Look AHEAD was a randomized controlled trial that recruited 5,145 individuals with overweight or obesity and type 2 diabetes from 16 study centers in the United States. Individuals were recruited from a variety of sources, including informational mailings, open screenings, advertisements, and referrals from health care professionals ((21)). The design and methods of the Look AHEAD trial have been published previously ((22)), as well as the results for its primary CVD outcomes ((23)). The ILI produced greater reductions in weight and glycated hemoglobin as well as greater initial improvements in fitness and most cardiovascular risk factors, but it did not reduce the rate of cardiovascular events. After the main phase of the trial, Look AHEAD-Continuation was funded to support additional data collection, including new measures considered to be of greatest importance to an aging cohort, to continue analysis of data from the intervention phase of Look AHEAD and to conduct closeout and additional analyses from the Look AHEAD-Continuation phase of the trial (see Study Protocol in online Supporting Information).
To be eligible, participants had to meet the following criteria: 45 to 76 years of age, BMI > 25 (> 27 if treated with insulin), glycated hemoglobin < 11% (97mM), blood pressure < 160/100 mm Hg, triglyceride level < 600 mg/dL, and successful completion of a maximal graded exercise test. Patients with cancer requiring treatment in the past 5 years, except for nonmelanoma skin cancers, were not eligible for the trial. Participants were randomly assigned to ILI or DSE. All participants provided informed consent and local institutional review boards approved the protocols. For the analysis of cancer outcomes, 4,859 trial participants who had not reported a diagnosis of cancer at baseline (except for nonmelanoma skin cancer) were included (Figure 1).
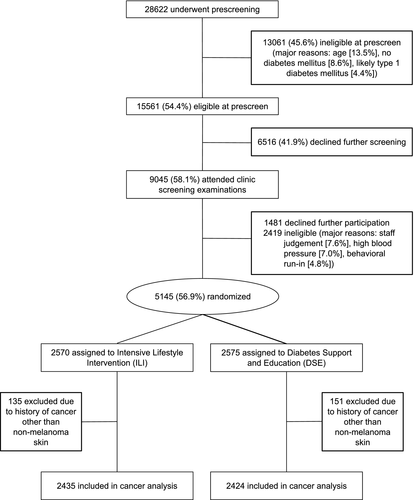
Randomization
Between August 22, 2001, and April 30, 2004, patients were randomly assigned (1:1) to ILI or DSE by a Web-based data management system at the coordinating center at Wake Forest School of Medicine (Winston-Salem, North Carolina). Randomization was stratified by clinical center, with random block sizes. Allocation was concealed to study staff; the assignment was revealed only after the participant was enrolled in the clinical trial. Data were collected by trained, certified staff who were masked to the intervention. Outcomes assessors and laboratory staff were masked to treatment, but participants and interventionists were not masked because the intervention was behavioral ((24)).
Interventions
The ILI was designed to achieve and maintain weight loss of at least 7% by facilitating reduced caloric intake and increased physical activity. The program included both group and individual counseling sessions, occurring weekly during the first 6 months, followed by three sessions per month for the next 6 months and twice-monthly contact and regular refresher group series and campaigns in years 2 to 10. Specific intervention strategies included a calorie goal of 1,200 to 1,800 kcal/d (with < 30% of calories from fat and > 15% from protein), the use of meal-replacement products, and at least 175 minutes of moderate-intensity physical activity per week. For the DSE comparison group, three group sessions per year focused on diet, exercise, and social support were provided during years 1 through 4. In subsequent years, the frequency was reduced to one session annually. Details about the ILI and DSE program have been published elsewhere ((25, 26)). The ILI and DSE programs were led by lifestyle counselors who were registered dietitians, behavioral counselors, or exercise specialists.
Participants in both the ILI and DSE groups were provided routine medical care by their own health care providers. The intervention began at enrollment (2001-2004) and ended in 2012 because of futility on the primary CVD outcome. The study continued after the intervention stopped; this analysis used data collected through January 2015. The mean (range) lengths of intervention for ILI and DSE participants included in the analyses for this manuscript were both 9.8 (8.4-11.1) years.
Outcomes
At annual visits, certified staff members measured weight, height, waist circumference, and blood pressure, as well as assessed medication use and obtained blood samples for analysis at a central laboratory. During annual visits and telephone calls every 6 months, staff members queried participants about all new diagnoses, medical events, and hospitalizations. Cancer incidence was one of the prespecified tertiary outcomes in Look AHEAD-Continuation (see Study Protocol in online Supporting Information). Cancer was listed in the study outcome form; information related to outpatient cancer care visits and cancer hospitalizations was additionally collected. Medical records were reviewed for all self-reported medical events including cancers and cancer deaths, with adjudication according to standard criteria by a central panel of physicians. For mortality, items such as death certificates, hospital records for inpatient deaths, relevant emergency department records, and/or autopsy reports were collected. For deaths occurring as an outpatient (including pronounced dead in emergency department), an informant interview was conducted, and the most recent hospital records prior to the death were reviewed. Staff who obtained and adjudicated all outcome measures were masked to study group assignment.
Cancer incidence was defined as the first reported occurrence of a malignant tumor other than nonmelanoma skin cancer. Before conducting cancer-related analysis, we predefined obesity-related cancers based on the list published by the IARC Working Group in 2016 ((1)) as having sufficient evidence to be associated with high BMI, including cancers of the esophagus, colon, rectum, kidney, pancreas, uterus, ovary, postmenopausal breast, stomach cardia, liver, gallbladder, meningioma, thyroid, and multiple myeloma. Because we did not collect data on a specific region of a tumor, we were not able to differentiate cancers in the gastric cardia from other stomach cancers. Similarly, biopsy specimen reports were not always available; cancer stage and pathology were not ascertained in all cases. We were not able to differentiate esophageal adenocarcinoma from squamous cell carcinoma. There were no meningioma cases in the study.
Statistical analysis
Analysis of the cancer outcomes was conducted on an intention-to-treat basis. Baseline characteristics were compared between the ILI and DSE groups using t tests for continuous variables or χ2 tests for dichotomous variables. Follow-up time, in person-years, was determined for each participant using the difference in date of randomization to date of first cancer diagnosis (or cancer death for mortality analyses). Censoring dates were calculated using date of death from other causes, the last available follow-up, or the end of follow-up for this analysis (January 23, 2015).
Our primary analyses examined the effect of ILI on (1) overall cancer incidence, (2) obesity-related cancer incidence, and (3) overall cancer mortality. Our secondary analysis considered site-specific cancer incidence. We hypothesized that the impact of ILI on cancer incidence might be greater among women based on results from previous studies ((3)); thus, we additionally stratified analyses by gender and tested for a gender interaction.
Cumulative cancer incidence and cumulative cancer mortality were calculated using Kaplan-Meier estimates. Cox proportional hazards models were subsequently used to calculate hazard ratios (HRs), 95% CIs, and two-sided P values for cancer incidence and cancer mortality comparing the ILI group with the DSE group. In order to examine the heterogeneity of any possible effects on cancer incidence and cancer mortality, we conducted post hoc analysis to test treatment-subgroup interactions for race/ethnicity, baseline BMI (< 35 vs. ≥ 35 based on the median), smoking status (ever vs. never), and age (< 58 vs. ≥ 58 based on the median). Proportional hazards assumptions were verified for each model, and results were not adjusted for multiple comparisons. A sensitivity analysis on overall cancer incidence and obesity-related cancer incidence was conducted using the Fine and Gray method. P < 0.05 indicated statistical significance. All analyses were performed using SAS software version 9.4.
Results
Baseline characteristics are shown in Table 1 by intervention assignment. Characteristics of the participants included in this analysis did not differ by randomization assignment, except for small differences in systolic and diastolic blood pressure, glycated hemoglobin, and family history of diabetes. The distribution of variables in this study population was similar to that in the original Look AHEAD cohort ((11)). Baseline characteristics by sex and by study arm are shown in Supporting Information Table S1.
| DSE (n = 2,424) | ILI (n = 2,435) | |
|---|---|---|
| Age (y) | 58.7 ± 6.82 | 58.42 ± 6.76 |
| Female | 1,439 (59.36) | 1,440 (59.14) |
| Race | ||
| African American | 381 (15.72) | 383 (15.74) |
| White | 1,516 (62.54) | 1,522 (62.53) |
| Hispanic | 332 (13.7) | 331 (13.6) |
| Other | 195 (8.04) | 198 (8.13) |
| Education | ||
| <13 years | 493 (20.89) | 483 (20.2) |
| 13-16 years | 913 (38.69) | 891 (37.26) |
| >16 years | 954 (40.42) | 1,017 (42.53) |
| Smoking | ||
| Never | 1,227 (50.77) | 1,218 (50.1) |
| Past | 1,087 (44.97) | 1,101 (45.29) |
| Current | 103 (4.26) | 112 (4.61) |
| Drinking | ||
| None/week | 1,637 (67.76) | 1,651 (68.05) |
| 1-3/week | 460 (19.04) | 479 (19.74) |
| 4+/week | 319 (13.2) | 296 (12.2) |
| Height (ft) | 5.49 ± 0.33 | 5.49 ± 0.32 |
| Weight (lb) | 222.19 ± 41.96 | 221.71 ± 43.35 |
| BMI (kg/m2) | 36 ± 5.77 | 35.93 ± 6.02 |
| Waist circumference (cm) | 114.08 ± 13.69 | 113.86 ± 14.35 |
| SBP (mm Hg) | 129.57 ± 17.08 | 128.1 ± 17.18 |
| DBP (mm Hg) | 70.46 ± 9.55 | 69.9 ± 9.54 |
| HbA1c (%) | 7.32 ± 1.21 | 7.25 ± 1.15 |
| eGFR (mL/min/1.73 m2) a | 93.94 ± 22.22 | 94.63 ± 23.16 |
| Cholesterol (mg/dL) | 190.28 ± 37.05 | 191.07 ± 38.34 |
| Triglycerides (mg/dL) | 152 (107, 217) | 154 (110, 220) |
| Insulin use | 385 (16.5) | 371 (15.75) |
| Statin use | 1,052 (44.5) | 1,080 (45.47) |
| History of CVD | 324 (13.37) | 343 (14.09) |
| Hypertension | 2,006 (82.76) | 2,036 (83.61) |
| Family history of diabetes | 1,631 (67.29) | 1,540 (63.24) |
| Self-reported diabetes duration (y) | 5 (2, 10) | 5 (2, 10) |
- Data given as n (%), mean ± SD, or median (Q1,Q3). No significant differences between the two groups, except for SBP (P < 0.01), DBP (P = 0.04), HbA1c (P = 0.04), and family history of diabetes (P < 0.01).
- a eGFR calculated by Modification of Diet in Renal Disease study equation.
- CVD, cardiovascular disease; DBP, diastolic blood pressure; DSE, diabetes support and education; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; ILI, intensive lifestyle intervention; SBP, systolic blood pressure.
Participants in the ILI group had significantly greater reductions in weight than those in the DSE group (Supporting Information Figure S1). Differences in mean weight loss (expressed in kilograms and percentage) were largest at 1 year (8.73 ± 7.54 kg [8.6% ± 6.8%] in the ILI group vs. 0.75 ± 5.00 kg [0.7% ± 4.8%] in the DSE group) but remained significant throughout the trial. After 12 years of follow-up, the mean weight loss from baseline was 6.84 ± 10.96 kg (6.5% ± 9.9%) in the ILI group and 4.87 ± 12.28 kg (4.6% ± 11.5%) in the DSE group.
During a median follow-up of 11 years, 684 participants developed cancer of any type (332 in the ILI group and 352 in the DSE group; Figure 2A), with corresponding overall cancer incidence rates of 13.2 and 14.2 per 1,000 person-years, respectively (HR, 0.93; 95% CI: 0.80-1.08) (Table 2). The incidence rates of obesity-related cancers were 6.1 and 7.3 per 1,000 person-years in ILI and DSE, respectively (HR, 0.84; 95% CI: 0.68-1.04). There was no difference for cancers not related to obesity (HR, 1.02; 95% CI: 0.83-1.27). The Kaplan-Meier survival curves show that the incidence of obesity-related cancers in the intervention group was lower throughout the follow-up period, but the difference between the two groups did not achieve statistical significance (Figure 2B). The intervention effect on obesity-related cancer incidence did not differ between men and women (P = 0.68 for interaction). The corresponding HRs stratified by sex were 0.78 (95% CI: 0.51-1.19) and 0.86 (95% CI: 0.67-1.10) in men and women, respectively. A total of 80 participants in the ILI group and 85 in the DSE group died of cancer during follow-up (Figure 2C), corresponding to cancer mortality rates of 3.0 and 3.2 per 1,000 person-years, respectively (HR, 0.92; 95% CI: 0.68-1.25) (Table 3).

| Cancer type | DSE | ILI | HR (95% CI) | P | ||
|---|---|---|---|---|---|---|
| Number of events | Ratea | Number of events | Ratea | |||
| All cancers | 352 | 14.2 | 332 | 13.2 | 0.93 (0.80-1.08) | 0.32 |
| Obesity related b | 185 | 7.3 | 158 | 6.1 | 0.84 (0.68-1.04) | 0.10 |
| Nonobesity related | 167 | 6.6 | 174 | 6.7 | 1.02 (0.83-1.27) | 0.83 |
| Men | ||||||
| All cancers | 177 | 18.2 | 165 | 16.6 | 0.91 (0.74-1.12) | 0.38 |
| Obesity related b | 49 | 4.8 | 39 | 3.7 | 0.78 (0.51-1.19) | 0.25 |
| Nonobesity related | 128 | 13.0 | 126 | 12.5 | 0.96 (0.75-1.22) | 0.73 |
| Women | ||||||
| All cancers | 175 | 11.6 | 167 | 11.0 | 0.94 (0.76-1.16) | 0.58 |
| Obesity related b | 136 | 9.0 | 119 | 7.7 | 0.86 (0.67-1.10) | 0.23 |
| Nonobesity related | 39 | 2.5 | 48 | 3.0 | 1.22 (0.80-1.86) | 0.36 |
- a Rate per 1,000 person-years
- b Included esophagus, colon, rectum, kidney, pancreas, stomach, liver, gallbladder, thyroid, and multiple myeloma in men and women and additional uterus, ovary, postmenopausal breast in women.
- DSE, diabetes support and education; HR, hazard ratio; ILI, intensive lifestyle intervention.
| Cancer type | DSE | ILI | HR (95% CI) | P | ||
|---|---|---|---|---|---|---|
| Number of events | Ratea | Number of events | Ratea | |||
| All cancers | 85 | 3.2 | 80 | 3.0 | 0.92 (0.68-1.25) | 0.59 |
| Obesity related b | 45 | 1.7 | 42 | 1.6 | 0.91 (0.60-1.39) | 0.67 |
| Nonobesity related | 40 | 1.5 | 38 | 1.4 | 0.93 (0.60-1.45) | 0.74 |
| Men | ||||||
| All cancers | 50 | 4.8 | 39 | 3.6 | 0.75 (0.50-1.15) | 0.19 |
| Obesity related b | 24 | 2.3 | 17 | 1.6 | 0.69 (0.37-1.28) | 0.24 |
| Nonobesity related | 26 | 2.5 | 22 | 2.1 | 0.82 (0.46-1.44) | 0.48 |
| Women | ||||||
| All cancers | 35 | 2.2 | 41 | 2.6 | 1.15 (0.73-1.81) | 0.54 |
| Obesity related b | 21 | 1.3 | 25 | 1.6 | 1.17 (0.65-2.08) | 0.60 |
| Nonobesity related | 14 | 0.9 | 16 | 1.0 | 1.13 (0.55-2.31) | 0.74 |
- a Rate per 1,000 person-years.
- b Included esophagus, colon, rectum, kidney, pancreas, stomach, liver, gallbladder, thyroid, and multiple myeloma in men and women and additional uterus, ovary, postmenopausal breast in women.
- DSE, diabetes support and education; HR, hazard ratio; ILI, intensive lifestyle intervention.
Post hoc analyses to test treatment-subgroup interaction for race/ethnicity, baseline age, BMI, and smoking status were conducted to examine the heterogeneity of any possible effects on overall cancer incidence, obesity-related cancer incidence, and cancer mortality. None of the interactions were statistically significant. Figure 3 showed results from subgroup analysis of obesity-related cancer incidence.
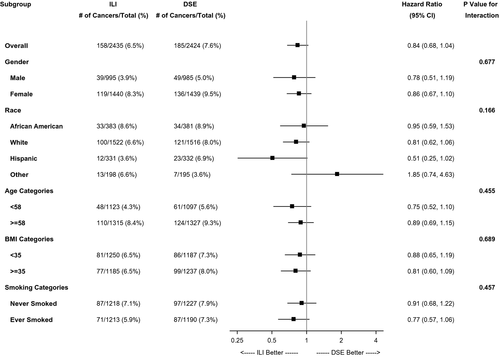
Results from site-specific analyses were shown in the Supporting Information Tables S2-S7. Moreover, in the sensitivity analysis incorporating competing risk from death for overall cancer incidence and obesity-related cancer incidence, the results were almost identical.
Discussion
We compared the cancer incidence and mortality of individuals with overweight or obesity and type 2 diabetes who were randomly assigned to a 10-year ILI designed for weight loss with those assigned to a control regimen of DSE. After a median follow-up of 11 years, the ILI was not associated with significant reduction in overall cancer incidence or cancer mortality, likely because of the insufficient power for either outcome. We found that the ILI lowered incidence of obesity-related cancers (HR, 0.84; 95% CI: 0.58-1.04). The relatively small number of cancer cases likely increased the 95% CIs to include 1. Because we did not collect data on the specific region of a tumor (e.g., cardia of stomach, esophageal adenocarcinoma, wherein associations are very strong with obesity), the misclassification may lead to underestimating the intervention effect.
Obesity is associated with metabolic and endocrine disruptions, which include alterations in sex hormone metabolism, insulin and insulin-like growth factor signaling, and inflammatory pathways ((27-29)). Weight loss decreases intra-abdominal fat and the levels of the endogenous insulins sensitizer adiponectin, which, in turn, improves insulin, pro-inflammatory cytokines, and may lead to lower cell proliferation and a lower likelihood of developing cancers ((30, 31)). Evidence from clinical trials in postmenopausal women with overweight or obesity has demonstrated weight loss through caloric restriction diet with or without exercise resulted in favorable effects on serum sex hormone ((32, 33)), improved insulin resistance ((34)), reduced inflammatory biomarkers ((35, 36)), oxidative stress ((37)), and angiogenesis ((38)). These underlying mechanisms provided molecular and endocrine evidence supporting the hypothesis that weight loss may reduce the risk of obesity-related cancers.
Epidemiological studies have also suggested that intentional weight loss positively affects these mechanisms ((2)) as well as reduces cancer risk. The Nurses’ Health Study, which included a total of 87,143 postmenopausal women followed up with for up to 24 years, found that women who had lost 10 kg or more since menopause and had never used postmenopausal hormones were at a lower risk of breast cancer than those who did not lose weight (RR, 0.43; 95% CI: 0.21-0.86; P = 0.01) ((3)). In our study, women randomized to the weight-loss intervention had a 22% reduced risk of postmenopausal breast cancer, but the association was not statistically significant (HR, 0.78; 95% CI: 0.56-1.09). Compared with the Nurses’ Health Study, our study had far fewer cases and the average amount of weight loss was about 6 kg in the intervention group, compared with ≥ 10 kg observed in the NHS showing reduced cancer risk. The relative moderate weight loss in the ILI arm may not be enough to mitigate cancer risks. Nonetheless, the subset of those who lost ≥ 10 kg in the Nurses’ Health Study in the absence of a study intervention are not comparable with participants in the intervention group of a randomized clinical trial.
The Women's Health Initiative observational study, which included 58,667 postmenopausal women with 12 years of follow-up, found that when compared with women who were weight stable (± 5%), those who had lost ≥ 5% had a significantly lower obesity-related cancer risk (HR, 0.88; 95% CI: 0.80-0.98) ((13)). This magnitude was similar to the effect size observed in our study.
The Swedish Obese Subjects study involved 2,010 patients with obesity (BMI ≥ 34 in men and ≥ 38 in women) who underwent bariatric surgery and 2,037 matched controls, but treatment was not assigned at random, complicating analyses of outcomes among those who did or did not undergo surgery. Over 10 years, compared with the control group, the incidence rate of cancer was 33% lower in the surgery group (HR, 0.67; 95% CI: 0.53-0.85). However, the reduced risk of cancer was seen only in women (HR, 0.58; 95% CI: 0.44-0.77) and not in men (HR, 0.97; 95% CI: 0.62-1.52) ((6)). Cancer risk was reduced in some obesity-related cancer sites including ovary, colorectal, breast, and endometrial, but site-specific numbers were small and the site-specific differences were not statistically significant. Unlike the Swedish Obese Subjects study, our trial did not observe a significant sex-intervention interaction on obesity-related cancer. In a study that included 6,596 Utah patients who had gastric bypass and 9,442 controls who were matched with surgery patients by sex, age, and BMI categories ((7)), obesity-related cancer incidence (HR, 0.62; 95% CI: 0.49-0.78) and cancer mortality (HR, 0.54; 95% CI: 0.32-0.90) were significantly lower in the surgical group compared with controls after a mean of 12.5 years of follow-up. The large reductions may be partly due to unaccounted residual confounders because the surgery group had better education, socioeconomic status, access to care, and health at baseline than the controls. Even though meta-analysis ((5)) showed that patients undergoing bariatric surgery had reduced cancer risk, the number and quality of these studies were insufficient for conclusions. Furthermore, the Look AHEAD participants were older at baseline (average 58 years) than patients included in bariatric surgery studies. Our findings pose the unanswered question of whether intentional weight loss should be provided earlier in life to achieve significant benefit in cancer prevention.
The World Cancer Research Fund has estimated that in the United States, excess adiposity accounts for about 17% of the risk for postmenopausal breast cancer, 15% to 17% for colorectal cancer, 20% to 28% for kidney cancer, and 17% to 20% for pancreatic cancer ((39)). The risk reduction observed in the Look AHEAD trial, though nonsignificant, is of a similar magnitude as would be expected if most of the excess cancer risk imparted by obesity were reversible with moderate amounts of intentional weight loss. Indeed, lifestyle intervention achieved a smaller magnitude of intentional weight loss as compared with bariatric surgery, but the noninvasive, public health approach is meaningful at the population level. In addition, the hypothesis of greater effect of the ILI in prevention of obesity-related cancers as a group than other cancers was observed. Additional cancer outcomes could accrue with a longer follow-up in Look AHEAD participants.
Many studies have supported an association between increased BMI near the time of cancer diagnosis and reduced survival in patients with breast cancer ((40)), whereas evidence for other cancers has been limited. Large-scale, ongoing clinical trials of weight loss in breast cancer survivors, such as the Breast Cancer Weight Loss (BWEL) study ((41)), will shed light on the impact of weight loss on breast cancer recurrence and survival.
Studies have suggested that certain glucose-lowering medications, including metformin, thiazolidinediones, insulin, and incretin-based therapies, are associated with decreased or increased risk of cancer. Data from randomized clinical trials are very scant because of relatively short follow-up times in trial settings. Results from observational studies have been heterogeneous; many studies suffered from biases, particularly time-related biases and confounding by indication ((42)). In addition, most patients with diabetes, like participants in the Look AHEAD trial, were treated with one or more medications, making it difficult to assess independent associations of individual medication. Continuous monitoring of the cancer issues related to antidiabetes medications are still required.
To our knowledge, this study is the only randomized clinical trial that has examined long-term cancer outcomes in an ILI focused on weight loss. Cancer outcomes were prespecified and underwent careful adjudication. Nonetheless, there are several limitations. The Look AHEAD trial was limited to adults with type 2 diabetes and high BMI at baseline. Whether these findings can be generalized to all adults with excess adiposity is not known. Another limitation of the Look AHEAD trial was the insufficient number of many site-specific cancers, which generated site-specific results that must be viewed with caution. We did not have complete information on cancer stage at diagnosis, which limited the opportunity to investigate whether the intervention was associated with a differential stage of cancer. Pooled data from weight-loss trials with long-term follow-up will be useful in assessing the effect of weight loss on cancer risk. To that end, future long-term trials of weight loss should include obesity-related cancers as a prespecified end point for adjudication. Finally, participants in the DSE comparison group also received some intervention and lost weight over the study period, which may have reduced obesity-related cancer risks and thus could explain some of the nonsignificant results.
This study showed that an ILI aimed at weight loss lowered incidence of obesity-related cancers by 16% in adults with overweight or obesity and type 2 diabetes. Although the result was not statistically significant, this finding provided evidence that patients with obesity can reduce their cancer risk through weight loss.
Acknowledgments
Writing group members: Hsin-Chieh Yeh, PhD (Chair), Christos Mantzoros, MD, DSc, Mara Vitolins, DrPH, Rebecca Sedjo, PhD, Lynne Wagenknecht, DrPH, Jeanne M. Clark, MD, MPH, Katelyn Garcia, MS, Antonio Wolff, MD, Edward Horton, MD, George Blackburn, MD, PhD (deceased), Tim Byers, MD, MPH.
A deidentified database will be prepared and submitted to the NIDDK Central Repository. Included will be documentation including protocols, forms, and data dictionaries. Access is guided by NIDDK Central Repository policy.
Funding agencies
This work was supported by the National Institutes of Health (NIH) through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, DK56992). Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the NIDDK. The Indian Health Service (IHS) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the IHS or other funding sources. Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the Harvard Clinical and Translational Science Center (RR025758-04); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (M01RR000056), the Clinical Translational Research Center funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346). The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America. Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Disclosure
JMJ reports personal fees from WW (formerly Weight Watchers International, Inc.), outside the submitted work. All other authors declared no conflicts of interest.
Clinical trial registration
ClinicalTrials.gov identifier NCT00017953.





