Daily Branched-Chain Amino Acid Intake and Risks of Obesity and Insulin Resistance in Children: A Cross-Sectional Study
Abstract
Objective
This study aimed to investigate the association of daily branched-chain amino acid (BCAA) intake with the risks of obesity and insulin resistance in children of mothers with gestational diabetes mellitus (GDM).
Methods
Daily BCAA intake was calculated by using a validated food frequency questionnaire in 996 children of mothers with GDM. The odds ratios (ORs) (95% CI) of childhood obesity and insulin resistance were obtained using logistic regression models.
Results
The multivariable-adjusted ORs for overweight and insulin resistance increased across quartiles of daily BCAA intake (P < 0.05 for trend). Multivariable-adjusted ORs for each 1-SD increase in BCAA intake were 1.37 (1.16-1.62) for overweight and 1.19 (1.02-1.38) for insulin resistance. After additional adjustment of children’s daily total energy intake, the OR was still significant for overweight risk but no longer significant for insulin resistance. There were positive associations of daily leucine, isoleucine, and valine intake with the risks for overweight and insulin resistance.
Conclusions
Daily BCAA intake was associated with increased risks for overweight and insulin resistance in children of mothers with GDM, but this association was not fully independent of children’s daily energy intake. Restriction in dietary BCAA may help prevent childhood obesity and insulin resistance.
Study Importance
What is already known?
- ► Several studies have reported a positive association of branched-chain amino acids via diet or plasma with the risks of obesity and insulin resistance in adults.
- ► It is uncertain whether excessive branched-chain amino acid intake increases the risks of obesity and insulin resistance in children.
- ► Daily intake of branched-chain amino acids was associated with increased risks for overweight and insulin resistance in children of mothers with gestational diabetes mellitus.
What does this study add?
- ► Our data indicated that excessive branched-chain amino acid intake increased the risks for obesity and insulin resistance in children, so restriction in nutrition ingredients such as branched-chain amino acids may be important to prevent childhood obesity and insulin resistance.
Introduction
The branched-chain amino acids (BCAA), including leucine, isoleucine, and valine, are essential amino acids for human beings ((1)). BCAA are comparatively abundant in dietary proteins, constituting up to 15% to 20% of protein intake, and they increase after intake of a meal containing proteins ((1)). A positive association has been found between a BCAA-rich diet and metabolic health, including the regulation of body weight, muscle protein synthesis, and glucose homeostasis ((2, 3)). Moreover, some recent human studies have found that elevated serum BCAA levels were associated with weight gain, insulin resistance, and glucose metabolism abnormality in adults ((4, 5)). Increased serum BCAA levels were also associated with insulin resistance in a nonobese, insulin-resistant, fructose-fed rat model ((6)). A prospective study further demonstrated that serum BCAA levels predicted the future risk for diabetes ((7)). Elevations in the circulating BCAA levels were significantly associated with obesity in children and adolescents, which may independently predict future insulin resistance ((8)).
One meta-analysis reported that oral BCAA supplementation exerted a modest influence on the circulating leucine profile, and the total BCAA intake level was positively associated with the risk for type 2 diabetes ((9)). It also has been reported that reduced dietary intake of BCAA was associated with an improvement of glucose tolerance and body composition ((10, 11)). However, a study in young northern Chinese adults demonstrated that the dietary BCAA ratio was inversely associated with the risks for obesity, postprandial glucose, and status of inflammation ((12)). Nevertheless, it is unclear whether excessive BCAA intake is a risk factor for children’s obesity and insulin resistance. We aimed to examine the association of daily BCAA intake with the risks for overweight and insulin resistance among 996 children of mothers with gestational diabetes mellitus (GDM).
Methods
GDM screening process
Tianjin is the fourth largest city in China, only a 30-minute distance by train from Beijing. There are six central districts in Tianjin with about 4.3 million residents. In 1999, the Tianjin Women’s and Children’s Health Center launched an urban universal screening of GDM using the World Health Organization (WHO)’s criteria in all six central districts. The screening rate was reported to be > 91% between 1999 and 2008 ((13)). We first invited all pregnant women (at 26-30 gestational weeks) to participate in a 1-hour oral glucose tolerance test with a 50-g glucose load in their community health centers. Then those with a glucose reading ≥ 7.8mmol/L were referred to the Tianjin Women’s and Children’s Health Center to undergo a 2-hour oral glucose tolerance test with a 75-g glucose load. If the pregnant women met the 1999 WHO criteria of diabetes (fasting glucose ≥ 7mmol/L or 2-hour glucose ≥ 11.1mmol/L) or impaired glucose tolerance (2-hour glucose ≥ 7.8mmol/L and < 11.1mmol/L), they would be diagnosed as having GDM ((14)).
Study population
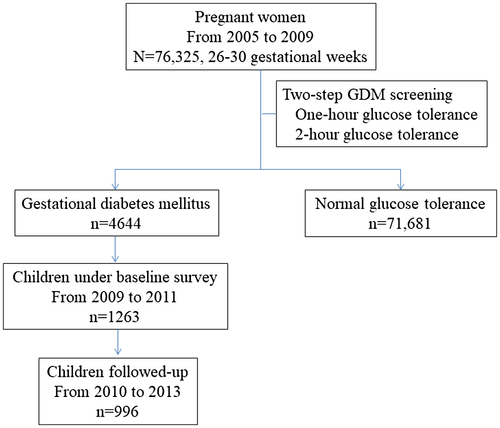
In total, 76,325 women were screened from 2005 to 2009, among whom 4,644 women were diagnosed as having GDM and 71,681 were free of GDM. We invited all 4,644 women with GDM to participate in the Tianjin Gestational Diabetes Mellitus Prevention Program. From August 2009 to July 2011, a total of 1,263 women with GDM finished the baseline survey. A total of 996 children finished the follow-up survey and had complete information on BMI and insulin resistance (Figure 1). The recruitment and inclusion and exclusion criteria have been described in detail elsewhere ((15)). We collected written informed consent from all participants, and this study was approved by the Human Subjects Committee of the Tianjin Women’s and Children’s Health Center.
Questionnaires and measurements
Mothers’ information was collected by a self-administered questionnaire, including sociodemographic characteristics, such as age, marital status, education (< 13 years, 13-15 years, and ≥ 16 years), family income (< 5,000 yuan/month, 5,000-7,999 yuan/month, and ≥ 8,000 yuan/month), and occupation; pregnancy outcomes (prepregnancy weight, weight gain during pregnancy, gestational age, and the number of births in the index pregnancy); and lifestyle in the past year, such as smoking status (nonsmokers, former smokers, and current smokers), passive smoking, alcohol drinking status, sitting time, and leisure-time physical activity (0 min/day, 1-29 min/day, and ≥ 30 min/day). Children’s information was collected by another questionnaire completed by their mothers, including children’s general information, such as gender, birth date, age, birth weight, birth length, and lactation (exclusive formula, mixed, or exclusive breast); history of diseases and medication; and routine activities (indoor and outdoor activities, screen watching time, and sleep duration) ((16)). A validated food frequency questionnaire to measure the children’s frequency and quantity of intake of 35 major food groups and beverages during the past year was collected by the children’s mothers. The food frequency questionnaire asked these children about their frequency of “usual” consumption of 35 food categories, with response categories of never, times/year, times/month, times/week, or times/day, and quantity of average consumption in grams or milliliters per time. The performance of the food frequency questionnaire has been validated in the China National Nutrition and Health Survey in 2002 ((17)). Energy and daily BCAA intake was calculated according to a food ingredient list published in 2002 ((17)). Children were divided into four groups according to BCAA quartiles stratified by sex and age.
All mother-child pairs underwent a physical examination. Using the standardized protocol, all participants’ height and weight were measured in light indoor clothing and without shoes by trained research doctors. BMI was obtained by dividing weight in kilograms by height in meters squared. All mothers’ prepregnancy BMI calculation used their self-reported prepregnancy weight and their measured height. Children’s BMI calculation used their body weight and height examined at the study visit. Children’s BMI-for-age z scores were calculated based on the WHO growth reference ((18, 19)). Children’s BMI was classified as normal weight, BMI < 85th percentile; overweight, 85th percentile ≤ BMI < 95th percentile; and obesity, BMI ≥ 95th percentile, according to the WHO age- and gender-specific growth reference ((18, 19)).
Whole blood specimens were collected from all participants after an overnight fast of at least 8 hours. Plasma glucose was measured using an automatic analyzer (TBA-120FR; Toshiba), and insulin was measured with chemiluminescence using a Siemens ADVIA Centaur CP Immunoassay System. The homeostatic model assessment was used to estimate insulin resistance (HOMA-IR) as previously described ((20)), and insulin resistance was defined as the upper quartile of HOMA-IR.
Statistical analysis
The general characteristics (continuous and categorical variables) of both mothers and children according to quartiles of children’s daily BCAA intake levels were analyzed using the χ2 test or general linear model. Logistic regression models were used to estimate odds ratios (ORs) of childhood overweight and insulin resistance according to children’s daily BCAA intake levels. BCAA were evaluated in the following two ways: (1) as quartiles and (2) as a continuous variable. All analyses were adjusted for maternal age, gestational age, education and smoking status, prepregnancy BMI and gestational weight gain, and children’s sex, age, birth weight, and feeding status (categorical variables) (Model 1); then they were adjusted for the children’s lifestyles, including outdoor physical activity time (continuous variables), screen watching time (continuous variables), and sleep time (categorical variables) (Model 2), as well as children’s daily total energy intake (Model 3). All the statistical analyses were performed with the SPSS Statistics software package version 25.0 for Windows (IBM). Two-sided P < 0.05 was considered statistically significant.
Results
General characteristics of the study population are presented in Table 1. There were differences in sex composition, BMI-for-age z score, outdoor activity time, daily energy intake, energy intake from fat, prevalence of overweight, and insulin resistance among children with different daily BCAA intake levels (all P < 0.05). Moreover, the linear regression analysis indicated that BCAA and components were positively associated with HOMA-IR and BMI z score (all P < 0.05, as shown in Supporting Information Table S1).
| Quartiles of branched-chain amino acids | P value | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| No. of participants | 249 | 249 | 249 | 249 | |
| Maternal characteristics | |||||
| Delivery age, y | 31.3 ± 3.6 | 30.6 ± 3.4 | 31.4 ± 3.5 | 30.7 ± 3.6 | 0.03 |
| Gestational age at delivery, wk | 39.0 ± 1.5 | 39.2 ± 1.3 | 39.1 ± 1.5 | 38.9 ± 1.6 | 0.19 |
| Prepregnancy BMI, kg/m2 | 23.2 ± 3.3 | 22.7 ± 3.2 | 23.2 ± 3.2 | 23.3 ± 3.5 | 0.24 |
| Gestational weight gain, kg | 16.8 ± 6.0 | 16.2 ± 5.5 | 16.9 ± 6.0 | 16.8 ± 6.4 | 0.56 |
| Current smoker, % | 0 | 1.2 | 1.6 | 3.2 | 0.004 |
| Education, % | 0.71 | ||||
| < 13 years | 23.3 | 18.9 | 24.5 | 21.3 | |
| 13-15 years | 69.1 | 75.1 | 67.9 | 69.9 | |
| ≥ 16 years | 7.6 | 6.0 | 7.6 | 8.8 | |
| Child characteristics | |||||
| Boy, % | 47.8 | 50.6 | 50.6 | 64.3 | < 0.001 |
| Age, y | 3.07 ± 1.04 | 3.08 ± 1.08 | 3.01 ± 1.02 | 3.14 ± 1.09 | 0.58 |
| Birth weight, g | 3,540 ± 513 | 3,503 ± 492 | 3,557 ± 552 | 3,571 ± 542 | 0.51 |
| Mode of infant feeding, % | 0.76 | ||||
| Exclusive breastfeeding | 45.4 | 42.6 | 38.6 | 47.0 | |
| Exclusive formula feeding | 39.4 | 48.2 | 45.4 | 37.8 | |
| Mixed feeding | 15.3 | 9.2 | 16.1 | 15.3 | |
| BMI, kg/m2 | 15.4 ± 1.2 | 15.6 ± 1.6 | 15.9 ± 1.5 | 16.2 ± 2.1 | < 0.001 |
| BMI-for-age z score | −0.07 ± 0.91 | 0.03 ± 1.12 | 0.24 ± 1.07 | 0.47 ± 1.33 | < 0.001 |
| Prevalence of overweight, % | 10.0 | 17.3 | 18.1 | 24.5 | < 0.001 |
| Fasting plasma glucose, mmol/L | 4.31 ± 0.40 | 4.33 ± 0.39 | 4.36 ± 0.35 | 4.38 ± 0.39 | 0.22 |
| HOMA-IR a | −0.44 ± 0.35 | −0.42 ± 0.36 | −0.42 ± 0.36 | −0.37 ± 0.38 | 0.14 |
| Insulin resistance, % | 18.9 | 24.5 | 25.3 | 28.5 | 0.015 |
| Outdoor activity, h | 1.69 ± 0.83 | 1.61 ± 0.89 | 1.54 ± 0.88 | 1.73 ± 0.86 | 0.07 |
| Screen watching time, h | 1.30 ± 1.04 | 1.24 ± 1.02 | 1.25 ± 1.05 | 1.39 ± 1.04 | 0.33 |
| Sleeping time, % | 0.58 | ||||
| ≤ 8 h/d | 3.6 | 1.6 | 1.2 | 1.6 | |
| 9-10 h/d | 43.4 | 45.4 | 47.4 | 49.8 | |
| ≥ 11 h/d | 53.0 | 53.0 | 51.4 | 48.6 | |
| Daily nutrition intake | |||||
| Energy, kcal | 686 ± 138 | 836 ± 131 | 953 ± 138 | 1,157 ± 237 | < 0.001 |
| Energy intake from protein, % | 13.8 ± 2.2 | 15.7 ± 2.2 | 16.6 ± 2.2 | 17.9 ± 2.7 | < 0.001 |
| Energy intake from carbohydrate, % | 53.7 ± 8.5 | 52.2 ± 7.3 | 51.6 ± 6.9 | 51.4 ± 7.0 | 0.002 |
| Energy intake from fat, % | 32.5 ± 8.3 | 32.1 ± 7.2 | 31.8 ± 6.6 | 30.6 ± 6.4 | 0.03 |
| Branched-chain amino acids, mg/d | 3,893 ± 702 | 5,428 ± 322 | 6,591 ± 363 | 8,595 ± 1,353 | < 0.001 |
| Isoleucine, mg/d | 1,021 ± 186 | 1,419 ± 86 | 1,718 ± 97 | 2,238 ± 349 | < 0.001 |
| Leucine, mg/d | 1,717 ± 310 | 2,404 ± 152 | 2,931 ± 169 | 3,833 ± 618 | < 0.001 |
| Valine, mg/d | 1,155 ± 211 | 1,605 ± 98 | 1,942 ± 111 | 2,523 ± 391 | < 0.001 |
- a Data were log-transformed.
- BMI-for-age z score and overweight in children were evaluated according to age- and sex-specific growth reference issued by World Health Organization.
- HOMA-IR, homeostasic model assessment for insulin resistance.
As shown in Table 2 and Supporting Information Figure S1, elevated levels of daily BCAA intake (assessed by quartiles) were associated with an increased risk of childhood overweight (P < 0.01 for trend). The multivariable-adjusted (maternal age at delivery, gestational age at delivery, education, and current smoking, as well as children’s age, sex, birth weight, feeding status, outdoor physical activity time, screen watching time, and sleep time; Model 2) ORs of childhood overweight associated with each 1-SD increase in daily intake of BCAA, isoleucine, leucine, and valine were 1.37 (95% CI: 1.16-1.62), 1.38 (95% CI: 1.16-1.63), 1.36 (95% CI: 1.15-1.61), and 1.36 (95% CI: 1.15-1.61), respectively. The association of daily intake of BCAA, isoleucine, and leucine with the risk of childhood overweight attenuated but was still significant after further adjustment for children’s daily energy intake (multivariable-adjusted Model 3).
| No. of participants | No. of cases | Odds ratio (95% CI) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||
| Branched-chain amino acids | |||||
| Quartile 1 | 249 | 25 | 1 | 1 | 1 |
| Quartile 2 | 249 | 43 | 2.11 (1.22-3.65) | 2.19 (1.26-3.82) | 2.07 (1.13-3.82) |
| Quartile 3 | 249 | 45 | 1.97 (1.15-3.39) | 2.06 (1.19-3.57) | 1.74 (0.88-3.43) |
| Quartile 4 | 249 | 61 | 2.76 (1.63-4.67) | 2.81 (1.65-4.78) | 2.21 (1.05-4.64) |
| P value for trend | <0.001 | <0.001 | 0.11 | ||
| One-SD increase | 1.38 (1.17-1.63) | 1.37 (1.16-1.62) | 1.31 (1.01-1.70) | ||
| Isoleucine | |||||
| Quartile 1 | 250 | 24 | 1 | 1 | 1 |
| Quartile 2 | 249 | 49 | 2.57 (1.50-4.43) | 2.63 (1.52-4.56) | 2.38 (1.31-4.34) |
| Quartile 3 | 250 | 43 | 1.85 (1.06-3.22) | 1.89 (1.08-3.32) | 1.51 (0.76-3.00) |
| Quartile 4 | 247 | 58 | 2.93 (1.71-5.00) | 2.90 (1.69-4.99) | 2.08 (0.99-4.40) |
| P value for trend | 0.001 | 0.001 | 0.25 | ||
| One-SD increase | 1.38 (1.17-1.63) | 1.38 (1.16-1.63) | 1.30 (1.01-1.69) | ||
| Leucine | |||||
| Quartile 1 | 249 | 26 | 1 | 1 | 1 |
| Quartile 2 | 249 | 43 | 2.07 (1.20-3.56) | 2.18 (1.26-3.78) | 2.02 (1.10-3.68) |
| Quartile 3 | 250 | 45 | 1.86 (1.09-3.18) | 1.93 (1.12-3.33) | 1.56 (0.80-3.04) |
| Quartile 4 | 248 | 60 | 2.62 (1.55-4.41) | 2.66 (1.57-4.52) | 1.99 (0.96-4.12) |
| P value for trend | 0.001 | 0.001 | 0.18 | ||
| One-SD increase | 1.38 (1.17-1.63) | 1.36 (1.15-1.61) | 1.32 (1.02-1.70) | ||
| Valine | |||||
| Quartile 1 | 249 | 25 | 1 | 1 | 1 |
| Quartile 2 | 249 | 43 | 2.12 (1.23-3.66) | 2.17 (1.25-3.77) | 2.05 (1.11-3.82) |
| Quartile 3 | 249 | 45 | 1.92 (1.11-3.30) | 2.03 (1.17-3.52) | 1.70 (0.85-3.39) |
| Quartile 4 | 249 | 61 | 2.77 (1.63-4.69) | 2.81 (1.65-4.79) | 2.21 (1.04-4.67) |
| P value for trend | 0.001 | <0.001 | 0.12 | ||
| One-SD increase | 1.37 (1.16-1.61) | 1.36 (1.15-1.61) | 1.29 (0.99-1.67) | ||
- Model 1 adjusted for maternal age at delivery, gestational age at delivery, education, current smoking, prepregnancy BMI, and gestational weight gain as well as children’s age, sex, birth weight, and feeding status.
- Model 2 adjusted for variables in Model 1 plus children’s outdoor physical activity time, screen watching time, and sleep time.
- Model 3 adjusted for variables in Model 2 plus children’s daily energy intake.
Multivariable-adjusted ORs of childhood insulin resistance across quartiles of daily intake of total BCAA were 1.00, 1.39, 1.52, and 1.71 (P = 0.018 for trend), respectively (Model 2, Table 3; Supporting Information Figure S2). There were positive associations of daily intake of isoleucine, leucine, and valine with the risk of childhood insulin resistance. Multivariable-adjusted (Model 2) ORs of childhood insulin resistance associated with each 1-SD increase in daily intake of BCAA, isoleucine, leucine, and valine were 1.19 (95% CI: 1.02-1.38), 1.19 (95% CI: 1.03-1.39), 1.19 (95% CI: 1.02-1.38), and 1.19 (95% CI: 1.02-1.39), respectively. The positive associations of daily intake of BCAA, isoleucine, leucine, and valine with the risk of childhood insulin resistance were no longer significant after further adjustment for children’s daily energy intake (multivariable-adjusted Model 3).
| No. of participants | No. of cases | Odds ratio (95% CI) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||
| Branched-chain amino acids | |||||
| Quartile 1 | 249 | 47 | 1 | 1 | 1 |
| Quartile 2 | 249 | 61 | 1.37 (0.88-2.14) | 1.39 (0.89-2.17) | 1.26 (0.77-2.07) |
| Quartile 3 | 249 | 63 | 1.49 (0.96-2.32) | 1.52 (0.97-2.37) | 1.28 (0.74-2.24) |
| Quartile 4 | 249 | 71 | 1.74 (1.12-2.69) | 1.71 (1.10-2.66) | 1.43 (0.76-2.67) |
| P value for trend | 0.014 | 0.018 | 0.31 | ||
| One-SD increase | 1.20 (1.03-1.39) | 1.19 (1.02-1.38) | 1.12 (0.89-1.42) | ||
| Isoleucine | |||||
| Quartile 1 | 250 | 45 | 1 | 1 | 1 |
| Quartile 2 | 249 | 64 | 1.60 (1.03-2.50) | 1.61 (1.03-2.52) | 1.48 (0.9-2.41) |
| Quartile 3 | 250 | 63 | 1.55 (0.99-2.42) | 1.55 (0.99-2.42) | 1.32 (0.76-2.31) |
| Quartile 4 | 247 | 70 | 1.82 (1.17-2.84) | 1.79 (1.15-2.79) | 1.49 (0.79-2.8) |
| P value for trend | 0.014 | 0.019 | 0.33 | ||
| One-SD increase | 1.20 (1.04-1.40) | 1.19 (1.03-1.39) | 1.13 (0.89-1.43) | ||
| Leucine | |||||
| Quartile 1 | 249 | 47 | 1 | 1 | 1 |
| Quartile 2 | 249 | 61 | 1.41 (0.90-2.20) | 1.44 (0.92-2.25) | 1.31 (0.8-2.15) |
| Quartile 3 | 250 | 60 | 1.37 (0.88-2.14) | 1.38 (0.89-2.17) | 1.18 (0.68-2.05) |
| Quartile 4 | 248 | 74 | 1.83 (1.18-2.83) | 1.81 (1.17-2.81) | 1.55 (0.83-2.87) |
| P value for trend | 0.011 | 0.014 | 0.24 | ||
| One-SD increase | 1.20 (1.03-1.39) | 1.19 (1.02-1.38) | 1.12 (0.89-1.41) | ||
| Valine | |||||
| Quartile 1 | 249 | 46 | 1 | 1 | 1 |
| Quartile 2 | 249 | 60 | 1.38 (0.88-2.16) | 1.40 (0.89-2.19) | 1.31 (0.79-2.18) |
| Quartile 3 | 250 | 66 | 1.67 (1.07-2.59) | 1.70 (1.09-2.65) | 1.53 (0.87-2.71) |
| Quartile 4 | 248 | 70 | 1.83 (1.18-2.86) | 1.81 (1.16-2.82) | 1.65 (0.87-3.14) |
| P value for trend | 0.005 | 0.006 | 0.13 | ||
| One-SD increase | 1.20 (1.03-1.39) | 1.19 (1.02-1.39) | 1.12 (0.88-1.43) | ||
- Model 1 adjusted for maternal age at delivery, gestational age at delivery, education, current smoking, prepregnancy BMI, and gestational weight gain as well as children’s age, sex, birth weight, and feeding status.
- Model 2 adjusted for variables in Model 1 plus children’s outdoor physical activity time, screen watching time, and sleep time.
- Model 3 adjusted for variables in Model 2 plus children’s daily energy intake.
Discussion
The present study indicated that daily intake of BCAA, leucine, isoleucine, and valine was associated with increased risks for overweight and insulin resistance among children of mothers with GDM; however, this association was not fully independent of children’s daily energy intake, especially for the risk of childhood insulin resistance.
The prevalence of pediatric obesity has increased rapidly in recent decades worldwide ((21)). Many factors account for the rapid increase in pediatric obesity, including congenital and acquired factors. Maternal prepregnancy overweight and obesity and excess gestational weight gain are risk factors for children’s obesity; other childhood factors such as elevated energy intake, elevated screen time, and reduced outdoor activity also are risk factors for childhood obesity ((22-24)). The present study indicated that daily dietary intake of BCAA was independently associated with increased risks for childhood overweight and insulin resistance after adjustment for these major risk factors. However, the positive association of daily dietary intake of BCAA with the risk for childhood insulin resistance was not independent of children’s daily energy intake.
A low-fat diet or low-carbohydrate diet has been accepted as an effective intervention for metabolic disorders ((25)). It also was reported that a protein-restricted diet could help improve metabolic indexes, including obesity and insulin resistance in humans ((10)). Several recent studies have further indicated that BCAA restriction may largely recapitulate the metabolic effects induced by the restriction of proteins ((10, 26, 27)). Cummings et al. ((28)) pointed out that the restriction of dietary BCAA significantly decreased body weight and adiposity, increased energy expenditure, and improved glucose tolerance and insulin sensitivity in animal experiments. There are very few studies on the effects of dietary BCAA restriction on metabolism in humans ((29)). In the present study, our data indicated dietary BCAA intake was independently associated with childhood obesity and insulin resistance. Large human clinical trials are needed to assess whether dietary BCAA restriction can cause weight loss and improve metabolism among both adults and children and whether this association is independent of daily energy intake.
The mechanisms mediating BCAA and metabolic disorders are complicated. First, BCAA supplementation has been shown to increase the activation of mammalian target of rapamycin and subsequent ribosomal protein S6 kinase phosphorylation, which was coupled with insulin receptor substrate-1 Ser-307 phosphorylation and decreased insulin-induced phosphoinositide 3-kinase activity, resulting in impaired insulin signaling ((30, 31)). Second, the metabolites of BCAA were associated with the risks for obesity and insulin resistance. Researchers have pointed out that 3-hydroxyisobutyrate (3-HIB) dehydrogenase in the muscle tissue of rats decreased 50%, resulting in an elevation of catabolic intermediate of valine 3-HIB ((32, 33)). In animals, 3-HIB is secreted from muscle cells, activates endothelial fatty acid transport, stimulates muscle fatty acid uptake in vivo, and promotes muscle lipid accumulation and insulin resistance ((34)). In human studies, 3-HIB was shown to be related to insulin resistance in individuals with overweight and obesity, and changes in 3-HIB were associated with metabolic improvements with weight loss ((35)). Finally, leucine supplementation led to abnormal catabolism of BCAA, and the incompletely oxidized lipid species contributed to mitochondrial dysfunction in skeletal muscle in high-fat diet–fed rats ((36)). BCAA could also stimulate metabolic stress in islet β cells in animals ((10)). Impaired BCAA catabolism may result in increased circulating levels of BCAA that enhance their pathological effects on obesity and insulin resistance ((6)).
There were some strengths in our study. Our study enrolled a large sample of children of mothers with GDM. Data on a variety of confounding variables, such as the parameters of mothers before and during pregnancy, and indices of the children, including birth weight, lifestyle factors, and anthropometric indexes, were collected and used in the final analysis. There were also some limitations in our study. This was a cross-sectional study, and more prospective studies should be warranted in the future. Moreover, the study samples in the present study were restricted to children of mothers with GDM, and the extrapolation of the conclusion to the whole child population should be scrupulous.
Conclusion
The present study indicated that dietary BCAA intake was associated with increased risks for obesity and insulin resistance in children of mothers with GDM; however, the association of dietary BCAA intake with the risk for insulin resistance was not independent of children’s daily energy intake. Thus, dietary BCAA restriction may prevent these children from developing obesity and insulin resistance, and more clinical trials are needed to further verify this issue.
Acknowledgments
The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
Funding agencies
This study was supported by the European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly program for Collaborative Research between China and Europe. GH was partly supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100790) and the National Institute of General Medical Sciences (U54GM104940) of the National Institutes of Health.
Disclosure
The authors declared no conflict of interest.
Author contributions
HL, LW, Wei Li, Weiqin Li, J Leng, and SZ researched the data. J Lu, YG, and GH wrote the manuscript. LQ and XY reviewed and revised the manuscript. GH is the guarantor of this work, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.





