Will Delaying Breakfast Mitigate the Metabolic Health Benefits of Time-Restricted Eating?
Abstract
Eating out of phase with the biological clock induces circadian misalignment in peripheral organs and impairs glucose tolerance in preclinical models. Time-restricted eating (TRE) is a dietary approach that consolidates energy intake to 6 to 10 hours during the biologically active phase of the day, without necessarily altering diet quality and quantity. TRE induces pleiotropic metabolic benefits in mice, flies, and humans. Most studies have initiated TRE early in the biological morning. This perspective discusses the potential challenges in translating early TRE to the community and considers the potential metabolic consequences of delaying TRE.
Time-restricted eating (TRE) is a novel dietary tool that limits energy intake to a shortened daily window (e.g., 6-10 hours), without necessarily altering diet quality or quantity. TRE may be particularly effective in modern society, which has shifted to a grazing and prolonged daily pattern in eating ((1)). TRE restores circadian rhythmicity in peripheral metabolic organs, improves glucose tolerance, reduces hepatosteatosis, and prevents metabolic dysfunction in animal models of diet-induced obesity and aging ((2)). One study in humans indicated there are improvements in metabolism that occur independently of weight loss ((3)). These studies have led to marked scientific and community interest in TRE as a tool to combat chronic metabolic disease.
In nocturnal rodent models, TRE is typically initiated at the onset of the dark phase for 6 to 12 hours ((2, 4)). In diurnal humans, most studies have also initiated TRE early (eTRE) in the morning. eTRE reduces body weight, improves glucose tolerance, reduces oxidative stress, and improves markers of cardiovascular health in individuals with obesity ((3, 5)). Physiologically, eTRE is most likely to maximize the metabolic health benefits. Glucose tolerance exhibits clear circadian variation, with insulin sensitivity and secretion being much higher in the morning than the evening, even when there are 12-hour equidistant fasts between meals ((6)). The reverse is true for nocturnal rodents ((7)). Epidemiological studies have also linked breakfast skipping with poorer glycemic control and cardiovascular health, although a combination of breakfast skipping with late-night eating may be necessary to significantly elevate risk ((8)). Individuals who choose to eat earlier in the day also lose more body weight on an energy-restricted diet versus individuals who chose to eat later in the day ((9)). A similar result was found in a group that was randomized to receive more calories at breakfast versus dinner, along with greater reductions in fasting glucose after 12 weeks ((10)). These studies all suggest that eTRE will provide optimal body weight and health benefits.
However, the acceptability and long-term feasibility of implementing eTRE in the community is unclear. eTRE may be less feasible because the stereotypical working day is 9 am to 5 pm, and thus many individuals will find it difficult to consume a home-cooked dinner before 6 or 7 pm. There are also other social limitations in following a strict eTRE approach, as many get-togethers with friends and the wider community are often geared toward the evening. Furthermore, the biological drive to eat is also lowest in the morning, when ghrelin is at its circadian nadir ((11)). This means that people are generally less hungry in the morning versus the evening. Although eTRE was recently shown to reduce mean fasting ghrelin and reduce perceived appetite after 4 days ((5)), in our experience, approximately three-quarters of participants expressed a preference for eating later rather than earlier in the day (unpublished data).
The alternative is to advocate for a short phase delay in initiation of TRE. However, the metabolic consequences of delaying TRE (dTRE) are unclear (Figure 1). With this approach, breakfast would be delayed or skipped entirely, which is known to acutely impair daylong glucose control in people with type 2 diabetes ((12)). Severe restriction of the daily eating window, late in the day (between 4 and 8 pm), has also been shown to impair glucose tolerance as compared with the control condition of eating the same energy over breakfast, lunch, and dinner in normal weight individuals after 8 weeks ((13)). Tinsley et al. ((14)) conducted dTRE during any 4-hour window between 4 pm and 12 am, which did not alter body weight or fat mass in resistance-trained athletes. However, when dTRE was performed over a longer time period (scheduled from 1 pm to 8 pm), versus the control condition (eating three meals over a 12-hour period), dTRE significantly reduced body weight, fat mass, leptin, triglyceride, and triiodothyronine and increased adiponectin ((15)). There was no differential effect on fasting glucose or blood lipids.
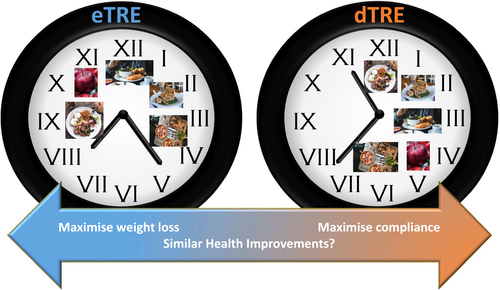
To our knowledge, only two studies have directly compared the effects of eTRE and dTRE. Delahaye et al. ((4)) compared the effects of 6 hours of TRE initiated at lights off (eTRF) or after a 6-hour delay (dTRE) in mice that were fed a high-fat diet for 8 weeks. Both were effective to limit body weight gain, fat gain, and hepatic lipid accumulation versus animals fed ad libitum. However, dTRE animals weighed more and had higher fasting glucose and insulin versus eTRE. Blood samples were collected after a 10-hour fast in the dTRE group and a 16-hour fast in the eTRE group, which could account for this difference. Improvements in glucose tolerance were not different between TRE groups. This result is similar to the only human trial comparing the eTRE and dTRE to date. In that study, eTRE and dTRE were equally effective in improving glucose tolerance as assessed in response to a mixed liquid meal in men with obesity ((16)). Importantly, meal tests were conducted after equidistant fasting lengths in both groups. However, this study was only 1 week in duration. While there was no statistical difference between TRE groups in the improvement in fasting glucose as measured by continuous glucose monitoring, only eTRE significantly reduced overnight fasting glucose levels from baseline. These studies suggest that delaying TRE may mitigate some of the weight benefits, but dTRE may be equally as effective for glucose tolerance.
In summary, TRE is a promising dietary approach that modestly reduces body weight and improves several markers of cardiometabolic health. However, studies in humans have been limited, and large-scale studies examining the long-term outcomes are warranted. While TRE is a practical approach, the majority of studies have implemented TRE early in the day. Future studies should compare eTRE and dTRE to assess social acceptability and long-term compliance as well as the potential metabolic impacts of delaying initiation of TRE.
Disclosure
The authors declare no conflict of interest.





