Intermittent Fasting Does Not Uniformly Impact Genes Involved in Circadian Regulation in Women with Obesity
Abstract
Objective
This study aimed to examine the effects of intermittent fasting (IF) on mRNA levels of peripheral clock genes in skeletal muscle and subcutaneous adipose tissue (SAT) in women with obesity.
Methods
Women were randomized to one of two IF protocols and provided with all foods at 100% or 70% of calculated weekly energy requirements for 8 weeks. Breakfast was consumed before a 24-hour fast, which was initiated on three nonconsecutive days per week. Muscle and SAT biopsies were performed at 8 am after an overnight fast at baseline and at week 8 on a refed day and again following a 24-hour fast at week 8 for analysis of the mRNA levels of key genes involved in circadian regulation.
Results
A group-by-time interaction was observed in Per2 in muscle (F = 3.497, P = 0.044) and SAT (F = 6.686, P = 0.008), but significance was lost upon post hoc adjustment. A time effect was observed in Rorα in muscle, which was decreased by refeeding in both groups (F = 7.225, P = 0.003).
Conclusions
There was no universal effect of IF to alter peripheral clocks, which may be partly because of the alignment of the fasting/feeding cycle with the biological clock. Optimizing intermittent fasting protocols could be important to prevent circadian misalignment in humans.
Study Importance
What is already known?
- ► Behavioral and metabolic processes are regulated via central and peripheral clocks.
- ► Prolonged fasting and overfeeding both dampen peripheral clocks in mouse tissues.
- ► Intermittent fasting reduces the risk factors for diabetes and heart disease in humans.
What does this study add?
- ► Intermittent fasting does not have a universal effect to blunt peripheral clocks in human skeletal muscle and adipose tissue.
- ► Intermittent overfeeding reduces genes involved in circadian regulation in muscle.
How might these results change the focus of clinical practice?
- ► Optimizing intermittent fasting protocols could be important to prevent circadian misalignment in humans.
Introduction
The circadian system controls behavioral and metabolic processes via a central clock located in the suprachiasmatic nucleus, which communicates with peripheral clocks that exist in most tissues, including liver, adipose tissue, muscle, and pancreas ((1-3)). At the molecular level, the circadian clock is generated by a transcriptional-translational feedback loop ((3)). The positive regulators of this loop are Circadian locomotor output cycles kaput (Clock) and Aryl hydrocarbon receptor nuclear translocator-like protein 1 (Arntl). These form a heterodimer to increase the expression of Cryptochrome homolog (Cry1/2) and Period homolog (Per1/2/3) genes. Per and Cry accumulate and translocate to the nucleus where they bind with Clock:Arntl heterodimers and repress their own transcription. Arntl is also positively regulated by Retinoic acid receptor-related orphan receptors (Ror-α/β/γ) and negatively regulated by nuclear receptor subfamily 1 group D member 1/ 2 (Nr1d1/2) nuclear hormone receptors ((3)).
Transcriptional programs of clock-controlled genes oscillate in a tissue-specific manner ((3, 4)) and have been confirmed to be rhythmic in human muscle ((2)) and subcutaneous adipose tissue (SAT) ((1)). Peripheral clocks are exquisitely sensitive to feeding/fasting cycles ((5-7)). During feeding, activation of mammalian target of rapamycin promotes the phosphorylation of glycogen synthase kinase 3, which increases the stability of Per ((8)), lengthening the period that leads to a phase delay ((9)). During fasting, protein kinase AMPK (5' adenosine monophosphate-activated protein kinase) is activated, reducing the stability of Cry1 and Per2 and leading to a phase advance ((9)).
Intermittent fasting (IF) is an eating pattern in which zero or minimal calories are consumed 1 to 4 days per week, followed by ad libitum eating on the remaining days ((10)). IF reduces risk factors for diabetes and heart disease in humans ((11-15)). It is controversial as to whether IF produces greater metabolic health benefits versus calorie restriction (CR) ((15-17)). Some of these controversies may be due to when the fasting protocol is implemented, with studies initiating fasts at breakfast ((15)), lunch ((12)), and dinner ((12-14)). There is only one study that has examined the effects of IF on peripheral clocks ((18)). In this study, IF abolished circadian rhythmicity of Per2, Cry1, and Arntl in mouse liver but only when the fasting/feeding cycle was initiated at the onset of the rest phase.
We recently completed an 8-week IF intervention in which we provided all food at 70% (IF70) or 100% (IF100) of energy requirements in women with obesity ((15, 19)). The exploratory aim of this substudy was to examine the effects of IF on mRNA levels of genes involved in circadian regulation in muscle and SAT. We hypothesized that IF would reduce the expression of genes involved in circadian regulation in muscle and SAT following 24-hour fasting days in both IF groups but that there would be a differential response between IF groups on refeeding days.
Methods
This study was approved by Royal Adelaide Hospital Research Ethics Committee, and participants provided written informed consent. It was registered with ClinicalTrials.gov (NCT01769976).
Participants and study design
The study design has been described previously ((15)). Because we were primarily interested in whether fasting alters clock expression, we limited analysis to IF groups (Supporting Information Figure S1). Briefly, 50 females, aged 35 to 70 years with BMI 25 to 42, were randomized into two IF groups for 8 weeks. Individuals who completed muscle and fat biopsies at baseline and week 8 were included for analysis (n = 37, aged 35-68 years and BMI 25-41) (Supporting Information Figure S1). The IF70 and IF100 groups were provided foods at 70% and 100% of calculated baseline energy requirements per week, respectively. Both groups were instructed to consume a breakfast (containing 30%-35% of energy requirements) before 8 am, initiating a ~24-hour “fast” until 8 am the following day on 3 nonconsecutive days per week. On the 4 refeeding days per week, the IF70 group consumed foods at ~100% of energy requirements, whereas the IF100 consumed ~145% of energy requirements to hit their weekly assigned energy targets.
Metabolic testing and muscle and adipose tissue biopsies
Participants underwent three metabolic visits in this study, including two after a 12-hour fast at baseline (Baseline, V0) and week 8 (Refed, V8A) and one after 24-hour fast at week 8 (Fast, V8B). Premenopausal women (n = 16/37) were studied in the follicular phase to minimize the influence of the menstrual cycle. Individuals were weighed in a hospital gown, and fasting blood samples were drawn. Fasting glucose and insulin were measured, and homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as (fasting serum insulin [milliunits/liter] × fasting plasma glucose [millimoles/liter]) / 22.5 ((20)). Vastus lateralis muscle and abdominal fat tissue were collected at ~8:00 am ((19)). All samples were immediately frozen in liquid nitrogen and stored at −80℃.
Quantitative real-time polymerase chain reaction
RNA extraction, cDNA reverse-transcription protocols, and gene expression analysis (quantitative real-time polymerase chain reaction) have been described elsewhere ((19)). Six clock genes were measured in SAT and skeletal muscle, and Cry1 was below detection limits in SAT. Clock genes evaluated were Arntl (Hs00154147_m1), Clock (Hs00231857_m1), Per2 (Hs01007553_m1), Cry1 (Hs00172734_m1), Nr1d1 (Hs00253876_m1), and Rorα (Hs00536545_m1). As described previously ((19)), the relative expression of each gene was normalized for the mean of B2m (beta 2-Microglobulin) and 18s for SAT and Actb (Beta-actin) and Hprt (Hypoxanthine-guanine phosphoribosyltransferase) for skeletal muscle.
Statistics
Data are presented as mean (SEM). A restricted maximum likelihood mixed-effects model was conducted to examine the group effects of 8-week intervention following an overnight 12-hour fast and 24-hour fast as well as the time effects within each group. The model included fixed factors for group (IF70, IF100), time (Baseline/V0, Refed/V8A, and Fast/V8B), and the group-by-time interaction and a random factor for participant with an unstructured covariance matrix to account for the repeated visits. Within-group comparisons of the effect of time were conducted using Bonferroni-adjusted pairwise comparisons of the estimated marginal means with significance set at P < 0.05 (two-sided). Between-group comparisons of the changes between visits were conducted using unadjusted planned contrasts. Data were log-transformed if the skewness or heteroscedasticity in the residuals were observed. Statistical analysis was performed using SPSS (version 25; IBM Corp., Armonk, New York).
Results
Anthropometric and biochemical results
As previously reported, body weight was significantly decreased by the intervention, with greater reduction in IF70 as compared with IF100 (Table 1). The same trend was also observed in fat mass but did not reach significance in this smaller cohort as was previously reported ((15, 19)). Group-by-time differences were also observed in the change in fasting insulin and HOMA-IR (Table 1). Following refeeding, fasting insulin and HOMA-IR were reduced in IF70 but were transiently elevated above baseline in the IF100 group, likely reflecting the overfeeding prescribed to this group. Following the 24-hour fast, insulin levels were reduced in both IF groups. Fasting glucose levels were reduced from baseline after a 24-hour fast in the IF70 group only (Table 1).
| IF100 (n = 17) | IF70 (n = 20) | Between-group comparison (P) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| V0 (12-h fast) | V8A (12-h fast) | V8B (24-h fast) | V0 (12-h fast) | V8A (12-h fast) | V8B (24-h fast) | Δ (V8A-V0) | Δ (V8B-V0) | Δ (V8A-V8B) | |
| Weight (kg) | 84.4 ± 3.2 | 81.2 ± 3.2* | – | 88.7 ± 3.0 | 83.5 ± 3.2* | – | 0.007 | – | – |
| BMI (kg/m2) | 31.3 ± 1.1 | 30.1 ± 1.1* | – | 32.7 ± 0.9 | 30.8 ± 0.9* | – | 0.012 | – | – |
| Fat (%) | 46.5 ± 1.6 | 44.8 ± 1.8* | – | 49.1 ± 1.5 | 47.0 ± 1.6* | – | 0.358 | – | – |
| FPG (mmol/L) | 4.9 ± 0.1 | 5.0 ± 0.1 | 4.8 ± 0.1 | 4.9 ± 0.1 | 4.7 ± 0.1 | 4.7 ± 0.1* | 0.124 | 0.414 | 0.415 |
| FINS (mU/L) | 18.4 ± 1.8 | 22.9 ± 2.6* | 14.5 ± 1.7*# | 20.1 ± 1.7 | 16.2 ± 1.2* | 14.0 ± 1.3* | < 0.001 | 0.486 | 0.021 |
| HOMA-IR | 4.1 ± 0.5 | 4.7 ± 0.5 | 3.4 ± 0.5# | 4.4 ± 0.4 | 3.4 ± 0.3* | 2.9 ± 0.3* | 0.001 | 0.226 | 0.143 |
- * P < 0.05 difference within each group versus baseline.
- # P < 0.001 difference within each group from V8A to V8B.
- Data shown as mean ± SEM. Mixed-effects model performed to test group differences of 8-week intervention following overnight 12-hour fast (Refed, V8A) and 24-hour fast (Fast, V8B) as well as time effects within each group. P values are bold if significance presented between group comparisons. IF70, intermittent fasting diet at 70% baseline energy requirements; IF100, intermittent fasting diet at 100% baseline energy requirements; V0,baseline; FPG, fasting plasma glucose; FINS, fasting plasma insulin; HOMA-IR, homeostatic model of assessment-insulin resistance.
mRNA levels of circadian genes in muscle and adipose tissue
Group-by-time differences were observed in Per2 in muscle (F = 3.497, P = 0.044; Figure 1C) and adipose tissue (F = 6.686, P = 0.008; Figure 1I), but significance was lost upon post hoc adjustment. A time effect was observed in Rorα in muscle, which was decreased by refeeding in both groups (time, F = 7.225, P = 0.003; Figure 1E). Within-group analysis showed reduced muscle mRNA levels of Per2 (P = 0.021), Rorα (P = 0.034; Figure 1E), and Cry1 (P = 0.022; Figure 1F) following refed days and reduced levels of Clock (V0 vs. V8B, P = 0.019; V8A vs. V8B, P = 0.043; Figure 1B) and Nr1d1 (P = 0.035; Figure 1D) following fast days in the IF100 group. In the IF70 group, Per2 mRNA levels were increased in muscle (P = 0.013; Figure 1C) but reduced in adipose tissue (P = 0.027; Figure 1I) during the fed to fast transition. No other genes were altered by the interventions.
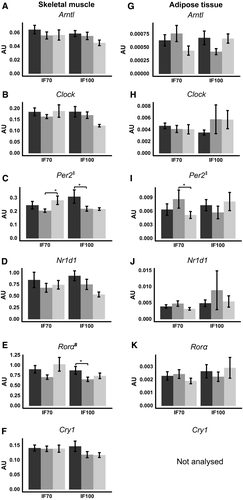
Discussion
Peripheral clocks exhibit circadian rhythmicity in human muscle ((2)) and adipose tissue ((1)) and are exquisitely sensitive to the feeding/fasting cycle ((5-7)). Based on preclinical findings ((4, 18)), we hypothesized that IF would reduce expression of peripheral clocks in human muscle and adipose tissue following a fasting day but that mRNA levels would be restored by refeeding, particularly in the IF70 group, which was under CR. Reassuringly, we observed that 24 hours of fasting did not produce universal changes in genes involved in circadian regulation in human muscle or adipose tissue.
Acute 24-hour fasting completely blunted the rhythmicity of Arntl protein and downstream genes, including Dbp, Nr1d1, Per2, and Per3 in mouse liver and muscle ((4)). This was sustained when the fasting duration was extended to 48 hours but was rapidly reversed by refeeding ((4)). In the present study in humans, 24 hours of fasting reduced Clock and Nr1d1 mRNA levels only in muscle in the IF100 group and Per2 levels only in adipose tissue in the IF70 group. In contrast, Per2 was increased by fasting in muscle in the IF70 group, potentially indicating there may be tissue-specific regulation in humans. We speculate that CR may have mediated the fasting-induced increase in Per2. In mouse liver, CR increased the amplitude in Per1, Per2, Cry2, and Arntl ((21)). To our knowledge, the effects of CR on the phase or amplitude of peripheral clocks have not been reported in human tissue. However, weight loss induced by low-calorie diet increased the mRNA levels of Per2 and Nr1d1 in human adipose tissue samples that were taken at 9 am ((22)). In skeletal muscle, morning Cry1 mRNA levels were increased in women with obesity and decreased to the level of lean controls following weight loss induced by bariatric surgery ((23)).
The effects of IF on peripheral clocks have previously only been tested during a fed day in mice ((18)). IF initiated at the onset of the rest phase dampened the amplitude of expression in Per1, Per2, Cry1, Clock, and Arntl during a refeeding day. Initiation of IF at the onset of active phase did not alter the amplitude of expression but induced a short phase advance in clock genes that was in alignment with an advance in peak food intakes ((18)). This mouse study shows the clear entraining effect of food intake on peripheral clocks and advocates for alignment of initiation of IF with the biological clock. In the present study, we selected to initiate the fasting/feeding protocol at breakfast and observed minimal changes in clock genes in muscle or fat in the IF70 group. This meal timing on fasting days potentially limited the negative consequences of IF on molecular clocks ((18)) and contrasts a number of IF studies in humans that advocate to start/end the fasting periods at lunch or dinner ((12-14)). At the transcriptomic level, delaying the initiation of food intake by 6 hours or “skipping breakfast” resulted in ~500 genes differentially expressed in human adipose tissue ((24)). Genes upregulated with fasting were positively correlated with Per1, suggesting that skipping breakfast delayed the molecular clock in adipose tissue ((24)). Similar results have been reported in other studies of breakfast delay in adipose tissue ((25)) and blood leukocytes ((26)). This difference in meal timing on fasting days could also explain some of the discrepancy in health outcomes between studies of IF ((15-17)), as circadian desynchrony has been linked to increased disease risk and reduced lifespan in animal models ((27)).
The IF100 group was included to test the effects of IF independently of weight loss. This group was therefore overfed on feeding days in order to maintain overall energy balance and experienced minimal weight loss, no improvements in blood lipids, and transient elevations in insulin following refeeding days ((15)). Insulin stimulates Per2 protein accumulation by increasing Per2 mRNA translation ((28)). The net result is to suppress expression of Arntl target genes, including Per2, Cry1, Nr1d1, and Rorα ((3)). Reductions in Per2, Cry1, and Rorα mRNA levels were observed in muscle in response to refeeding in the IF100 group. These results could also have occurred because of a lengthened daily eating cycle. While we aligned the start of feeding/fasting cycle, we neglected to control, or track, the time that individuals completed their prescribed food intakes each day. It is possible that the IF100 group required longer to eat the large volume of food prescribed on this day, contributing to a phase delay or dampening of clock gene expression ((7)).
Because only one time point was sampled, we cannot determine whether there was a dampening or shifting in the phase of these genes limiting interpretation. As we were primarily interested in the effects of fasting, we chose not to include samples from the CR group arm for analysis, meaning we cannot differentiate between the effects of IF and CR. The present study was also limited by small sample size and to females. While premenopausal participants were studied in the follicular phase, the impact of the menstrual cycle on the molecular clock was not taken into consideration ((29)). Finally, glucose profiles were not assessed by continuous glucose monitoring.
The present study is the first to examine the impacts of IF on clock gene expression in human muscle and adipose tissue. Genes involved in circadian regulation were sensitive to intermittent overfeeding in skeletal muscle, but we did not observe a universal effect of fasting across groups or tissues. This may have partly been due to the alignment of the fasting/feeding cycle with the biological clock (i.e., at breakfast). Given the potential for IF to promote day-to-day variation in meal timing, further consideration should be given to the optimal design of IF protocols in humans. These studies should compare the effects of initiating IF after breakfast versus dinner on health and the amplitude and phase of peripheral clocks during both fed and fasting days.
Acknowledgments
Individual deidentified participant data will be made available on request.
Funding agencies
The research was funded by a National Health and Medical Research Council (NHMRC) Project Grant APP1023401. LZ is supported by a Beacon of Enlightenment Scholarship from University of Adelaide.
Disclosure
The authors declared no conflicts of interest.
Author contributions
LKH and GAW designed the research. ATH and BL collected data and analyzed plasma biomarkers. BL and LZ performed experiments. GAW and CHT provided clinical support, supervised clamps, and performed muscle and adipose tissue biopsies. LZ and KL performed statistical analysis. All authors contributed to data interpretation and preparation of the manuscript. LKH had full access to the data and had primary responsibility for the final publication.
Clinical trial registration
ClinicalTrials.gov identifier NCT01769976.





