Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures – 2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists
Abstract
Objective
The development of these updated clinical practice guidelines (CPGs) was commissioned by the American Association of Clinical Endocrinologists (AACE), The Obesity Society (TOS), American Society for Metabolic and Bariatric Surgery (ASMBS), Obesity Medicine Association (OMA), and American Society of Anesthesiologists (ASA) Boards of Directors in adherence with the AACE 2017 protocol for standardized production of CPGs, algorithms, and checklists.
Methods
Each recommendation was evaluated and updated based on new evidence from 2013 to the present and subjective factors provided by experts.
Results
New or updated topics in this CPG include: contextualization in an adiposity-based chronic disease complications-centric model, nuance-based and algorithm/checklist-assisted clinical decision-making about procedure selection, novel bariatric procedures, enhanced recovery after bariatric surgery protocols, and logistical concerns (including cost factors) in the current health care arena. There are 85 numbered recommendations that have updated supporting evidence, of which 61 are revised and 12 are new. Noting that there can be multiple recommendation statements within a single numbered recommendation, there are 31 (13%) Grade A, 42 (17%) Grade B, 72 (29%) Grade C, and 101 (41%) Grade D recommendations. There are 858 citations, of which 81 (9.4%) are evidence level (EL) 1 (highest), 562 (65.5%) are EL 2, 72 (8.4%) are EL 3, and 143 (16.7%) are EL 4 (lowest).
Conclusions
Bariatric procedures remain a safe and effective intervention for higher-risk patients with obesity. Clinical decision-making should be evidence based within the context of a chronic disease. A team approach to perioperative care is mandatory, with special attention to nutritional and metabolic issues.
Abbreviations
-
- A1C
-
- hemoglobin A1C
-
- AACE
-
- American Association of Clinical Endocrinologists
-
- ABCD
-
- adiposity-based chronic disease
-
- ACE
-
- American College of Endocrinology
-
- ADA
-
- American Diabetes Association
-
- AHI
-
- Apnea-Hypopnea Index
-
- ASA
-
- American Society of Anesthesiologists
-
- ASMBS
-
- American Society for Metabolic and Bariatric Surgery
-
- BMI
-
- body mass index
-
- BPD
-
- biliopancreatic diversion
-
- BPD/DS
-
- biliopancreatic diversion with duodenal switch
-
- CI
-
- confidence interval
-
- CPAP
-
- continuous positive airway pressure
-
- CPG
-
- clinical practice guideline
-
- CRP
-
- C-reactive protein
-
- CT
-
- computed tomography
-
- CVD
-
- cardiovascular disease
-
- DBCD
-
- dysglycemia-based chronic disease
-
- DS
-
- duodenal switch
-
- DVT
-
- deep vein thrombosis
-
- DXA
-
- dual-energy x-ray absorptiometry
-
- EFA
-
- essential fatty acid
-
- EL
-
- evidence level
-
- EN
-
- enteral nutrition
-
- ERABS
-
- enhanced recovery after bariatric surgery
-
- FDA
-
- U.S. Food and Drug Administration
-
- G4G
-
- Guidelines for Guidelines
-
- GERD
-
- gastroesophageal reflux disease
-
- GI
-
- gastrointestinal
-
- HCP
-
- health care professional(s)
-
- HTN
-
- hypertension
-
- ICU
-
- intensive care unit
-
- IGB
-
- intragastric balloon(s)
-
- IV
-
- intravenous
-
- LAGB
-
- laparoscopic adjustable gastric band
-
- LAGBP
-
- laparoscopic adjustable gastric banded plication
-
- LGP
-
- laparoscopic greater curvature (gastric) plication
-
- LRYGB
-
- laparoscopic Roux-en-Y gastric bypass
-
- LSG
-
- laparoscopic sleeve gastrectomy
-
- MetS
-
- metabolic syndrome
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NASH
-
- nonalcoholic steatohepatitis
-
- NSAID
-
- nonsteroidal anti-inflammatory drug
-
- OA
-
- osteoarthritis
-
- OAGB
-
- one-anastomosis gastric bypass
-
- OMA
-
- Obesity Medicine Association
-
- OR
-
- odds ratio
-
- ORC
-
- obesity-related complication
-
- OSA
-
- obstructive sleep apnea
-
- PE
-
- pulmonary embolism
-
- PN
-
- parenteral nutrition
-
- PRM
-
- pulmonary recruitment maneuver
-
- RCT
-
- randomized controlled trial
-
- RD
-
- registered dietitian
-
- RYGB
-
- Roux-en-Y gastric bypass
-
- SG
-
- sleeve gastrectomy
-
- SIBO
-
- small intestinal bacterial overgrowth
-
- TOS
-
- The Obesity Society
-
- TSH
-
- thyrotropin
-
- T1D
-
- type 1 diabetes
-
- T2D
-
- type 2 diabetes
-
- VTE
-
- venous thromboembolism
-
- WE
-
- Wernicke encephalopathy
-
- WHO
-
- World Health Organization
LAY ABSTRACT
Obesity is an officially recognized global disease and continues to be a risk factor for chronic medical conditions such as cardiovascular diseases, diabetes, chronic kidney disease, nonalcoholic fatty liver disease, metabolic syndrome, and many cancers. This updated guideline is based on an increased number and quality of the best available scientific studies to guide physicians in the clinical care of patients with obesity who undergo surgical and nonsurgical bariatric procedures. This guideline identifies patient candidates for bariatric procedures, discusses which types of bariatric procedures should be offered, outlines management of patients before procedures, and recommends how to optimize patient care during and after procedures. Since publication of the previous guideline in 2013, the role of bariatric surgery in the treatment of patients with type 2 diabetes has grown substantially. Studies have demonstrated that bariatric/metabolic surgery achieves superior improvements in glycemic control of patients with type 2 diabetes and obesity, compared with various medical and lifestyle interventions, and leads to substantial cost savings. Improved cardiovascular outcomes and quality of life have also been reported in patients undergoing bariatric surgery. New and emerging surgical and nonsurgical bariatric procedures are described. Criteria for bariatric procedures are better defined. This update includes checklists to assist health care professionals achieve greater precision in clinical decision-making and discusses the importance of a team approach to patient care, with special attention on nutrition, metabolism, and interventions to improve recovery after bariatric surgery. Enhanced recovery after bariatric surgery procedures is discussed in detail. Bariatric procedures remain a safe and effective intervention for higher-risk patients with obesity.
| Outline | |
| Introduction | |
| Methods | |
| Executive Summary | |
| Q1. Which patients should be offered bariatric procedures? | (R1-5) |
| Q2. Which bariatric procedure should be offered? | (R6) |
| Q3. How should potential candidates be managed before bariatric procedures? | (R7-12) |
| Q4. What are the elements of medical clearance for bariatric procedures? | (R13-34) |
| Q5. How can care be optimized during and within 5 days of a bariatric procedure? | (R35-48) |
| Q6. How can care be optimized 5 or more days after a bariatric procedure? | (R49-82) |
| Q7. What are the criteria for hospital admission after a bariatric procedure? | (R83-85) |
| Updated Evidence Base for 2019 | |
| References |
Introduction
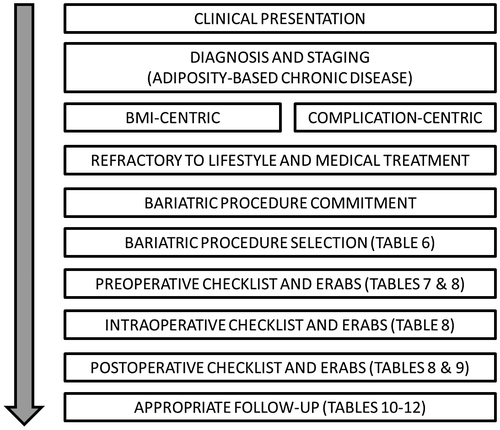
This 2019 clinical practice guideline (CPG) update provides revised clinical management recommendations that incorporate evidence from 2013 to the present, a period marked by a significant increase in the total number of publications on bariatric surgery, especially randomized controlled trials (RCTs), meta-analyses, and reviews (Table 1). In addition, this update requires reinterpretation of the utility and decision-making process within the context of an evolving obesity care model, increasingly detailed management strategies and protocols, and the need for a more transparent tactical plan in a probing and scrutinizing health care environment. New diagnostic terms, structured lifestyle approaches, pharmaceutical options, and surgical and nonsurgical procedures have reshaped the obesity care space. A general overview of the clinical pathway for bariatric surgery is provided in Figure 1. Readers are advised to refer to earlier editions of this CPG for additional supporting evidence, including the basics of bariatric surgery mechanisms of actions, risks, and benefits.
| Years | Non-English (% total) | RCT (% Δ) | Meta-analysis (% Δ) | Review (% Δ) | Guideline (% Δ) | Total (% Δ) |
|---|---|---|---|---|---|---|
| < 2008 | 975 (13) | 204 | 20 | 1,148 | 34 | 7,746 |
| 2008-2012 | 576 (8) | 201 (−0.01) | 46 (130) | 1,210 (5) | 40 (18) | 7,254 (−6) |
| 2013-2018 | 605 (4) | 746 (271) | 218 (374) | 2,396 (98) | 44 (0.1) | 14,105 (94) |
| All years | 2156 (7) | 1,154 | 284 | 4,754 | 118 | 29,105 |
- Abbreviation: RCT = randomized controlled trial.
- a The search term used was “bariatric surgery” on December 31, 2018. Standard PubMed filters were used with customized publication dates. Non-English figures were the difference of unfiltered amounts and the “English” language filter. Non-English percentages use “Total” publications as the denominator. Percentage change (% Δ) uses the figure at the previous publication date range as the denominator. Simple analysis shows that the greatest increase in total, RCT, meta-analyses, and reviews occurred since publication of the last AACE/ASMBS/TOS bariatric surgery clinical practice guideline update in 2013 in bold (1). The number of guidelines and non-English publications on bariatric surgery has remained generally constant over the years.
Update on obesity as a disease and clinical assessment
Since the publication of the 2013 American Association of Clinical Endocrinologists (AACE)/The Obesity Society (TOS)/American Society for Metabolic and Bariatric Surgery (ASMBS) bariatric surgery CPG (1), obesity continues to be a major national and global health challenge, as well as a risk factor for an expanding set of chronic diseases, including cardiovascular disease (CVD), diabetes, chronic kidney disease, nonalcoholic fatty liver disease (NAFLD), metabolic syndrome (MetS), and many cancers, among other comorbid conditions. Obesity is now included among the global noncommunicable disease targets identified by the World Health Organization (WHO) (2-4). In 2015, a total of 107.7 million children and 603.7 million adults had obesity worldwide (5). The prevalence of obesity in the United States is among the highest in the world. According to the National Health and Nutrition Examination Survey 2013-2016 dataset, 38.9% of U.S. adults and 18.5% of youth aged 2 to 19 years had obesity (6, 7). This translates into 93.3 million adults and 13.7 million children and youth, respectively. More women (40.8%) than men (36.5%) had obesity, with non-Hispanic black women (55.9%) showing the highest rates of prevalence (6, 7). Although the prevalence of obesity has been steady among adults since 2011-2012, rates of prevalence in certain subpopulations continue to rise, particularly for those with severe (class III, body mass index [BMI] ≥ 40 kg/m2) obesity where overall age-adjusted rates of prevalence are 5.5% and 9.8% for men and women, respectively, and 16.8% for non-Hispanic women (8).
The global burden of obesity is driven by the association between BMI and increased morbidity and mortality. Although BMI is simplistic (it is only an anthropometric calculation of height-for-weight; or more specifically, weight in kilograms [kg] divided by height in meters squared) and has been criticized as an insensitive marker of disease, it currently provides the most useful population-level measurement of overweight and obesity, and its utility as an estimate of risk has been validated in multiple large population studies across multiple continents. The j-shaped curve for BMI and mortality has recently been confirmed in a large meta-analysis (9) and a systematic review (10) that included 10.6 million and 30 million participants, respectively. These two studies confirm that both overweight and obesity increase the risk of all-cause mortality and should be prioritized on a population level.
Based on the complexity of body-weight regulation, increased morbidity and mortality associated with obesity, and the substantial burden on public health, obesity was officially recognized as a disease by the American Medical Association in 2013 along with multiple other organizations, and most recently by the World Obesity Federation (11). Several guidelines for treatment of obesity have also been published as a resource for clinicians since 2013. Most notable are the American Heart Association/American College of Cardiology/TOS Guideline for the Management of Overweight and Obesity in Adults (12), The AACE and the American College of Endocrinology (ACE) Clinical Practice Guidelines for Comprehensive Care of Patients with Obesity (13), the Obesity Medicine Association (OMA) Obesity Management Algorithm (14), and the Pharmacological Management of Obesity guidelines from the Endocrine Society (15). In 2017, the American Gastroenterological Association (AGA) issued a Practice Guide on Obesity and Weight Management, Education, and Resources (POWER) that emphasized a comprehensive approach to assessment, treatment, and prevention (16). This AGA guideline is particularly important for the increasing number of gastroenterologists who are performing endoscopic procedures for the treatment of obesity that include placement of intragastric balloons (IGB), plications and suturing of the stomach, and insertion of a duodenal-jejunal bypass liner, among other emerging procedures (17).
In addition to these guidelines, efforts are also underway to develop more practical and useful assessments to identify patients who require increased medical attention for obesity-related conditions. Analogous to other staging systems commonly used for congestive heart failure or chronic kidney disease, the AACE/ACE obesity CPG proposes an obesity staging system that is based on ethnic-specific BMI cutoffs along with assessment for adiposity-related complications (13). Stage 0 is assigned to individuals who have overweight or obesity by BMI classification but have no complications, whereas Stage 1 and 2 are defined as individuals with overweight or obesity by BMI classification and have one or more mild-moderate complications (Stage 1) or at least one severe complication (Stage 2). Building off this complications-centric approach to obesity care, AACE/ACE recently proposed a new diagnostic term for obesity using the abbreviation “ABCD,” which stands for adiposity-based chronic disease (18). A different functional staging system for obesity was proposed by Sharma and Kushner (19). Using a risk-stratification construct, referred to as the “Edmonton Obesity Staging System” (EOSS), individuals with obesity are classified into five graded categories, based on their morbidity and health-risk profile along three domains: medical, functional, and behavioral. The staging system was shown to predict increased mortality in two large population cohorts (20, 21). The need to shift from BMI- to complications-centric decision-making has applications beyond the U.S.; for example, in China, acceptance levels for bariatric surgery are principally based on the need for and expectations of weight loss, rather than treatment of severe obesity-related complications (ORC) (22, 23).
Update on nonsurgical therapies
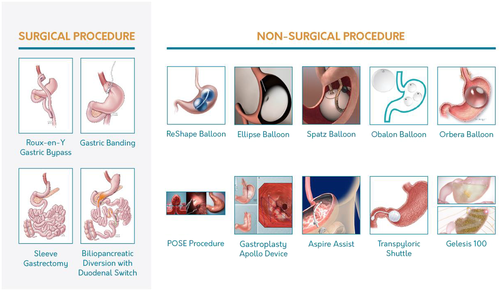
There are many bariatric surgical and nonsurgical procedures that are reimbursed by third-party payers, use U.S. Food and Drug Administration (FDA)-approved devices, or remain available through clinical investigative protocols (Figure 2). Advancements in nonsurgical approaches to obesity include development of endoscopic bariatric therapies and approval of newer antiobesity medications. Various endoscopic bariatric therapies function to reduce gastric volume by one of three techniques: (1) reduce the stomach’s capacity via space-occupying devices, such as IGB, (2) remodel the stomach utilizing endoscopic suturing/plication devices, such as endoscopic sleeve gastroplasty (SG), and (3) divert excess calories away from the stomach, such as aspiration therapy (17). Three IGB have been approved by the FDA since 2015 for patients with a BMI 30 to 40 kg/m2: the ReShape DuoTM (ReShape Medical, San Clemente, CA), the Orbera® IGB (Apollo EndoSurgery, Austin, TX), and the Obalon® Balloon (Obalon Therapeutics, Inc). Although these endoscopically placed devices are associated with short-term (6-month) weight loss, their utility and safety in long-term obesity management remain uncertain (24). The other nonsurgical resources for treatment of obesity are antiobesity medications, which are well defined in guidelines for obesity treatment based on demonstrable weight-loss efficacy and associated metabolic improvements. Four medications have been approved by the FDA since 2012: phentermine/topiramate ER, lorcaserin, naltrexone/bupropion ER, and liraglutide 3.0 mg (25). Antiobesity medications are approved by the FDA for patients with a BMI ≥ 30 kg/m2 without ORCs, or ≥ 27 kg/m2 when associated with at least one ORC. Based primarily on retrospective data and personal experience, these medications are increasingly used in patients who have undergone bariatric surgery but have experienced either insufficient weight loss or frank weight regain.
Update on bariatric surgery
Significant additions to the evidence base have occurred since the publication of the 2013 TOS/ASMBS/AACE bariatric surgery CPG (1). A PubMed computerized literature search (performed between January 1, 2013, and December 31, 2018) using the search term “bariatric surgery” revealed a total of 14,105 citations. Update of this 2019 CPG focuses on the most significant advances and changes in clinical care of the patient who undergoes bariatric surgery. Regarding procedure type, the SG has continued to trend upward, while the Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric band (LAGB) have trended downward. In one large database from 2015, the SG accounted for 63% of procedures performed, compared to 30% and 2% for RYGB and LAGB, respectively (26). The increase in SG is principally due to comparable metabolic and weight-loss outcomes, but with lower complication rates (27) and fewer nutritional deficiencies, compared with RYGB.
One of the most significant advances since the 2013 CPG has been the growing role of bariatric surgery in the treatment of patients with type 2 diabetes (T2D). A substantial body of evidence from 12 RCTs demonstrates that bariatric/metabolic surgery achieves superior improvements in glycemic-control metrics in patients with T2D, compared with various medical and lifestyle interventions. The improvement in glycemic control appears to be due to both weight loss–dependent and –independent effects (28). Based on these data, the Second Diabetes Surgery Summit Consensus Conference published guidelines in 2015 that were endorsed by more than 50 other organizations interested in the treatment of T2D (29). According to these guidelines, metabolic surgery should be considered in patients with T2D and obesity (BMI > 35.0 kg/m2) when hyperglycemia is inadequately controlled with lifestyle and optimal medical therapy. The 2016 Standards of Care for Diabetes from the American Diabetes Association (ADA) includes bariatric surgery in the treatment algorithm for T2D. Warren et al. (30) demonstrated that in a population-based model where an increased number of bariatric surgeries are performed in patients with T2D, there is a substantial cost savings over a 10-year period, roughly $5.4 million per 1,000 patients.
There have also been two cohort studies, six RCTs, and five meta-analyses published since 2013 that report mortality and CVD outcomes, such as myocardial infarction, stroke, CVD risk and events, hypertension (HTN), and dyslipidemia (31-43). Despite heterogeneity in study design, these data favor significantly improved CVD outcomes in patients undergoing bariatric surgery. DiaSurg 2, a randomized controlled multicenter trial comparing RYGB versus medical treatment in German patients with insulin-requiring T2D with BMI 26 to 35 kg/m2, is currently underway (44). The primary end point is composite time-to-event using 8-year data, including CVD mortality, myocardial infarction, coronary bypass, percutaneous coronary intervention, nonfatal stroke, amputation, and surgery for peripheral atherosclerotic artery disease.
The evolving role of bariatric procedures, or more generally speaking gastrointestinal (GI) procedures, to decrease cardiometabolic risk is more clearly envisioned within the nexus of ABCD and a newly proposed model of dysglycemia-based chronic disease (DBCD) (45). In this model, abnormal adiposity intersects with stage-I DBCD as a driver for insulin resistance, T2D, and CVD (45). The recent findings of a large, multicenter, retrospective matched cohort study by Fisher et al. (46) corroborate this concept. They found a lower risk of macrovascular outcomes associated with bariatric surgery in patients with T2D and severe obesity (46). From a pragmatic standpoint, once this ABCD-DBCD model can be scientifically validated, decision-making for the use of GI interventional procedures on cardiometabolic risk reduction will be based on complication risk assessments, rather than just hemoglobin A1C (A1C), BMI, or other simplistic metrics.
Quality of life was reported in two RCTs and improved in the patients undergoing bariatric surgery (33, 34). The impact of bariatric surgery on skeleton and fracture risk has also been recently studied (47-49). Follow-up data from the National Institutes of Health–supported, prospective cohort Longitudinal Assessment of Bariatric Surgery continue to inform clinical care regarding various aspects of postoperative management, including weight-loss trajectories (50), behavioral variables, 3-year weight changes (51), and risks for developing alcohol-use disorder (52). Lastly, postoperative weight regain is recognized as a significant clinical issue that requires focused attention.
The American Board of Obesity Medicine
Based on the increased prevalence and burden of overweight and obesity among U.S. adults and children, a distinct need for more advanced competency in the field of obesity, burgeoning approaches in obesity care expected to continue over the next decade, and complex perioperative care of the patient undergoing bariatric surgery, the American Board of Obesity Medicine (ABOM) was established in 2011 (www.abom.org). Certification as an ABOM diplomate signifies specialized knowledge in the practice of obesity medicine and distinguishes a physician as having achieved competency in obesity care. As of 2018, over 2,600 physicians have become Diplomates, of which over half co-manage patients who have undergone bariatric surgery (53). This team-based approach to bariatric surgery that also includes dietitians, mental health professionals, and advanced practitioners (e.g., nurse practitioner and physician assistant) is important in perioperative management. Thus, the tactical approach to an obesity epidemic that can effectively implement evidence-based strategies, as well as increase exposure of health care professionals (HCP) to patients having bariatric surgery, mandates leadership roles of experts and champions for obesity care, development of formal obesity care teams, and a friendly logistical infrastructure to facilitate favorable outcomes.
Methods
The Boards of Directors for the AACE, TOS, ASMBS, OMA, and American Society of Anesthesiologists (ASA) approved this update of the 2008 (54) and 2013 (1) AACE/TOS/ASMBS Medical Guidelines for Clinical Practice for the Perioperative Nutritional, Metabolic, and Nonsurgical Management of the Bariatric Surgery Patient. Selection of the co-chairs, primary writers, and reviewers, as well as the logistics for creating this 2019 evidence-based CPG update were conducted in strict adherence with the AACE Protocol for Standardized Production of Clinical Practice Guidelines, Algorithms, and Checklists—2017 Update (2017 Guidelines for Guidelines; 2017 G4G) (55) (Tables 2-5). This updated CPG methodology provides for patient-first language (“patient undergoing bariatric procedures” instead of disease-first language: “bariatric patient”) and greater detail for evidence ratings and structure for the involvement of the American College of Endocrinology Scientific Referencing Subcommittee, a dedicated resource for the rating of evidence, mapping of grades, and general oversight of the entire CPG production process. In addition, the term “bariatric procedure” is used to broadly apply to both surgical and nonsurgical procedures. However, when the evidence specifically pertains to surgical procedures, then the term “bariatric surgery” is used. A critical improvement in the 2017 G4G is to create documents that are easier to use and less cumbersome. Nevertheless, as with all white papers and increasing diligence on the part of the writing team and sponsoring professional medical organizations, there remains an element of subjectivity that must be recognized by the reader when interpreting the information.
| Numerical descriptorb | Semantic descriptor | Methodology descriptor |
|---|---|---|
| STRONG EVIDENCE | ||
| 1 (1) | RCT | Randomized controlled trialc |
| 1 (1) | MRCT | Meta-analysis of only randomized controlled trials |
| INTERMEDIATE EVIDENCE | ||
| 2 (2) | MNRCT | Meta-analysis including nonrandomized prospective or case-controlled trials |
| 2 (new) | NMA | Network meta-analysis |
| 2 (2) | NRCT | Nonrandomized controlled trial (or unconfirmed randomization) |
| 2 (2) | PCS | Prospective cohort study (does not include open-label extension study) |
| 2 (2) | RCCS | Retrospective case-control study |
| 2 (new) | NCCS | Nested case-control study |
| 2 (3; reassigned) | CSS | Cross-sectional study |
| 2 (3; reassigned) | ES | Epidemiologic study (hypothesis driven; includes survey, registry, data mining, with or without retrospective uni-multivariate analyses or propensity matching |
| 2 (new) | OLES | Open-label extension study |
| 2 (new) | PHAS | Post hoc analysis study |
| WEAK EVIDENCE | ||
| 3 (new) | DS | Discovery science (explorative/inductive; includes -omics, “big data,” network analysis, systems biology, Bayesian inference, modeling) |
| 3 (new) | ECON | Economic study (includes Markov models, pharmaco-economics) |
| 3 (3) | CCS | Consecutive case series (N > 1) |
| 3 (3) | SCR | Single case report (N = 1) |
| 3 (new) | PRECLIN | Preclinical study (e.g., feasibility, safety) |
| 3 (new) | BR | Basic research (must be high impact and relevant) |
| NO EVIDENCE | ||
| 4 (4) | NE | No evidence (theory, opinion, consensus, review, position, policy, guideline) |
| 4 (new) | O | Other (e.g., lower impact/relevant basic research; any highly flawed study) |
- Abbreviations: AACE = American Association of Clinical Endocrinologists; G4GAC = Guidelines for Guidelines, Algorithms, and Checklists.
- a Based on principle that interventions, scientific control, generalizability, methodological flaws, and evidentiary details determine strength, consistent with other evidence-based methodology systems. Numerical and semantic descriptors of evidence levels provided in online supplementary material from (55).
- b The original numerical descriptions from G4GAC 2004, 2010, and 2014 are provided in parentheses.
- c The superiority of RCT over all other studies, and in particular MRCT, is discussed in references elsewhere.
- Reprinted with permission from Mechanick et al. Endocr Pract. 2017;23:1006-1021 (55).
| Study designa | Data analysisb | Interpretation of results |
|---|---|---|
| Allocation concealment (randomization) |
Intent-to-treat Modeling (e.g., Markov) Network analysis Statistics Appropriate follow-up Appropriate trial termination |
Generalizability Incompleteness Logical Overstated Validity |
| Blindingc | ||
| Comparator group | ||
| End points (real clinical vs. surrogate) | ||
| Hypothesis | ||
| Power analysis (too small sample size) | ||
| Premise | ||
| Type 1 error (e.g., adjusted for PHAS) |
- Abbreviations: AACE = American Association of Clinical Endocrinologists; G4GAC = Guidelines for Guidelines, Algorithms, and Checklists; PHAS = post hoc analysis study.
- a These subjective factors pertain to an individual citation. Subjective factors are provided in online supplementary material from (55).
- b Are these elements appropriate for the given study?
- c Including patients, clinicians, data collectors, adjudicators of outcome, and data analysts.
- Reprinted with permission from Mechanick et al. Endocr Pract. 2017;23:1006-1021 (55).
| Cascades (are there other recommendation versions based on ethnocultural factors?) |
| Dissenting opinions (based on HCP and patient preferences) |
| Economic (e.g., cost-effectiveness, cost-benefit, value) |
| Evidence base (are there significant gaps or is there overwhelming evidence?) |
| Relevance (patient-oriented evidence that matters vs. disease-oriented evidence; social acceptability) |
| Resource availability (limited or sufficient) |
| Risk to benefit |
- Abbreviations: AACE = American Association of Clinical Endocrinologists; G4GAC = Guidelines for Guidelines, Algorithms, and Checklists; HCP = health care professional(s).
- Each of these elements pertains to the recommendation statement with the evidence considered in aggregate. The element may be positive or negative and therefore modify a final recommendation grade. Recommendation qualifiers are provided in online supplementary material from (55).
- Reprinted with permission from Mechanick et al. Endocr Pract. 2017;23:1006-1021 (55).
| BEL | Predominantly negative SF and/or RQ | Predominantly positive SF and/or RQ | Consensus for recommendation and for grade | EL to grade mapping | Map to final recommendation grade |
|---|---|---|---|---|---|
| 1 | No | No | > 66% | Direct | 1 → A |
| Any b | No | No | 100% | Rule | Any → A (new) |
| 2 | No | Yes | > 66% | Adjust up | 2 → A |
| 2 | No | No | > 66% | Direct | 2 → B |
| 1 | Yes | No | > 66% | Adjust down | 1 → B |
| 3 | No | Yes | > 66% | Adjust up | 3 → B |
| 3 | No | No | > 66% | Direct | 3 → C |
| 2 | Yes | No | > 66% | Adjust down | 2 → C |
| 4 | No | Yes | > 66% | Adjust up | 4 → C |
| 4 | No | No | > 66% | Direct | 4 → D |
| 3 | Yes | No | > 66% | Adjust down | 3 → D |
| Any b | Yes/no | Yes/no | > 66% | Rule | Any → AD (new) |
- Abbreviations: AACE = American Association of Clinical Endocrinologists; BEL = best evidence level; EL = evidence level; G4GAC = Guidelines for Guidelines, Algorithms, and Checklists; RQ = recommendation qualifiers; SF = subjective factors.
- a Recommendation Grade A, “Very Strong”; B, “Strong”; C, “Not Strong”; D, “Primarily Based on Expert Opinion.” Mappings are provided in online supplementary material from (55).
- b Rule-based adjustment wherein any recommendation can be a “Very Strong” Grade A if there is 100% consensus to use this designation. Similarly, if > 66% consensus is not reached, even with some degree of scientific substantiation, a “Primarily Based on Expert Opinion” Grade D designation is assigned. The reasons for downgrading to D may be an inconclusive or inconsistent evidence base or simply failure of the expert writing committee to sufficiently agree. Note that any formulated recommendation is omitted from the document if sufficiently flawed, so any Grade D recommendation in the final document must be deemed sufficiently important. Rule-based adjustments are provided in online supplementary material from (55).
- Reprinted with permission from Mechanick et al. Endocr Pract. 2017;23:1006-1021 (55).
Key Updates are provided to highlight the most important new recommendations in this CPG. The Executive Summary is reorganized into seven clinical questions and provides updated recommendation numbers (R1, R2, R3, … R85) in their entirety followed by the respective publication year of the creation or last update in parentheses and an indication of updated explanations and/or references by an asterisk. In many cases, recommendations have been condensed for clarity and brevity. In other cases, recommendations have been expanded for more clarity to assist with complex and/or nuanced-based decision-making. The relevant evidence base, supporting tables, and figures for the updated recommendations follow the Executive Summary in an Appendix. The reader is encouraged to refer to the 2008 (54) and 2013 (1) AACE/TOS/ASMBS CPG for background material not covered in this 2019 update.
Key updates for 2019
- Technical: there is an increased amount and quality of recent evidence to guide clinical decision-making; the analysis of evidence is based on the updated 2017 G4G; there are now five sponsoring professional medical societies that provide a greater fund of expert knowledge and higher level of diligence in the iterative review process.
- Disease Context: the role for surgical and nonsurgical bariatric procedures has been reexamined in a complications-centric framework of ABCD and DBCD, providing the potential for greater precision for clinical decision-making based on biological correlates, clinical relevance, cardiometabolic risk assessment, and ethnicity-related differences in anthropometrics.
- Procedure Selection: new and emergent surgical and nonsurgical bariatric procedures are introduced and described, nuanced criteria for bariatric procedures are better defined, and an algorithm with supporting tables and checklists are provided to assist the reader with decision-making.
- Perioperative Protocols: proactive interventions to improve postoperative outcomes with an emphasis on perioperative enhanced recovery after bariatric surgery (ERABS) clinical pathways are presented and elaborated.
Executive Summary
There are 85 numbered recommendations in this 2019 update, compared with 74 updated recommendations in 2013 and 164 original recommendations in 2008. There are 12 new recommendations in this 2019 update (14%), and among the others, 61 were revised (72%). Unanimous consensus among primary writers was obtained for each of the recommendations. Updated recommendation numbers are indicated by the most recent update year, updated evidence by an asterisk after the year, and new recommendations by “NEW.” The semantic descriptors of “must,” “should,” and “may” generally, but not strictly, correlate (or map) with Grade A (strong), Grade B (intermediate), and Grade C (weak) recommendations, respectively; each semantic descriptor can be used with Grade D (no conclusive evidence and/or expert opinion) recommendations. Deviations from this mapping are not unusual and take into consideration further decision-making requirements, logistics, and subjective factors. Bariatric procedures include both surgical and nonsurgical procedures; the latter are generally performed endoscopically. Recommendations are oriented to the procedure type based on the respective evidence base and expert opinion.
Q1. Which patients should be offered bariatric procedures?
R1. (2019*). Patients with a BMI ≥ 40 kg/m2 without co-existing medical problems and for whom bariatric procedures would not be associated with excessive risk are eligible for a bariatric procedure (Grade A; BEL 1).
R2. (2019*). Patients with a BMI ≥ 35 kg/m2 and one or more severe obesity-related complications (ORCs) remediable by weight loss, including type 2 diabetes (T2D), high risk for T2D (insulin resistance, prediabetes, and/or metabolic syndrome [MetS]), poorly controlled HTN, NAFLD/nonalcoholic steatohepatitis (NASH), obstructive sleep apnea (OSA), osteoarthritis (OA) of the knee or hip, and urinary stress incontinence, should be considered for a bariatric procedure (Grade C; BEL 3). Patients with the following comorbidities and BMI ≥ 35 kg/m2 may also be considered for a bariatric procedure, though the strength of evidence is more variable: obesity-hypoventilation syndrome and Pickwickian syndrome after a careful evaluation of operative risk; idiopathic intracranial HTN; gastroesophageal reflux disease (GERD); severe venous stasis disease; impaired mobility due to obesity; and considerably impaired quality of life (Grade C; BEL 3).
R3. (2019*). Patients with BMI 30 to 34.9 kg/m2 and T2D with inadequate glycemic control despite optimal lifestyle and medical therapy should be considered for a bariatric procedure; current evidence is insufficient to support recommending a bariatric procedure in the absence of obesity (Grade B; BEL 2).
R4. (NEW). The BMI criterion for bariatric procedures should be adjusted for ethnicity (e.g., 18.5 to 22.9 kg/m2 is normal range, 23 to 24.9 kg/m2 overweight, and ≥ 25 kg/m2 obesity for Asians) (Grade D).
R5. (2019*). Bariatric procedures should be considered to achieve optimal outcomes regarding health and quality of life when the amount of weight loss needed to prevent or treat clinically significant ORCs cannot be obtained using only structured lifestyle change with medical therapy (Grade B; BEL 2).
Q2. Which bariatric procedure should be offered?
R6. (2019*). Selecting a bariatric procedure should be based on individualized goals of therapy (e.g., weight-loss target and/or improvements in specific ORCs), available local-regional expertise (obesity specialists, bariatric surgeon, and institution), patient preferences, personalized risk stratification that prioritizes safety, and other nuances as they become apparent (Tables 6-8) (Grade C; BEL 3). Notwithstanding technical surgical reasons, laparoscopic bariatric procedures should be preferred over open bariatric procedures due to lower early postoperative morbidity and mortality (Grade B; BEL 2). LAGB, laparoscopic sleeve gastrectomy (LSG), laparoscopic Roux-en-Y gastric bypass (LRYGB), and laparoscopic biliopancreatic diversion without/with duodenal switch (BPD/DS), or related procedures should be considered as primary bariatric and metabolic procedures performed in patients requiring weight loss and/or amelioration of ORCs (Grade A; BEL 1). Physicians must exercise caution when recommending BPD, BPD/DS, or related procedures because of the greater associated nutritional risks related to the increased length of bypassed small intestine (Grade A; BEL 1). Newer nonsurgical bariatric procedures may be considered for selected patients who are expected to benefit from short-term (i.e., about 6 months) intervention with ongoing and durable structured lifestyle with/without medical therapy (Grade C; BEL 3). Investigational procedures may be considered for selected patients based on available institutional review board–approved protocols, suitability for clinical targets, and individual patient factors, and only after a careful assessment balancing the importance for innovation, patient safety, and demonstrated effectiveness (Grade D).
| Procedure (ref) | Target weight loss (%TBWL) | Favorable aspects | Unfavorable aspects |
|---|---|---|---|
| LAGB (845) | 20%-25% |
|
|
| SG (845) | 25%-30% |
|
|
| RYGB (845) | 30%-35% |
|
|
| BPD/DS (845) | 35%-45% |
|
|
- Selection of the specific bariatric procedure is done after a decision is made to have a bariatric procedure. Estimate of bariatric surgery numbers can be found at http://asmbs.org/resources/estimate-of-bariatric-surgery-numbers (accessed March 25, 2018).
-
Abbreviations: ASMBS = American Society for Metabolic and Bariatric Surgery; BMI = body mass index; GERD = gastroesophageal reflux disease; LAGB = laparoscopic adjustable gastric banding; BPD/DS = biliopancreatic diversion with duodenal switch; RYGB = Roux-en-Y gastric bypass; SG = sleeve gastrectomy; TBWL = total body weight loss.
- STEP 1: Identify durable target weight loss beyond that achieved with lifestyle and medications to mitigate relevant obesity-related complications, a primary determinant of an optimal procedure selection:
- ◦ > 5%-10% weight loss: type 2 diabetes, dyslipidemia, hypertension, nonalcoholic fatty liver disease, low testosterone, obstructive sleep apnea/reactive airway disease, urinary stress incontinence, polycystic ovary syndrome
- ◦ > 10%-15% weight loss: metabolic syndrome, prediabetes, nonalcoholic steatohepatitis, osteoarthritis, GERD, depression (13).
- STEP 2: Identify other factors that can affect decision-making, including: durability, eating behaviors, surgeon skills, institutional experience, cardiometabolic effects, prior gastrointestinal surgery, and gastrointestinal disease. “Favorable” aspects show key parameters to favor selection of the respective procedure. “Unfavorable” aspects show key parameters against selection of the respective procedure.
- STEP 1: Identify durable target weight loss beyond that achieved with lifestyle and medications to mitigate relevant obesity-related complications, a primary determinant of an optimal procedure selection:
| Procedure (ref) | |||
|---|---|---|---|
| Target weight loss (%TBWL) | Favorable aspects | Unfavorable aspects | |
| Primary obesity surgery endoluminal (POSE) (846) | 5% |
|
|
| Gelesis100 (ingested Hydrogel capsules) | 6% |
|
|
| vBLOC (847, 848) | 8%-9% |
|
|
| Intragastric balloon (17, 849, 850) | 10%-12% |
|
|
| AspireAssist (851) | 12%-14% |
|
|
| Transpyloric shuttle (852) | 14% |
|
|
| Endoscopic sleeve gastroplasty (ESG) (853) | 16%-20% |
|
|
- Abbreviations: FDA = U.S. Food and Drug Administration; TBWL = total body weight loss; vBLOC = vagal nerve-blocking device; n/v = nausea/vomiting; RCT = randomized controlled trial.
| Procedure (ref) | Target weight loss (%TBWL) | Favorable aspects | Unfavorable aspects |
|---|---|---|---|
| Laparoscopic greater curvature plication (854) | 15-25% |
|
|
| OAGB (845) | 35-40% |
|
|
| OADS (SIPS, SADI-S) (265, 854) | 35-45% |
|
|
- Abbreviations: ASMBS = American Society for Metabolic and Bariatric Surgery; BPD/DS = biliopancreatic diversion with duodenal switch; GERD = gastroesophageal reflux disease; OAGB = one-anastomosis gastric bypass; OADS = one-anastomosis duodenal switch; RYGB = Roux-en-Y gastric bypass; SIPS = stomach intestinal pylorus-sparing; SADI-S = single-anastomosis duodeno-ileal bypass with sleeve gastrectomy; TBWL = total body weight loss.
- a Institutional review board (IRB) or IRB exemption required (https://asmbs.org/resources/endorsed-procedures-and-devices).
Q3. How should potential candidates be managed before bariatric procedures?
R7. (2008). Patients must undergo evaluation for ORCs and causes of obesity before the procedure, with special attention directed to those factors that could influence a recommendation for bariatric procedures (see preoperative checklist in Table 9) (Grade A; BEL 1) and consider a referral to a specialist in obesity medicine (Grade D).
| ✓ Complete history and physical (obesity-related comorbidities, causes of obesity, weight, BMI, weight-loss history, commitment, and exclusions related to surgical risk) |
| ✓ Routine labs (including fasting blood glucose and lipid panel, kidney function, liver profile, lipid profile, urine analysis, prothrombin time/INR, blood type, and CBC) |
| ✓ Nutrient screening with iron studies, B12 and folic acid (RBC folate, homocysteine, methylmalonic acid optional), and 25-vitamin D (vitamins A and E optional); consider more extensive testing in patients undergoing malabsorptive procedures based on symptoms and risks |
| ✓ Cardiopulmonary evaluation with sleep apnea screening (ECG, CSR, and echocardiography if cardiac disease or pulmonary hypertension suspected; deep vein thrombosis evaluation, if clinically indicated) |
| ✓ GI evaluation (H. pylori screening in areas of high prevalence; gallbladder evaluation and upper endoscopy, if clinically indicated) |
| ✓ Endocrine evaluation (A1C with suspected or diagnosed prediabetes or diabetes; TSH with symptoms or increased risk of thyroid disease; androgens with PCOS suspicion [total/bioavailable testosterone, DHEAS, Δ4-androstenedione]); screening for Cushing syndrome if clinically suspected (1-mg overnight dexamethasone test, 24-hour urinary free cortisol, 11 pm salivary cortisol) |
| ✓ Lifestyle medicine evaluation: healthy eating index; cardiovascular fitness; strength training; sleep hygiene (duration and quality); mood and happiness; alcohol use; substance abuse; community engagement |
| ✓ Clinical nutrition evaluation by RD |
| ✓ Psychosocial-behavioral evaluation |
| ✓ Assess for individual psychological support/counseling |
| ✓ Document medical necessity for bariatric surgery |
| ✓ Informed consent |
| ✓ Provide relevant financial information |
| ✓ Continue efforts for preoperative weight loss |
| ✓ Optimize glycemic control |
| ✓ Pregnancy counseling |
| ✓ Smoking-cessation counseling |
| ✓ Verify cancer screening by primary care physician |
- Abbreviations: BMI = body mass index; CBC = complete blood count; CSR = Cheyne Stokes respiration; ECG = electrocardiogram; GI = gastrointestinal; INR = international normalized ratio; PCOS = polycystic ovary syndrome; RBC = red blood cell; RD = registered dietitian; DHEAS = dehydroepiandrosterone-sulfate; TSH = thyrotropin.
- a Based on information included in Mechanick et al. Endocr Pract. 2013;19:337-372 (1).
R8. (2008). The evaluation must include a comprehensive medical history, psychosocial history, physical examination, and appropriate laboratory testing to assess surgical risk (see preoperative checklist in Table 9) (Grade A; BEL 1).
R9. (2008). Medical records should contain clear documentation of the indications for bariatric surgery (Grade D).
R10. (2019*). Because informed consent is a dynamic process, there must be a thorough discussion with the patient regarding the risks and benefits, procedural options, choices of surgeon and medical institution, and the need for long-term follow-up and vitamin supplementation (including costs required to maintain appropriate follow-up and nutrient supplementation) (Grade D). Patients must also be provided with educational materials, which are culturally and educationally appropriate, as well as access to similar preoperative educational sessions at prospective bariatric surgery centers (Grade D). Consent should include experience of the surgeon with the specific procedure offered and whether the hospital has an accredited bariatric surgery program (Grade D).
R11. (2013). The bariatric surgery program must be able to provide all necessary financial information and clinical material for documentation so that, if needed, third-party payer criteria for reimbursement are met (Grade D).
R12. (2013). Weight loss before the procedure can reduce liver volume and may help improve the technical aspects of surgery in patients with an enlarged liver or fatty liver disease and therefore may be recommended before a bariatric procedure (Grade B; BEL 1; downgraded due to inconsistent evidence). Weight loss or medical nutritional therapy may be recommended to patients in selected cases to improve comorbidities, such as glycemic targets (Grade D).
Q4. What are the elements of medical clearance for bariatric procedures?
R13. (NEW). A lifestyle medicine checklist should be completed as part of a formal medical clearance process for all patients considered for any bariatric procedure (Table 9) (Grade D).
R14. (2019*). Glycemic control before the procedure must be optimized using a diabetes comprehensive care plan, including healthy low-calorie dietary patterns, medical nutrition therapy, physical activity, and, as needed, pharmacotherapy (Grade A; BEL 1). Reasonable targets for preoperative glycemic control, which may be associated with shorter hospital stays and improved bariatric procedure outcomes, include a hemoglobin A1C (A1C) value of 6.5% to 7.0% (48 to 53 mmol/mol) or less and peri-procedure blood glucose levels of 80 to 180 mg/dL (Grade B; BEL 2). More liberal preoperative targets, such as an A1C of 7% to 8% (53 to 64 mmol/mol), are recommended in patients with advanced microvascular or macrovascular complications, extensive comorbid conditions, or long-standing diabetes in which the general goal has been difficult to attain despite intensive efforts (Grade A; BEL 1). In patients with A1C > 8% or otherwise uncontrolled diabetes, clinical judgment determines the need and timing for a bariatric procedure (Grade D).
R15. (2013*). Routine screening for primary hypothyroidism with a thyrotropin (TSH) level before a bariatric procedure is not recommended, though many insurance plans require a serum TSH level (Grade D). A serum TSH level should be obtained only if clinical evidence of hypothyroid is present (Grade B; BEL 2). Patients found to be hypothyroid must be treated with levothyroxine monotherapy (Grade A; BEL 1).
R16. (2019*). A fasting lipid panel should be obtained in all patients with obesity (Grade A; BEL 1). Treatment should be initiated according to available and current clinical practice guidelines (CPGs) (see www.aace.com/files/lipid-guidelines.pdf and www.lipid.org/recommendations) (Grade D).
R17. (2013*). Candidates for bariatric procedures should avoid pregnancy pre procedure and for 12 to 18 months post procedure (Grade D). Women who become pregnant after bariatric procedures should be counseled and monitored for appropriate weight gain, nutritional supplementation, and fetal health (Grade C; BEL 3). All women of reproductive age should be counseled on contraceptive choices before and after bariatric procedures (Grade D). Patients undergoing Roux-en-Y gastric bypass (RYGB) or another malabsorptive procedure should be counseled about nonoral contraceptive therapies (Grade D). Patients who become pregnant following bariatric procedure should have nutritional surveillance and laboratory screening for nutrient deficiencies every trimester, including iron, folate, vitamin B12, vitamin D, and calcium, and if after a malabsorptive procedure, fat-soluble vitamins, zinc, and copper (Grade D). Patients who become pregnant post LAGB should have band adjustments as necessary for appropriate weight gain for fetal health (Grade B; BEL 2).
R18. (2008*). Estrogen therapy should be discontinued before a bariatric procedure (1 cycle of oral contraceptives in premenopausal women; 3 weeks of hormone replacement therapy in postmenopausal women) to reduce the risks for postprocedure thromboembolic phenomena (Grade D).
R19. (2008*). Women should be advised that their fertility status might be improved after a bariatric procedure (Grade D).
R20. (2019*). Case-by-case decisions to screen for monogenic and syndromic causes of obesity should be based on specific historical and physical findings. (Grade D).
R21. (2019*). The need for an electrocardiogram and other noninvasive cardiac testing is determined on the basis of the individual risk factors and findings on history and physical examination and should be based on the latest American College of Cardiology/American Heart Association guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery (458) (Grade D). Patients with known heart disease require a formal cardiology consultation before bariatric procedures (Grade D). Patients at risk for heart disease must undergo evaluation for peri-procedure β-adrenergic blockade (Grade A; BEL 1).
R22. (2019*). In patients evaluated for bariatric procedures, clinical screening for OSA (with confirmatory polysomnography if screening tests are positive) should be considered (Grade C, BEL 3). Patients with intrinsic lung disease or disordered sleep patterns should have a formal pulmonary evaluation, including arterial blood gas measurement, when knowledge of the results would alter patient care (Grade C; BEL 3).
R23. (2019*). Tobacco use must be avoided at all times by all patients. In particular, patients who smoke cigarettes should stop as soon as possible, preferably 1 year, but at the very least, 6 weeks before bariatric procedures (Grade A; BEL 2, upgraded by consensus). Also, tobacco use must be avoided after bariatric procedures given the increased risk of poor wound healing, anastomotic ulcer, and overall impaired health (Grade A; BEL 1). Structured intensive cessation programs are preferable to general advice and should be implemented (Grade D).
R24. (2013*). Patients with a history of deep vein thrombosis (DVT) or cor pulmonale should undergo a risk assessment for bariatric surgery and an appropriate diagnostic evaluation for DVT (Grade D). In selecting treatment approaches to prevent thrombosis, the routine placement of a vena cava filter is discouraged; however, prophylactic placement of a vena cava filter may be considered in individual patients after careful evaluation of the risks and benefits (Grade C; BEL 3).
R25. (2019*). Clinically significant gastrointestinal (GI) symptoms should be evaluated before bariatric procedures with imaging studies, upper GI series, or endoscopy (Grade D). The use of preoperative endoscopy may be considered in all patients being evaluated for sleeve gastrectomy (SG) (Grade D).
R26. (2019*). Imaging studies are not recommended as a routine screen for liver disease (Grade B, BEL 2). Abdominal ultrasound is indicated to evaluate symptomatic biliary disease and elevated liver function tests (Grade C, BEL 3). Abdominal ultrasonography or elastography may be helpful and may be considered to identify NAFLD, but may not be diagnostic (Grade B, BEL 2). Consideration can be made for liver biopsy at the time of a bariatric procedure to document steatohepatitis and/or cirrhosis that may otherwise be unknown due to normal appearance on imaging and/or liver function tests (Grade C, BEL 3). A comprehensive evaluation is recommended in those patients with clinically significant and persistent abnormal liver function tests (Grade A; upgraded by consensus rule).
R27. (2013*). Routine screening for the presence of Helicobacter pylori before bariatric procedures may be considered in areas of high prevalence (Grade C; BEL 3).
R28. (2013*). Prophylactic treatment for gouty attacks should be considered before bariatric procedures in patients with a history of gout (Grade C, BEL 3).
R29. (2008*). There are insufficient data to warrant assessment of bone mineral density with dual-energy x-ray absorptiometry (DXA) or serum or urinary bone turnover markers before the procedure outside formal recommendations by the National Osteoporosis Foundation (http://www.iscd.org/documents/2014/10/nof-clin-guidelines.pdf/) (Grade D).
R30. (2019*). A formal psychosocial-behavioral evaluation performed by a qualified behavioral health professional (i.e., licensed in a recognized behavioral health discipline, such as psychology, social work, psychiatry, psychiatric nursing, etc., with specialized knowledge and training relevant to obesity, eating disorders, and/or bariatric procedures), which assesses environmental, familial, and behavioral factors, as well as risk for suicide, should be required for all patients before a bariatric procedure (Grade C; BEL 3). Any patient considered for a bariatric procedure with a known or suspected psychiatric illness, or substance abuse or dependence, should undergo a formal mental health evaluation before the procedure (Grade C; BEL 3). Following RYGB and SG, high-risk groups should eliminate alcohol consumption due to impaired alcohol metabolism and risk of alcohol-use disorder postoperatively (Grade C; BEL 3).
R31. (2013*). All patients should undergo evaluation of their ability to incorporate nutritional and behavioral changes before and after any bariatric procedure (Grade C; BEL 3).
R32. (2013*). All patients must undergo an appropriate nutritional evaluation, including micronutrient measurements, before any bariatric procedure (Table 9) (Grade A; BEL 1). In comparison with purely restrictive procedures, more extensive nutritional evaluations are required for malabsorptive procedures (Grade A; BEL 1). Whole-blood thiamine levels may be considered in patients prior to bypass procedures (RYGB and BPD/DS) (Grade C; BEL 3).
R33. (2013*). Patients should be followed by their primary care physician and have age- and risk-appropriate cancer screening before bariatric procedures (Grade C; BEL 3).
R34. (NEW). Preoperative enhanced recovery after bariatric surgery (ERABS) clinical pathways should be implemented in all patients who are having bariatric surgery to improve postoperative outcomes (Grade D). Comprehensive preoperative optimization (prehabilitation) should be implemented, including but not limited to deep breathing exercises, continuous positive airway pressure (CPAP) as appropriate, incentive spirometry, leg exercises, continued oral nutrition with carbohydrates, including sips of clear liquids up to 2 hours preoperatively, H2 blocker or proton-pump inhibitor, opioid-sparing multimodal analgesia, thromboprophylaxis, and education about perioperative protocols (Table 10) (Grade B; BEL 2).
| Protocol component/intervention | Outcome |
|---|---|
| Immediate Preoperative | |
| Carbohydrate loading | Decreased insulin resistance |
| Decreased protein catabolism, LOS | |
| Faster return of bowel function | |
| Reduced fasting | No adverse outcomes |
| Multimodal preanesthesia medication | Decreased pain, PONV, opioid use |
| Intraoperative | |
| Standard intraoperative anesthesia pathway | Decreased pain, PONV, opioid use |
| Protective ventilation strategies | Decreased pulmonary complications |
| Goal-directed fluid management | Decreased morbidity, LOS |
| Postoperative nausea and vomiting prophylaxis | Decreased PONV |
| Regional block | Decreased pain, opioid use |
| Postoperative | |
| Standard multimodal analgesia regimen | Decreased pain, PONV, opioid use |
| Early ambulation | Decreased VTE |
| Early return of oral intake | Easier return of bowel function |
- Abbreviations: AHRQ = Agency for Healthcare Research and Quality; LOS = length of stay; PONV = postoperative nausea and vomiting; VTE = venous thromboembolism.
- a Based on information included in Grant et al. Anesth Analg. 2019;129:51-60 (855); Thorell et al. World J Surg. 2016;40:2065-2083 (568); Ljungqvist et al. JAMA. 2017;152:292-298 (856); Alvarez et al. Curr Opin Anaesthesiol. 2017;30:133-139 (593); and Bellamy et al. Perioper Med (Lond). 2013;2:12 (549).
Q5. How can care be optimized during and within 5 days of a bariatric procedure?
R35. (NEW). Appropriate perioperative ERABS clinical pathways should be implemented in all patients undergoing bariatric surgery (Table 10) (Grade D). Routine pulmonary recruitment maneuvers (PRMs) should be performed intraoperatively as needed (Grade D). Intraoperative use of dexmedetomidine may be considered to decrease perioperative opioid use (Grade C; BEL 3). Intraoperative protocols to detect possible silent bleeding sites should be performed (Grade D). Consider dynamic indicators to guide goal-directed fluid therapy to avoid excess intraoperative fluid administration (Grade B; BEL 2).
R36. (NEW). A postoperative checklist should be reviewed and implemented (Table 11). Appropriate postoperative ERABS clinical pathways should be implemented in all patients who have had bariatric surgery (Table 10) (Grade D).
| Checklist item | LAGB | SG | RYGB | BPD/DS | |
|---|---|---|---|---|---|
| Early postoperative care | |||||
| ✓ | Monitored telemetry at least 24 hours if high risk for MI | ✓ | ✓ | ✓ | ✓ |
| ✓ | Protocol-derived staged meal progression supervised by RD | ✓ | ✓ | ✓ | ✓ |
| ✓ | Healthy eating education by RD | ✓ | ✓ | ✓ | ✓ |
| ✓ | Multivitamin plus minerals (# tablets for minimal requirement) | 1 | 2 | 2 | 2 |
| ✓ | Elemental calcium (as calcium citrate) | 1,200-1,500 mg/d | 1,200-1,500 mg/d | 1,200-1,500 mg/d | 1,800-2,400 mg/d |
| ✓ | Vitamin D, at least 3,000 IU/d, titrate to > 30 ng/mL | ✓ | ✓ | ✓ | ✓ |
| ✓ | Vitamin B12 as needed for normal range levels | ✓ | ✓ | ✓ | ✓ |
| ✓ | Maintain adequate hydration (usually > 1.5 L/d PO) | ✓ | ✓ | ✓ | ✓ |
| ✓ | Monitor blood glucose with diabetes or hypoglycemic symptoms | ✓ | ✓ | ✓ | ✓ |
| ✓ | Pulmonary toilet, spirometry, DVT prophylaxis | ✓ | ✓ | ✓ | ✓ |
| ✓ | If unstable, consider PE, IL | PE | PE | PE/IL | PE/IL |
| ✓ | If rhabdomyolysis suspected, check CPK | ✓ | ✓ | ✓ | ✓ |
| Follow-up | |||||
| ✓ | Visits: initial, interval until stable, once stable (months) | 1, 1-2, 12 | 1, 3, 6, 12 | 1, 3, 6-12 | 1, 3, 6 |
| ✓ | Monitor progress with weight loss and evidence of complications each visit | ✓ | ✓ | ✓ | ✓ |
| ✓ | SMA-21, CBC/plt with each visit (and iron at baseline and after as needed) | ✓ | ✓ | ✓ | ✓ |
| ✓ | Avoid nonsteroidal anti-inflammatory drugs | ✓ | ✓ | ✓ | ✓ |
| ✓ | Adjust postoperative medications | ✓ | ✓ | ✓ | ✓ |
| ✓ | Consider gout and gallstone prophylaxis in appropriate patients | ✓ | ✓ | ✓ | ✓ |
| ✓ | Need for antihypertensive therapy with each visit | ✓ | ✓ | ✓ | ✓ |
| ✓ | Lipid evaluation every 6-12 months based on risk and therapy | ✓ | ✓ | ✓ | ✓ |
| ✓ | Monitor adherence with physical activity recommendations | ✓ | ✓ | ✓ | ✓ |
| ✓ | Evaluate need for support groups | ✓ | ✓ | ✓ | ✓ |
| ✓ | Bone density (DXA) at 2 years | ✓ | ✓ | ✓ | ✓ |
| ✓ | 24-hour urinary calcium excretion at 6 months and then annuallyb | x | x | x | ✓ |
| ✓ | B12 (annually; MMA and HCy optional; then q 3-6 months if supplemented) | ✓ | ✓ | ✓ | ✓ |
| ✓ | Folic acid (RBC folic acid optional), iron studies, 25-vitamin D, iPTH | x | x | ✓ | ✓ |
| ✓ | Vitamin A (initially and q 6-12 months thereafter) | x | x | optional | ✓ |
| ✓ | Copper, zinc, selenium evaluation with specific findings | x | x | ✓ | ✓ |
| ✓ | Thiamine evaluation with specific findings | ✓ | ✓ | ✓ | ✓ |
| ✓ | Consider eventual body contouring surgery | ✓ | ✓ | ✓ | ✓ |
| ✓ | Lifestyle medicine evaluation: healthy eating index; cardiovascular fitness; strength training; sleep hygiene (duration and quality); mood and happiness; alcohol use; substance abuse; community engagement | ✓ | ✓ | ✓ | ✓ |
| ✓ | Hemoglobin A1C, TSH evaluation in long-term follow-up | ✓ | ✓ | ✓ | ✓ |
- Abbreviations: BPD/DS = biliopancreatic diversion with duodenal switch; CBC = complete blood count; CPK = creatine phosphokinase; DVT = deep vein thrombosis; DXA = dual-energy x-ray absorptiometry; HCy = homocysteine; IL = intestinal leak; iPTH = intact parathyroid hormone; LAGB = laparoscopic adjustable gastric band; MI = myocardial infarction; MMA = methylmalonic acid; PE = pulmonary embolus; plt = platelets; PO = orally; q = daily; RBC = red blood cell; RD = registered dietitian; RYGB = Roux-en-Y gastric bypass; SG = sleeve gastrectomy; SMA-21 = chemistry panel; TSH = thyrotropin.
- a Based on information included in Mechanick et al. Endocr Pract. 2013;19:337-372 and Parrott et al. Surg Obes Rel Dis. 2017;13:727-741 (1, 448).
- b This testing should be considered for any patient after a bariatric procedure at 6 months and then annually if there is a history of renal stones.
R37. (NEW). Preemptive antiemetic and nonopioid analgesic medications immediately before and during bariatric procedures as part of a multimodal pain management strategy should be implemented to decrease early postprocedure opioid use and postoperative nausea and vomiting (Grade C; BEL 3).
R38. (2013*). A low-sugar clear liquid meal program can usually be initiated within 24 hours after any of the surgical bariatric procedures, but this diet and meal progression should be discussed with the surgeon and guided by the registered dietitian (RD) (Table 12) (Grade C; BEL 3). A consultation for postoperative meal initiation and progression must be arranged with an RD who is knowledgeable about the postoperative bariatric diet (Grade A, BEL 1). Patients should receive education in a protocol-derived staged meal progression based on their surgical procedure (Grade D). Patients should be counseled to eat 3 small meals during the day and chew small bites of food thoroughly before swallowing (Grade D). Patients should be counseled about the principles of healthy eating, including at least 5 daily servings of fresh fruits and vegetables (Grade D). Protein intake should be individualized, assessed, and guided by an RD, regarding gender, age, and weight (Grade D). A minimal protein intake of 60 g/d and up to 1.5 g/kg ideal body weight per day should be adequate; higher amounts of protein intake—up to 2.1 g/kg ideal body weight per day—need to be assessed on an individualized basis (Grade D). Concentrated sweets should be eliminated from the diet after RYGB to minimize symptoms of the dumping syndrome, as well as after any bariatric procedure to reduce caloric intake (Grade D). Crushed or liquid rapid-release medications should be used instead of extended-release medications to maximize absorption in the immediate postprocedure period (Grade D).
| UpToDate: Postoperative Nutritional Management (857) | 2008 ASMBS Allied Health Nutritional Guidelines (858) | Guidelines for Perioperative Care in Bariatric Surgery: ERAS Society Recommendations (568) | Academy of Nutrition and Dietetics Pocket Guide to Bariatric Surgery, 2nd ed (859) | |
|---|---|---|---|---|
| Diet progression |
Surgeon or institution specific Stage 1 and 2: Hydration and liquids
Stage 3: Solid foods with an emphasis on protein sources, some carbohydrates, and fiber (~10-14 days after surgery) Stage 4: Micronutrient supplementation (when patient reaches a stable or maintenance weight) Long-term diet:
The Vegetarian Resource Group
|
Diet Stage: Clear liquid (1 to 2 days after surgery)
Full liquid (10-14 days after surgery)
Pureed (10-14+ days)
Mechanically altered soft (> 14 days after surgery)
Regular textured (6-8 weeks after surgery) Purpose of nutrition care after surgical weight loss procedures:
|
Clear liquid meal regimen initiated a couple of hours postoperatively Balanced meal plan to include:
Avoid concentrated sweets to reduce caloric intake and to minimize symptoms of dumping (gastric bypass) |
Postoperative nutrition care of the bariatric patient has 2 distinct stages during the first year:
Typically described in stages:
|
| Fluids | Throughout all the diet stages, patients should be counseled to consume adequate fluid to prevent dehydration | N/A | > 1.5 L daily |
48-64 oz/d
|
| Protein |
46 g/d—women 56 g/d—men Protein needs:
|
Exact needs have yet to be defined | Should average 60-20 g daily | Guidelines for protein consumption not defined |
| Carbohydrates |
|
N/A | N/A | N/A |
| Fat | 20%-35% of the daily caloric intake; bulk of the fat intake should be unsaturated fat | N/A | N/A | N/A |
| Behavior |
|
Avoid/delay
|
|
|
| Other |
Close monitoring with a registered dietitian |
Dietitian’s role is a vital component of the bariatric surgery process | Nutritional and meal planning guidance should be provided to patient and family before bariatric surgery and during the postoperative hospital course and reinforced at subsequent outpatient visits | RD responsible for the nutrition care of the postsurgery patient and plays an important role in every aspect of care, from pre-operative assessment of the patient to long-term follow-up, evaluation, and monitoring |
| Follow up with registered dietitian | Consultation should be provided with a dietitian and a protocol-derived staged meal progression, based on the type of surgical procedure, should be adhered to |
- Abbreviations: American Society for Metabolic and Bariatric Surgery; BPD/DS = biliopancreatic diversion with duodenal switch; DASH = detary approaches to stop hypertension; ERAS = enhanced recovery after surgery; LAGB = laparoscopic adjustable gastric band; N/A = not applicable; RD = registered dietitian; RYGB = Roux-en-Y gastric bypass; SG = sleeve gastrectomy.
R39. (2019*). After consideration of deficiency states before the procedure, as well as risks and benefits in the early (< 5 days) postprocedure period, patients with, or at risk for, demonstrable micronutrient insufficiencies or deficiencies must be treated with the respective micronutrient, and then adjusted based on recommendations for the late postprocedure period (Tables 11, 13, and 14) (Grade A, BEL 2, upgraded by consensus). Minimal daily nutritional supplementation for patients with BPD/DS, RYGB, and SG should be in chewable form initially, and as 2 adult multivitamins plus minerals (each containing iron, folic acid, and thiamine) (Grade B, BEL 2), elemental calcium (1,200 to 1,500 mg/d for SG and RYGB and 1,800 to 2,400 mg/d for BPD/DS in diet and as citrated supplement in divided doses) (Grade B, BEL 2), at least 2,000 to 3,000 IU of vitamin D (titrated to therapeutic 25-hydroxyvitamin D levels > 30 ng/mL) (Grade A, BEL 1), total iron as 18 to 60 mg via multivitamins and additional supplements (Grade A, BEL 1), and vitamin B12 (parenterally as sublingual, subcutaneous, or intramuscular preparations, or orally, if determined to be adequately absorbed) (Grade B; BEL 2). Minimal daily nutritional supplementation for patients with LAGB should include 1 adult multivitamin plus minerals (including iron, folic acid, and thiamine) (Grade B, BEL 2), 1,200 to 1,500 mg/d of elemental calcium (in diet and as citrated supplement in divided doses), and at least 2,000 to 3,000 IU of vitamin D (titrated to therapeutic 25-dihydroxyvitamin D levels) (Grade B, BEL 2). Additional recommendations to prevent micronutrient deficiencies are included in Tables 11, 13, and 14.
| Vitamin/mineral | Prevalence of deficiency | Screening |
|---|---|---|
| Vitamin B1 (thiamine) | < 1%-49% depending on procedure and post WLS time frame |
Recommended for high-risk groups
Post-WLS patients with signs and symptoms or risk factors should be assessed for thiamine deficiency at least during the first 6 months and then every 3-6 months until symptoms resolve |
| Vitamin B12 (cobalamin) |
At 2-5 years post WLS
|
Recommended for patients who have undergone RYGB, SG, or BPD/DS |
| More frequent screening (every 3 months) recommended in the first year post surgery, and then at least annually or as clinically indicated for patients who chronically use medications that exacerbate risk of B12 deficiency, such as nitrous oxide, neomycin, metformin, colchicine, proton-pump inhibitors, and seizure medications | ||
| Screening should include serum MMA with or without homocysteine to identify metabolic deficiency of B12 in symptomatic and asymptomatic patients and in patients with history of B12 deficiency or preexisting neuropathy | ||
| Vitamin B12 deficiencies can occur due to food intolerances or restricted intake of protein and vitamin B12-containing foods | ||
| Folate (folic acid) | Up to 65% of patients | Screening recommended for all patients |
| Particular attention should be given to female patients of childbearing age | ||
| Poor dietary intake of folate-rich foods and suspected nonadherence with multivitamin may contribute to folate deficiency | ||
| Iron |
3 months-10 years post WLS
|
Iron deficiency can occur after any bariatric procedure, despite routing supplementation |
| Routine postbariatric screening is recommended within 3 months after surgery, and then every 3 to 6 months until 12 months, and annually thereafter for all patients | ||
| Iron status should be monitored in postbariatric patients at regular intervals using an iron panel, complete blood count, total iron-binding capacity, ferritin, and soluble transferrin receptor (if available), along with clinical signs and symptoms | ||
| Additional screening should be performed based on clinical signs and symptoms and/or laboratory findings or in cases where deficiency is suspected | ||
| Vitamin D and calcium | Up to 100% of patients | Routine screening is recommended for all patients |
| 25(OH)D is the preferred biochemical assay | ||
| Elevated PTH levels and increased bone formation/resorption markers may also be considered | ||
| Vitamin A | Up to 70% of patients within 4 years post surgery | Screening is recommended within the first postoperative year, particularly for those who underwent BPD/DS, regardless of symptoms |
| Screening is recommended in patients who have undergone RYGB and BPD/DS, particularly in those with evidence of protein-calorie malnutrition | ||
| Vitamin E | Uncommon | Screening is recommended in patients who are symptomatic |
| Vitamin K | Uncommon | Screening is recommended in patients who are symptomatic |
| Zinc |
Up to 70% of patients after BPD/DS Up to 40% of patients after RYGB Up to 19% of patients after SG Up to 34% of patients after AGB |
Zinc deficiency is possible, even during zinc supplementation and especially if primary sites of absorption (duodenum and proximal jejunum) are bypassed Screening should be performed at least annually post RYGB and post BPD/DS Serum and plasma zinc are the preferred biomarkers for screening in patients after bariatric surgery |
| Copper |
Up to 90% in patients after BPD/DS 10-20% in patients after RYGB 1 case report for patients after SG No data for patients after AGB |
Screening is recommended at least annually after BPD/DS and RYGB, even in the absence of clinical signs or symptoms |
| Serum copper and ceruloplasmin are recommended biomarkers for determining copper status because they are closely correlated with physical symptoms of copper deficiency |
- Abbreviations: 25(OH)D = 25-hydroxyvitamin D; AGB = adjustable gastric band; BPD/DS = biliopancreatic diversion/duodenal switch; GI = gastrointestinal; MMA = methylmalonic acid; PTH = parathyroid hormone; RYGB = Roux-en-Y gastric bypass; SBBO = small bowel bacterial overgrowth; SG = sleeve gastrectomy; WLS = weight loss surgery.
| Micronutrient | Supplementation to prevent deficiency | Repletion for patients with deficiency |
|---|---|---|
| Vitamin B1 (thiamine) | ≥ 12 mg of thiamine daily; preferably a 50- to 100-mg daily dose of thiamine from a B-complex supplement or high-potency multivitamin | Bariatric patients with suspected thiamine deficiency should be treated before or in the absence of laboratory confirmation and monitored/evaluated for resolution of signs and symptoms |
| Repletion dose for thiamine deficiency varies based on route of administration and severity of symptoms: | ||
|
||
| Magnesium, potassium, and phosphorus should be given simultaneously to patients at risk for refeeding syndrome | ||
| Vitamin B12 (cobalamin) |
Supplement dose varies based on route of administration
|
1,000 μg/d to achieve normal levels and then resume dosages recommended to maintain normal levels |
| Folate (folic acid) | 400-800 micrograms of oral folate daily from multivitamin | Oral dose of 1000 μg of folate daily to achieve normal levels and then resume recommended dosage to maintain normal levels |
| 800-1,000 micrograms of oral folate daily in women of childbearing age | > 1-mg/d supplementation is not recommended because of the potential masking of vitamin B12 deficiency | |
| Iron |
Males and patients without a history of anemia: 18 mg of iron from multivitamin |
Oral supplementation should be increased to provide 150-200 mg of elemental iron daily to amounts as high as 300 mg 2-3 times daily |
| Menstruating females and patients who have undergone RYGB, SG, or BPD/DS: 45-60 mg of elemental iron daily (cumulatively, including iron from all vitamin and mineral supplements) | ||
|
Oral supplementation should be taken in divided doses separately from calcium supplements, acid-reducing medications, and foods high in phytates or polyphenols |
||
| Oral supplementation should be taken in divided does separately from calcium supplements, acid-reducing medications, and foods high in phytates or polyphenols | ||
|
Vitamin C supplementation may be added to increase iron absorption and decrease risk of iron overload IV iron infusion should be administered if iron deficiency does not respond to oral therapy |
||
| Vitamin D and calcium |
Appropriate dose of daily calcium from all sources varies by surgical procedure
To enhance calcium absorption in post-WLS patients
Recommended preventive dose of vitamin D should be based on serum vitamin D levels
|
All bariatric patients with vitamin D deficiency or insufficiency should be repleted as follows:
Repletion of calcium deficiency varies by surgical procedure:
|
| Vitamin A |
Dosage is based on type of procedure:
Higher maintenance doses of fat-soluble vitamins may be required for bariatric patients with a previous history of vitamin A deficiency |
For bariatric patients with vitamin A deficiency without corneal changes, a dose of 10,000-25,000 IU/d of vitamin A should be given orally until clinical improvement is evident |
|
For bariatric patients with vitamin A deficiency with corneal changes, a dose of 50,000-100,000 IU of vitamin A should be administered IM for 3 days, followed by 50,000 IU/d IM for 2 weeks |
||
|
Water-miscible forms of fat-soluble vitamins are also available to improve absorption Special attention should be paid to supplementation of vitamin A in pregnant women after bariatric surgery |
||
| Bariatric patients with vitamin A deficiency should also be evaluated for concurrent iron and/or copper deficiencies because these can impair resolution of vitamin A deficiency | ||
| Vitamin E |
15 mg/d Higher maintenance doses of fat-soluble vitamins may be required for postbariatric patients with a previous history of vitamin E deficiency Water-miscible forms of fat-soluble vitamins are also available to improve absorption |
Optimal therapeutic dose of vitamin E for bariatric patients is not defined Potential antioxidant benefits can be achieved with supplements of 100-400 IU/d, which is higher than the amount found in multivitamins. Additional supplementation may be required for repletion |
| Vitamin K |
Dosage is based on type of procedure:
Higher maintenance doses of fat-soluble vitamins may be required for post-WLS patients with a previous history of vitamin K deficiency Water-miscible forms of fat-soluble vitamins are also available to improve absorption Special attention should be paid to post-WLS supplementation of vitamin K in pregnant women |
A parenteral dose of 10 mg is recommended for bariatric patients with acute malabsorption A dose of either 1-2 mg/d orally or 1-2 mg/wk parenterally is recommended for post-WLS patients with chronic malabsorption |
| Zinc |
All post-WLS patients should take 4 RDA zinc, with dosage based on type of procedure
The supplementation protocol should contain a ratio of 8-15 mg of supplemental zinc per 1 mg of copper to minimize the risk of copper deficiency The formulation and composition of zinc supplements should be considered in post-WLS patients to calculated accurate levels of elemental zinc provided by the supplement |
A dose-related recommendation for repletion cannot be made due to insufficient evidence Repletion doses should be chosen carefully to avoid inducing a copper deficiency Zinc status should be routinely monitored using consistent parameters throughout treatment |
| Copper |
All post-WLS patients should take 4 RDA copper as part of routine multivitamin and mineral supplementation, with dosage based on type of procedure:
Supplementation with 1 mg of copper is recommended for every 8-15 mg of elemental zinc to prevent copper deficiency in all post-WLS patients Copper gluconate or sulfate is the recommended source of copper for supplementation |
Recommended repletion regimen varies with severity of deficiency:
|
- Abbreviations: 25(OH)D = 25-hydroxyvitamin D; BPD/DS = biliopancreatic diversion/duodenal switch; IM = intramuscular; IV = intravenous; LAGB = laparoscopic adjust gastric band; RDA = recommended dietary allowance; RYGB = Roux-en Y gastric bypass; SG = sleeve gastrectomy; SQ = subcutaneous; WLS = weight loss surgery.
R40. (2019*). Goal-directed intra- and early postprocedure fluid management should be guided by continuous noninvasive measurements to avoid over- and underhydration (Grade B, BEL 2). Once patients can tolerate orals, fluids should be consumed slowly, preferably at least 30 minutes after meals to prevent GI symptoms, and in sufficient amounts to maintain adequate hydration (more than 1.5 liters daily) (Grade D).
R41. (2019*). Nutrition support (enteral nutrition [EN; tube feeds] or parenteral nutrition [PN]) should be considered in bariatric surgery patients at high nutritional risk; PN should be considered in those patients who are unable to meet their needs using their GI tract for at least 5 to 7 days with noncritical illness or 3 to 7 days with critical illness (Grade D). In patients with severe protein malnutrition and/or hypoalbuminemia, not responsive to oral or EN protein supplementation, PN should be considered (Grade D). PN formulation for patients after bariatric procedures should be hypocaloric with relatively high nitrogen (Grade D).
R42. (2019*). Intra-/perioperative intravenous (IV) insulin is recommended for glycemic control (Grade B; BEL 2). In immediate postoperative patients with T2D, the use of all insulin secretagogues (sulfonylureas and meglitinides), sodium-glucose cotransporter-2 inhibitors, and thiazolidinediones should be discontinued and insulin doses adjusted (due to low calorie intake) to minimize the risk for hypoglycemia (Grade D). Except for metformin and incretin-based therapies, antidiabetic medications should be withheld if there is no evidence of hyperglycemia (Grade D). Metformin and/or incretin-based therapies may be continued postoperatively in patients with T2D until prolonged clinical resolution of T2D is demonstrated by normalized glycemic targets (including fasting and postprandial blood glucose and A1C (Grade D). Subcutaneous insulin therapy, using a rapid-acting insulin analogue (insulin lispro, aspart, or glulisine) before meals and a basal long-acting insulin analogue (insulin glargine, detemir, or degludec) should be used to achieve glycemic targets (140 to 180 mg/dL) in hospitalized patients not in intensive care (Grade D). In the intensive care unit (ICU), IV regular insulin as part of a standard intensive insulin therapy protocol should be used to control hyperglycemia to a 140- to 180-mg/dL blood glucose target (Grade D). Endocrinology consultation should be considered for patients with type 1 diabetes (T1D), or with T2D and uncontrolled hyperglycemia (Grade D). Once home, in patients with T2D, periodic fasting blood glucose concentrations must be determined (Grade A; BEL 1). Preprandial, 2-hour postprandial, and bedtime reflectance meter glucose (RMG; “fingerstick”) determinations, or the use of continuous glucose monitors, in the home setting is also recommended, depending on the patient’s ability to test the level of glycemic control targeted, use of oral agents or insulin, and overall care plan (Grade A; BEL 1). RMG determinations or the use of continuous glucose monitors is recommended if symptoms of hypoglycemia occur (Grade A; BEL 1).
R43. (2013*). Patients with high perioperative risk for myocardial infarction should be managed in a telemetry-capable setting for at least the first 24 hours after a bariatric surgical procedure (Grade B; BEL 2).
R44. (2019*). Pulmonary management includes aggressive pulmonary toilet and incentive spirometry, oxygen supplementation to avoid hypoxemia, and early institution of CPAP when clinically indicated (Grade C, BEL 3). Routine admission to an ICU should not be implemented in patients solely due to the presence of severe OSA provided there is adequate CPAP use (Grade D).
R45. (2019*). Prophylaxis against DVT is recommended for all patients after bariatric surgical procedures (Grade B; BEL 2). Prophylactic regimens after bariatric surgery may include sequential compression devices (Grade C; BEL 3), as well as subcutaneously administered unfractionated heparin or low-molecular-weight heparin given within 24 hours after bariatric surgery (Grade B; BEL 2). Extended chemoprophylaxis after hospital discharge should be considered for high-risk patients, such as those with history of DVT, known hypercoagulable state, or limited ambulation (Grade C, BEL 3). The use of DVT risk calculators (Grade C; BEL 3) and early ambulation are encouraged (Grade C; BEL 3). Serum anti-Xa levels should be considered to guide low-molecular-weight heparin dosing in the prophylactic range (Grade A; BEL 1). Fondaparinux at 5 mg daily should be considered as a preventive option (Grade A; BEL 1).
R46. (NEW). Respiratory distress or failure to wean from ventilatory support should prompt a diagnostic work-up for pulmonary embolism (PE) (Grade B; BEL 2).
R47. (2019*). Patients with respiratory distress or failure to wean from ventilatory support after a bariatric procedure should prompt a standard diagnostic work-up with a particular emphasis to detect anastomotic leak (Grade D). In the clinically stable patient, computed tomography (CT) (preferred over upper-GI studies [water-soluble contrast followed by thin barium]) may be considered to evaluate for anastomotic leaks in suspected patients (Grade C; BEL 3). Exploratory laparotomy or laparoscopy is justified and may therefore be considered in the setting of high clinical suspicion for anastomotic leaks (Grade A; BEL 1). A selected diatrizoate meglumine and diatrizoate sodium upper-GI study in the absence of abnormal signs or symptoms may be considered to identify any subclinical leaks before discharge of the patient from the hospital, but routine studies are not cost-effective (Grade C; BEL 3). C-reactive protein (CRP) and/or procalcitonin testing should be considered if a postoperative leak is suspected or the patient is at increased risk for a leak after hospital discharge (Grade B; BEL 2).
R48. (2019*). Patients should have adequate padding at pressure points during bariatric surgery (Grade D). When rhabdomyolysis is suspected, creatine kinase (CK) levels should be determined, urine output monitored, and adequate hydration provided (Grade C; BEL 3). The risk for rhabdomyolysis increases as BMI increases (particularly with BMI > 55 to 60 kg/m2); therefore, screening CK levels may be tested in these higher risk groups (Grade D). Excessive postoperative IV fluids should be avoided (Grade D).
Q6. How can care be optimized 5 or more days after a bariatric procedure?
R49. (2019*). Follow-up should be scheduled depending on the bariatric procedure performed and the severity of comorbidities (Table 11) (Grade D). Following LAGB procedures, frequent nutritional follow-up and band adjustments are recommended to optimize safety and achieve weight-loss targets (Grade C; BEL 3). Significant weight regain or failure to lose weight should prompt a comprehensive evaluation for (a) decreased patient adherence with lifestyle modification, (b) evaluation of medications associated with weight gain or impairment of weight loss, (c) development of maladaptive eating behaviors, (d) psychological complications, and (e) radiographic or endoscopic evaluation to assess pouch enlargement, anastomotic dilation, formation of a gastrogastric fistula among patients who underwent RYGB, or inadequate band restriction among patients who underwent LAGB (Grade B; BEL 2). Interventions should first include dietary change, physical activity, behavioral modification with frequent follow-up, and then, if appropriate, pharmacologic therapy and/or surgical revision (Grade B; BEL 2). In those patients with or without complete resolution of their comorbidities, such as T2D, dyslipidemia, OSA or HTN, continued surveillance and management should be guided by current CPGs for those conditions (Grade D). Routine metabolic and nutritional monitoring is recommended after all bariatric procedures (Grade A; BEL 1).
R50. (2013*). Patients who have undergone RYGB, BPD/DS, or SG and who present with postprandial hypoglycemic symptoms that have not responded to nutritional manipulation should undergo an evaluation to differentiate noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS) from factitious or iatrogenic causes, dumping syndrome, and insulinoma (Grade C; BEL 3). In patients with NIPHS, therapeutic strategies should be implemented, and include dietary changes (low-carbohydrate diet), octreotide, diazoxide, acarbose, calcium-channel antagonists, gastric restriction, and/or reversal procedures, with partial or total pancreatectomy reserved for the rare recalcitrant cases (Grade C; BEL 3). Continuous glucose monitoring may be considered in those patients with hypoglycemia syndromes after bariatric procedures (Grade C, BEL 3).
R51. (2013*). Unless specifically contra-indicated, patients must be advised to incorporate at least some amount of physical activity, with a target of moderate aerobic physical activity that includes a minimum of 150 minutes per week and goal of 300 minutes per week, including strength training 2 to 3 times per week (Grade A; BEL 1).
R52. (2019*). All patients should be encouraged to participate in ongoing support groups (Grade B; BEL 2), self-monitoring (Grade B; BEL 2), and/or mobile technologies (Grade B; BEL 2) to improve weight loss and cardiometabolic risks after bariatric procedures.
R53. (2019*). Baseline and annual postoperative evaluation for vitamin D deficiency is recommended after RYGB, SG, or BPD/DS (Grade B; BEL 2). In patients who have undergone RYGB, BPD, or BPD/DS, treatment with oral calcium citrate and vitamin D (ergocalciferol [vitamin D2] or cholecalciferol [vitamin D3]) is indicated to prevent or minimize secondary hyperparathyroidism without inducing frank hypercalciuria (Grade C; BEL 3). In patients with severe vitamin D malabsorption, initial oral doses of vitamin D2 at 50,000 IU 1 to 3 times weekly or D3 (minimum of 3,000 IU/d to 6,000 IU/d) should be recommended. Of note, vitamin D3 is recommended as a more potent treatment than vitamin D2 based on frequency and amount of dosing needed for repletion; however, both can be utilized (Grade B; BEL 2). Recalcitrant cases may require concurrent oral administration of calcitriol (1,25-dihydroxyvitamin D) (Grade D). Hypophosphatemia is usually due to vitamin D deficiency, and oral phosphate supplementation should be provided for mild to moderate hypophosphatemia (1.5 to 2.5 mg/dL) (Grade D).
R54. (2008). In patients who have had RYGB or BPD/DS, bone density measurements with use of axial (spine and hip) DXA may be indicated to monitor for osteoporosis at baseline and at about 2 years (Grade D).
R55. (2013*). Evaluation of patients for bone loss after bariatric procedures may include serum parathyroid hormone, total calcium, phosphorus, 25-hydroxyvitamin D, and 24-hour urine calcium levels (Grade C; BEL 3). Antiresorptive agents (bisphosphonates or denosumab) should only be considered in patients after bariatric procedures with osteoporosis once appropriate therapy for calcium and vitamin D insufficiency has been implemented (Grade D). If antiresorptive therapy is indicated after bariatric procedures, then intravenously administered bisphosphonates should be used (zoledronic acid, 5 mg once a year, or ibandronate, 3 mg every 3 months), as concerns exist about adequate oral absorption and potential anastomotic ulceration with orally administered bisphosphonates (Grade D). If concerns about absorption or potential anastomotic ulceration are obviated, oral bisphosphonate administration can be provided (alendronate, 70 mg/wk; risedronate, 35 mg/wk or 150 mg/mo; or ibandronate, 150 mg/mo). Alternatively, if bisphosphonates are poorly tolerated or ineffective, denosumab (60 mg subcutaneously every 6 months) may be considered, but again once appropriate therapy for calcium and vitamin D insufficiency has been implemented (Grade D).
R56. (2013*). Management of oxalosis and calcium oxalate stones includes avoidance of dehydration (Grade D), a low-oxalate meal plan (Grade D), oral calcium (Grade B; BEL 1; downgraded due to small evidence base), and potassium citrate therapy (Grade B; BEL 1; downgraded due to small evidence base). Probiotics containing Oxalobacter formigenes may be used, as they have been shown to improve renal oxalate excretion and improve supersaturation levels (Grade C; BEL 3).
R57. (2019*). Aggressive case finding (i.e., detecting a disorder in patients at risk) for vitamin A undernutrition may be performed in the first postoperative year after RYGB or BPD/DS or with evidence of malnutrition due to high prevalence for this deficiency state in these settings (Grade C; BEL 3). Aggressive case finding for vitamin E and K deficiencies should be reserved for those postoperative patients demonstrating symptoms (hemolytic anemia and neuromuscular, particularly ophthalmologic, for vitamin E; excessive bleeding or bruising for vitamin K) (Grade D). When indicated, the dosing strategies for vitamin A are 5,000 IU/day for LAGB, 5,000 to 10,000 IU/day for RYGB and SG, and 10,000 IU/day for BPD/DS; for vitamin E, 15 mg/day for all procedures; and for vitamin K, 90 to 120 µg/d for LAGB, RYGB, and SG and up to 300 µg/d for BPD/DS (Grade D).
R58. (2008*). In the presence of any established fat-soluble vitamin deficiency (vitamins A, D, E, and/or K) with, for example, hepatopathy, neuromuscular impairment, coagulopathy, or osteoporosis, or suspected essential fatty acid (EFA) deficiency (symptoms include hair loss, poor wound healing, and dry scaly skin), clinical and biochemical assessments of the other fat-soluble vitamins may be considered and then supplemented if abnormally low (Grade D). In patients with suspected EFA deficiency in the setting of malabsorptive procedures, therapeutic trials with topical borage, soybean, or safflower oil may be considered due to the low risk profile, but these trials are unproven at present (Grade D).
R59. (2019*). Anemia without evidence of blood loss warrants evaluation of nutritional deficiencies, as well as age-appropriate causes during the late postprocedure period (Grade D). Iron status should be monitored in all patients within the first 3 months after bariatric procedures, then every 3 to 6 months until 12 months, and then annually thereafter for all patients (Grade B; BEL 2). Treatment regimens include oral ferrous sulfate, fumarate, or gluconate to provide up to 150 to 200 mg of elemental iron daily (Grade A; BEL 1). Vitamin C supplementation may be added simultaneously to increase iron absorption (Grade C; BEL 3). IV iron infusion (preferably with ferric gluconate or sucrose) may be needed for patients with severe intolerance to oral iron or refractory deficiency due to severe iron malabsorption (Grade D).
R60. (2019*). Baseline and annual evaluation for vitamin B12 deficiency should be performed in all patients after bariatric surgery (Grade B; BEL 2). More frequent aggressive case finding (e.g., every 3 months) should be performed in the first postoperative year, and then at least annually or as clinically indicated for patients who chronically use medications that exacerbate the risk of B12 deficiency: nitrous oxide, neomycin, metformin, colchicine, proton-pump inhibitors, and seizure medications (Grade B, BEL 2). Since serum B12 may not be adequate to identify B12 deficiency, consider measuring serum methylmalonic acid, with or without homocysteine, to identify a metabolic deficiency of B12 in symptomatic and asymptomatic patients and in patients with a history of B12 deficiency or preexisting neuropathy (Grade B, BEL 2). Oral supplementation (via disintegrating tablet, sublingual, or liquid) with crystalline vitamin B12 at a dosage of 350 to 1,000 µg daily or more is recommended to maintain normal vitamin B12 levels (Grade A; BEL 1). Intranasally administered vitamin B12 may also be considered (Grade D). Parenteral (intramuscular or subcutaneous) B12 supplementation, 1,000 µg/month to 1,000 to 3,000 µg every 6 to 12 months, is indicated if B12 sufficiency cannot be maintained using oral or intranasal routes (Grade C; BEL 3).
R61. (2013). Folic acid supplementation (400 to 800 µg/d) should be part of a routine multivitamin-multimineral preparation (Grade B; BEL 2) and must be supplemented further (1,000 µg/d) when a deficiency state is suspected (e.g., with skin, nail, or mucosal changes) or found, as well as in all women of childbearing age (800 to 1,000 µg/d) to reduce the risk of fetal neural tube defects (Grade A; BEL 1). B12 status should be assessed in patients on higher-dose folic acid supplementation (> 1,000 µg/d) to detect a masked B12 deficiency state (Grade D).
R62. (2013). Nutritional anemias resulting from malabsorptive bariatric procedures can involve deficiencies in vitamin B12, folate, protein, copper, selenium, and zinc and may be evaluated when routine aggressive case finding for iron-deficiency anemia is negative (Grade C; BEL 3).
R63. (2013). There is insufficient evidence to support routine selenium screening or supplementation after a bariatric procedure (Grade D). However, selenium levels may be checked as part of aggressive case finding in patients with a malabsorptive bariatric surgical procedure who have unexplained anemia or fatigue, persistent diarrhea, cardiomyopathy, or metabolic bone disease (Grade C; BEL 3).
R64. (2019*). Zinc supplementation should be included as part of a routine multivitamin-multimineral preparation with 8 to 22 mg/d to prevent a deficiency state; the amount indicated varies depending on the bariatric procedure performed, with greater amounts required for RYGB and BPD/DS (Grade C; BEL 3). Routine aggressive case finding for zinc deficiency utilizing serum and plasma zinc determinations should be performed after malabsorptive bariatric surgical procedures (RYGB and BPD/DS) (Grade C; BEL 3), and zinc deficiency should also be considered in any patient after a bariatric procedure with chronic diarrhea, hair loss, pica, significant dysgeusia, or in male patients with unexplained hypogonadism or erectile dysfunction (Grade D). Treatment of zinc deficiency should target normal biochemical levels with 1 mg/d of copper also supplemented for every 8 to 15 mg/d of elemental zinc provided (Grade D).
R65. (2019*). Routine aggressive case finding for copper deficiency using serum copper and ceruloplasmin may be considered for all patients who have undergone RYGB or BPD/DS at least annually, even in the absence of clinical signs or symptoms of deficiency (Grade C, BEL 3), but especially in patients who are experiencing anemia, neutropenia, myeloneuropathy, or impaired wound healing (Grade D). Copper supplementation (2 mg/d) should be included as part of a routine multivitamin-multimineral preparation; further supplementation varies depending on the surgical procedure performed, with greater amounts required for patients who have had RYGB or BPD/DS (Grade D). In severe deficiency, treatment can be initiated with IV copper (3 to 4 mg/d) for 6 days (Grade D). Subsequent treatment of severe deficiency, or treatment of mild-to-moderate deficiency, can usually be achieved with 3 to 8 mg/day of oral copper sulfate or gluconate until levels normalize and symptoms resolve (Grade D). Patients being treated for zinc deficiency or using supplemental zinc for hair loss should receive 1 mg of copper for each 8 to 15 mg of elemental zinc, since zinc replacement can cause copper deficiency (Grade C; BEL 3). Copper gluconate or sulfate is the recommended source of copper for supplementation (Grade C; BEL 3).
R66. (2019*). Thiamine (vitamin B1) supplementation above the recommended dietary allowance is suggested to prevent thiamine deficiency (Grade D). Routine thiamine screening may be considered following bariatric procedures (Grade C; BEL 3). Aggressive case finding for thiamine deficiency and/or empiric thiamine supplementation is indicated for high-risk postprocedure patients, such as those with established preprocedure risk factors for thiamine deficiency, females, African Americans, patients not attending a nutritional clinic, patients with GI symptoms, patients with heart failure, protracted vomiting, PN, excessive alcohol use, neuropathy or encephalopathy (Grade C; BEL 3), or small intestinal bacterial overgrowth (SIBO) (Grade C; BEL 3). All post-WLS patients should take at least 12 mg of thiamine daily (Grade C; BEL 3). A 50- to 100-mg daily dose of thiamine from a B-complex supplement or high-potency multivitamin may be needed to maintain sufficient blood levels of thiamine and prevent thiamine deficiency in some patients (Grade D). Patients with severe thiamine deficiency (suspected or established) should be treated with IV (or intramuscular if IV access is not available) thiamine, 500 mg/d, for 3 to 5 days, followed by 250 mg/d for 3 to 5 days or until resolution of symptoms, and then to consider treatment with 100 mg/d, orally, usually indefinitely or until risk factors have resolved (Grade C; BEL 3). Mild deficiency can be treated with IV thiamine, 100 mg/d, for 7 to 14 days (Grade C; BEL 3). In patients with recalcitrant or recurrent thiamine deficiency with one of the above risks, the addition of antibiotics for SIBO should be considered (Grade C; BEL 3).
R67. (NEW). Commercial products that are used for micronutrient supplementation need to be discussed with a health care professional (HCP) familiar with dietary supplements, since many products are adulterated and/or mislabeled (Grade D).
R68. (2013*). Lipid levels and the need for lipid-lowering medications should be periodically evaluated (Grade D). The effect of weight loss on dyslipidemia is variable and incomplete; therefore, lipid-lowering medications should not be stopped unless clearly indicated (Grade C; BEL 3).
R69. (2019*). The need for antihypertensive medications should be evaluated repeatedly and frequently during the active phase of weight loss (Grade D). Because the effect of weight loss on blood pressure is variable, incomplete, and at times transient, antihypertensive medications should not be stopped unless clearly indicated; however, dosages may need to be titrated downward as blood pressure improves (Grade D).
R70. (NEW). Close attention to dosing of diabetes medication is recommended for those having had SG, RYGB, or BPD/DS, since these patients generally have dosing reduced in the early postoperative period, whereas those having had LAGB require significant weight loss before dosing must be reduced (Grade B; BEL 2). Patients with T2D who had their diabetes medication stopped after bariatric procedures must be monitored closely for recurrence of hyperglycemia, particularly with weight regain or suboptimal weight loss (Grade B; BEL 2).
R71. (NEW). In patients on thyroid hormone replacement or supplementation, TSH levels must be monitored after bariatric procedures and medication dosing adjusted, as dose reductions are more likely with weight loss but can increase with malabsorption (Grade B; BEL 2). Oral liquid forms of levothyroxine may be considered in those patients who have difficulty swallowing tablets after bariatric procedures (Grade D). Oral liquid or softgel forms of levothyroxine may be considered in patients with significant malabsorption in whom adequate TSH suppression to normal ranges is difficult after bariatric procedures (Grade C; BEL 3).
R72. (2019*). Persistent and severe GI symptoms (e.g., nausea, vomiting, abdominal pain, diarrhea, and constipation) warrant evaluation utilizing a pertinent history and physical exam, appropriate laboratory testing, and imaging (most commonly CT and/or upper GI series) (Grade C; BEL 3). Upper endoscopy with small-bowel biopsies and aspirates remains the gold standard and should be part of the evaluation of celiac disease and bacterial overgrowth in patients who have had a bariatric procedure (Grade C; BEL 3). Screening with a stool specimen should be obtained if the presence of Clostridium difficile colitis is suspected (Grade C; BEL 3). Persistent steatorrhea after BPD without/with DS should prompt evaluation for nutrient deficiencies (Grade C; BEL 3).
R73. (NEW). Patients with de novo gastroesophageal reflux and severe symptoms after SG should be treated with proton-pump inhibitor therapy, and those recalcitrant to medical therapy considered for conversion to RYGB (Grade C; BEL 3).
R74. (2019*). Nonsteroidal anti-inflammatory drugs (NDAIDs) should be avoided after bariatric procedures, if possible, because they (and steroids to a lesser extent) have been implicated in the development of anastomotic ulcerations, perforations, and leaks (Grade C; BEL 3); ideally, alternative pain medication should be identified before the bariatric procedure (Grade D). If the use of NSAIDs is unavoidable, then the use of proton-pump inhibitors may be considered (Grade C; BEL 3).
R75. (2019*). Endoscopy is safe and should be the preferred procedure to evaluate GI symptoms suggestive of stricture or foreign body (e.g., suture or staple), as it can be both diagnostic and therapeutic (e.g., endoscopic dilation or foreign body removal) (Grade C; BEL 3). Endoscopy may also be used for Helicobacter pylori testing as a possible contributor to persistent GI symptoms after bariatric procedures (Grade D).
R76. (NEW). Anastomotic ulcers after bariatric procedures should be treated with proton-pump inhibitors; prophylactic therapy with proton-pump inhibitors should be considered for 90 days to 1 year, depending on risk (Grade B; BEL 2). H2 receptor blockers and sucralfate may also be considered for postprocedure anastomotic ulcers, and if Helicobacter pylori is identified, triple therapy, including antibiotics, bismuth, and proton-pump inhibitors, may be used (Grade C; BEL 3).
R77. (2013*). Patients who have undergone RYGB with a nonpartitioned stomach and developed a gastro-gastric fistula with symptoms (e.g., weight regain, marginal ulcer, stricture, or gastroesophageal reflux) may be considered for a revisional procedure (Grade C; BEL 3).
R78. (2019*). Persistent vomiting, regurgitation, and upper-GI obstruction after LAGB should be treated with immediate removal of fluid from the adjustable band (Grade D). Persistent symptoms of gastroesophageal reflux, regurgitation, chronic cough, or recurrent aspiration pneumonia in a patient after LAGB raise concern for band slippage, esophageal dilation, and, in some cases, erosion, and should prompt evaluation of the patient with upper-GI endoscopy or fluoroscopy (Grade C; BEL 3), immediate referral to a bariatric surgeon, and depending on the clinical course, consideration of conversion to SG or RYGB (Grade D).
R79. (2019*). Ultrasound should be used to evaluate patients with right upper-quadrant pain for cholecystitis (Grade D). Patients who undergo SG, RYGB, or BPD/DS are at increased risk for cholelithiasis due to rapid weight loss, and oral administration of ursodeoxycholic acid is recommended: 500 mg once daily for SG and 300 mg twice a day for RYGB or BPD/DS (Grade A; BEL 1). In asymptomatic patients with known gallstones and a history of RYGB or BPD/DS, prophylactic cholecystectomy may be considered to avoid choledocholithiasis, since traditional endoscopic retrograde cholangiopancreatography can no longer be performed in these patients. Otherwise, cholecystectomy should be reserved for patients with symptomatic biliary disease due to a generally low incidence of biliary complications. (Grade B; BEL 2).
R80. (2013*). Although uncommon, suspected SIBO in the biliopancreatic limb after BPD/DS may be treated empirically with metronidazole or rifaximin (Grade C; BEL 3). For antibiotic-resistant cases of bacterial overgrowth, probiotic therapy with Lactobacillus plantarum 299v and/or Lactobacillus GG may be considered (Grade D). Thiamine deficiency may be suspected in patients with SIBO after bariatric procedures, especially when gut dysmotility occurs (Grade C; BEL 3).
R81. (2008*). Definitive repair of asymptomatic abdominal wall hernias can be deferred until weight loss has stabilized and nutritional status has improved to allow for adequate wound healing (12 to 18 months after bariatric surgery) (Grade D). Symptomatic hernias that occur after bariatric surgery may require prompt surgical evaluation (Grade C; BEL 3). Patients with sudden-onset of severe cramping, periumbilical pain, or recurrent episodes of severe abdominal pain any time after bariatric surgery should be evaluated with an abdominal and pelvic CT scan to exclude the potentially life-threatening complication of a closed-loop bowel obstruction (Grade D). Exploratory laparotomy or laparoscopy is indicated in patients who are suspected of having an internal hernia because this complication can be missed with upper-GI x-ray studies and CT scans (Grade C; BEL 3).
R82. (2013*). Body-contouring surgery may be performed after bariatric procedures to manage excess tissue that impairs hygiene, causes discomfort, and is disfiguring (Grade C; BEL 3). Body-contouring surgery is best pursued after weight loss has stabilized (12 to 18 months after bariatric surgery) (Grade D).
Q7. What are the criteria for hospital admission after a bariatric procedure?
R83. (2013). Severe malnutrition or hypoglycemia after a bariatric procedure should prompt hospital admission (Grade D). The initiation and formulation of EN (tube feeding) or PN should be guided by current CPGs (Grade D). Hospital admission is required for the management of GI complications after bariatric procedures in clinically unstable patients (Grade D). Surgical management should be pursued for GI complications not amenable or responsive to medical therapy (Grade D). However, if not dehydrated, patients may undergo endoscopic stomal dilation for stricture as an outpatient procedure (Grade D).
R84. (2008). Revision of a bariatric surgical procedure can be recommended when serious complications related to previous bariatric surgery cannot be managed medically (Grade C; BEL 3).
R85. (2008). Reversal of a bariatric surgical procedure is recommended when serious complications related to previous bariatric surgery cannot be managed medically and are not amenable to surgical revision (Grade D).
Updated evidence base for 2019
This evidence base pertains to the 7 questions and 85 updated numbered recommendations. There are 858 citations, of which 81% were published in 2013 or later, with 81 (9.4%) EL 1, 562 (65.5%) EL 2, 72 (8.4%) EL 3, and 143 (16.7%) EL 4, compared with 32 (7.9%) EL 1, 129 (32%) EL 2, 173 (43%) EL 3, and 69 (17.1%) EL 4 in the 2013 AACE/TOS/ASMBS CPG and 13 (1.7%) EL 1, 112 (14.4%) EL 2, 460 (59.2%) EL 3, and 192 (24.7%) EL 4 in the 2008 AACE/TOS/ASMBS CPG. There is a relatively high proportion (75%) of strong (EL 1 and 2) studies, compared with 40% in the 2013 AACE/TOS/ASMBS CPG and only 16% in the 2008 AACE/TOS/ASMBS CPG. The primary evidence base, supporting tables, and unrevised recommendations for general information are not provided in this document and may be found in the 2008 (54) and 2013 AACE/TOS/ASMBS CPG (1). Readers are strongly encouraged to review these past CPGs to place the updated explanations and references into better context. The technical evidence ratings for these updated references are found in the reference section of this document, appended at the end of each citation.
Q1. Which patients should be offered bariatric procedures?
R1. (2019*). Mortality rates, the risk and prevalence of ORCs conferring disease morbidity, and social costs of obesity are highest in those patients with class-III severe obesity (i.e., BMI ≥ 40 kg/m2) (56-58). The evidence base for recommending bariatric surgery for patients with BMI ≥ 40 kg/m2 without co-existing medical problems or severe ORCs is supported by recent studies demonstrating benefit with respect to reduced mortality (32, 38, 58-63), improvements in CVD risk factors (33, 38, 64), reduced rates of some cancers (65-67), substantial weight loss that is persistent in most patients (38, 58, 62, 63, 68-71), diabetes prevention (72-74), improved pulmonary function (75), and better mobility and quality of life (76-78). Currently, the WHO classification scheme for obesity determines diagnostic and therapeutic management based on BMI. However, BMI is a surrogate measure of adipose tissue mass, is confounded by ethnic differences and aspects of body composition (79-83), and does not provide information regarding the impact of excess adiposity on the health of the patient (13). Improved risk stratification strategies for bariatric surgery involving patients with BMI ≥ 40 kg/m2 may incorporate the risk, presence, and severity of ORCs (13, 19, 84), the functional status of the patient, and body-composition technologies (83) to more precisely evaluate the mass and distribution of adipose tissue (79, 80, 85). The benefits of bariatric procedures must be balanced against the inherent risks of complications and mortality, potential nutritional deficiencies, weight regain in some patients, and the need for lifelong lifestyle support and medical care. Factors found to be associated with poor outcome include open procedures, male gender, older age, congestive heart failure, peripheral vascular disease, DVT, PE, OSA, impaired functional status, chronic kidney disease, and suicidality (86, 87). Therefore, further studies are needed that utilize clinical risk-stratification systems to optimize patient selection criteria in patients with BMI ≥ 40 kg/m2 who do not have severe complications and that evaluate consequent patient outcomes.
R2. (2019*). Bariatric procedures can prevent and/or ameliorate ORCs that are responsive to weight loss, and these clinical benefits augment the benefit-risk ratio of the procedure and the salutary effects on the health of the patient. The strength of evidence for efficacy of bariatric procedures to ameliorate ORCs varies according to the complication. As described below, there exists strong evidence to support bariatric procedures in the prevention and/or treatment of several ORCs. Specifically, interventional cohort studies and randomized clinical trials (RCTs) have demonstrated clinical benefits in patients with BMI ≥ 35 kg/m2 and the following complications: T2D (31, 36, 40, 42, 88-90), high risk for T2D (prediabetes and/or MetS) (72, 73, 91-94), poorly controlled HTN (88, 95-97), NAFLD/NASH (98-104), OSA (105-110), OA of the knee or hip (111-116), and improving outcomes of knee or hip replacement (114, 116-119) and urinary stress incontinence (120-123).
Several other comorbidities may be ameliorated by bariatric procedures, although the evidence is weaker, often consisting of case reports and case series; these comorbidities include obesity-hypoventilation syndrome and Pickwickian syndrome after a careful evaluation of operative risk (75, 124, 125), idiopathic intracranial HTN (126-130), GERD preferentially employing RYGB (13, 110, 131-136), severe venous stasis disease (137, 138), impaired mobility due to obesity (77, 78, 139), and considerably impaired quality of life (77, 78, 139).
Clinical benefits with BMI ≥ 35 kg/m2
T2D. Bariatric surgery can be considered in patients with T2D when the BMI is ≥ 35 kg/m2, especially if diabetes is difficult to control with lifestyle and pharmacologic therapy (1, 31, 36, 40, 42, 88-90, 140). The Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial is a randomized controlled single-center study comparing outcomes of intensive medical therapy alone versus intensive medical therapy plus RYGB or SG (34, 88, 141). One-, 3-, and 5-year outcomes showed that a significantly higher percentage of patients after bariatric surgery met the primary end point of A1C ≤6% (≤42 mmol/mol), which was associated with a decrease in the number of diabetes medications when compared to the patients treated by medical therapy alone. These data underscore the effectiveness of bariatric surgery but should be interpreted cautiously when comparing medical and bariatric approaches because glycemic control in the medically treated patients was not optimal, and the study did not include a weight-loss arm using intensive lifestyle/behavior therapy plus weight-loss medications. The Swedish Obese Subjects study is a nonrandomized, prospective, controlled study in 4,047 patients with obesity who underwent bariatric surgery or received conventional treatment (31, 94). In a subgroup analysis of 343 patients with T2D at baseline, bariatric surgery brought 72% into remission (i.e., blood glucose ≤110 mg/dL on no diabetes drugs) compared with 16% in remission in medically treated controls at 2 years, decreasing to 30% in remission versus 7% in controls at 15 years (31). Additional trials and cohort studies have demonstrated clinical benefits of bariatric surgery in T2D (40, 89, 142-146).
Meta-analyses that include RCTs, nonrandomized interventional trials, and/or single-arm observational studies concluded that bariatric surgical procedures led to T2D remission rates of 60 to 66% (37, 147-150), with an order of effectiveness as follows: BPD/DS > RYGB ≥ SG > LAGB (149). The relative effectiveness of individual procedures producing T2D remission is not entirely clear, since some studies favor RYGB over SG (149, 151, 152) and many others conclude that these procedures are equally effective (153-156). Many (149, 151, 157) but not all (152, 153) studies indicate that greater degrees of weight loss following surgery are more likely to result in T2D remission. One study found that a composite scoring system (e.g., age, BMI, C-peptide level, and duration of T2D) predicted response in glycemic markers to bariatric surgery (158). In another study, higher baseline BMI was associated with a greater improvement in T2D after RYGB (159). In any event, “remission” is the proper terminology as opposed to “cure,” since overt T2D returns in over half of these patients in less than 10 years (31). Bariatric surgery must be balanced against the inherent risks of surgical complications and mortality, potential nutritional deficiencies, weight regain in some patients, and the need for lifelong lifestyle support and medical monitoring (1, 157, 160, 161).
Prediabetes, MetS, and T2D Prevention. Rates of incident T2D were reduced following a variety of bariatric surgical procedures (72, 73, 91-93, 155, 161). In two studies, bariatric surgery led to a 76 to 80% reduction in rates of T2D (72, 73), which was similar to the degree of prevention when lifestyle intervention (162) and/or weight-loss medications (163, 164) achieved 10% weight loss, even though bariatric surgery produced greater weight loss than observed with lifestyle and pharmacotherapy. These combined data suggest that 10% weight loss will reduce the risk of future T2D by ~80%, and this represents a threshold above which further weight loss will not result in additional preventive benefits.
HTN. Bariatric surgery is effective in lowering blood pressure in patients with obesity. This has been demonstrated in multiple uncontrolled interventional cohort studies (165, 166), controlled clinical trials (95, 96, 167-172), RCTs (88, 146, 173, 174), and in meta-analyses (36, 97, 175). Bariatric surgery promotes weight loss and lowering of blood pressure across all levels of obesity, as demonstrated by systematic reviews in class-I (36, 175) and class-II (175) obesity and in patients with severe obesity and BMI > 50 kg/m2 (176). When different bariatric surgical approaches are compared, patients experiencing greater weight loss generally have better outcomes regarding blood pressure and HTN (167, 175). Analysis of the Bariatric Outcomes Longitudinal Database found that HTN was better resolved after BPD/DS, compared with SG or RYGB (177). Beneficial effects of bariatric surgery in patients with HTN are maintained long term in many but not all patients (50, 178). In the Longitudinal Assessment of Bariatric Surgery multicenter observational cohort study, HTN was present in 68% of 2,458 subjects with obesity (median BMI 45.9 kg/m2) (50). After 3 years, HTN remained in remission in 269 of 705 patients (38%) undergoing RYGB (weight loss 31.5%) and 43 of 247 patients (17%) who had LAGB (weight loss 15.9%) (50). Effects of SG to produce complete remission of HTN in a retrospective cohort study occurred in 46% of patients at year 1, 48% at year 3, and 46% at year 5 (178).
T1D. There are limited data on the effects of bariatric or metabolic procedures on T1D. In a 2018 meta-analysis by Hussain (179), only 9 studies (N = 78 patients) demonstrated improvements in A1C, insulin dosing, and BMI. Improvements in diabetes management were not exclusively related to excess weight loss, arguing for roles of other factors. More data are needed to better define a role for GI procedures in the management of T1D.
NASH. In patients with NAFLD and NASH, bariatric surgery results in reductions in liver fat and improvements in histologic manifestations of liver injury, inflammation, and fibrosis (98-104, 180-182). In 39 patients undergoing RYGB, a postoperative weight loss of 50 kg over 18 months led to marked improvements in histologic steatosis, hepatocellular ballooning, centrilobular fibrosis, lobular inflammation, and the fibrosis stage (98). Nineteen patients with biopsy-proven NASH at the time of RYGB lost 40% total body weight after 21 months, and repeat biopsy demonstrated marked improvements in histologic steatosis, lobular inflammation, and portal and lobular fibrosis (99). Importantly, histopathologic criteria for NASH were no longer present in 89% of patients. Mummadi et al. (100) conducted a meta-analysis of 15 interventional studies that included 766 paired liver biopsies; the reductions in BMI after bariatric surgeries ranged from 19.11 to 41.76%, and the pooled proportion of patients with improvement or resolution in steatosis was 91.6%, steatohepatitis 81.3%, fibrosis 65.5%, and for complete resolution of NASH, 69.5%. Bariatric surgery has been observed to result in long-term reductions in liver transaminases in the Swedish Obese Subjects study, consistent with persisting salutary effects in NAFLD (104). Transient deterioration in liver function has also been observed following bariatric surgery in some patients with NASH (101).
OSA. Weight loss of ~10% or more can improve OSA as assessed by polysomnography and the apnea-hypopnea index (AHI) (183). Multiple trials assessing the efficacy of bariatric surgery have demonstrated efficacy for improvements in symptomatology and AHI scores in patients with OSA (105-110, 184). For example, bariatric surgery resulting in 27 to 47% weight loss produced a 49 to 98% reduction in the AHI (107). In another study, LAGB resulted in 20.2% weight loss and 54% improvement in sleepiness scores (99). Dixon et al. (183) found that LAGB was effective but not superior to conventional weight-loss programs in patients with OSA as measured by the AHI score.
OA. Multiple studies have demonstrated that bariatric surgery can reduce pain and improve function in patients with OA (112, 113, 185-187). In 59 consecutive patients followed prospectively after bariatric surgery, there was a significant increase in medial joint space on knee x-ray and clear improvements in the Knee Society Score (186). A meta-analysis of studies assessing effects of bariatric surgery on OA included 13 studies and 3,837 patients, but only 2 studies had a control group, and 11 were uncontrolled prospective studies (113). All studies measuring intensity of knee pain, knee physical function, and knee stiffness showed a significant improvement after bariatric surgery, with weight loss ranging from 14.5 to 35.2%. The quality of evidence was considered low for most of the included studies and moderate for one study. A case-control study by Peltonen et al. (112) that included patients who underwent bariatric surgery enrolled in the Swedish Obese Subjects study was the one deemed to be of moderate quality in this meta-analysis. Weight loss associated with bariatric surgery was associated with a significant improvement in pain, including work-restricting pain, in knees and ankles of men and women, with odds ratios (ORs) of 1.4 to 4.8 (112). A second systematic review of the literature in patients with obesity undergoing bariatric surgery (187) identified six studies for analysis; five were case series and one was the case-controlled trial by Peltonen et al. (112). All studies demonstrated improvements in pain, functional scores, and/or joint space width, resulting in a conclusion by these authors that bariatric surgery can benefit patients with knee and hip OA, but recognized the need for further investigation with RCTs.
Obesity is associated with higher rates of treatment involving arthroplasty or knee and hip replacement (188). The evidence base addressing efficacy and safety of knee replacement consists of observational and retrospective analyses. Patients with obesity undergoing total knee replacement can experience significant improvements in pain and functionality, often assessed using the Knee Society Score, the Western Ontario and McMaster Universities Osteoarthritis Index, or other instruments (117-119, 189, 190). However, knee replacement surgery in patients with obesity is more often associated with complications such as deep prosthetic infections, wound healing, superficial infections, and DVT (117-119, 189, 190). Patients with severe obesity can experience inferior survival of the prosthesis after total knee replacement compared with patients without obesity (114-116), although this was not consistently observed (190, 191). For these reasons, weight loss is recommended both before and after knee replacement surgery in patients with overweight and obesity. Many centers require the BMI to be below a specified threshold (e.g., < 35 to 40 kg/m2) before arthroplasty is entertained (192), although this is controversial (193). Bariatric surgery can therefore be used to reduce BMI to a level that will permit arthroplasty.
Urinary stress incontinence. Interventional cohort studies employing bariatric surgery have demonstrated improvements in urinary incontinence (120-122, 194-196). A systematic review identified five interventional cohort studies involving bariatric surgery, all of which reported improvements in stress incontinence symptoms in the clear majority of patients (123). In one such study, RYGB in 1,025 patients (78% women) produced a decrease in mean BMI from 51 kg/m2 to 33 kg/m2 and a decrease in urinary incontinence from 23% of the patients affected at baseline to only 2% of patients 1 to 2 years postoperatively (121).
R3. (2019*). Since 2013, there is increasing evidence from RCTs and meta-analyses regarding the metabolic benefits of bariatric procedures in patients with BMI of 30 to 34.9 kg/m2 (i.e., class-I obesity). With respect to weight loss per se, multiple studies (40, 197, 198) document efficacy in patients with class-I obesity. As a result, the FDA-approved LAGB for patients with a BMI of 30 to 34.9 kg/m2 with an ORC. However, the preponderance of studies in patients with class-I obesity have focused on the clinical benefits of bariatric procedures in those patients with T2D. A substantial number of RCTs and cohort interventional trials have demonstrated that bariatric surgical procedures can effectively result in sustained improvement in glycemic control concomitant with reductions in diabetes medications in patients with BMI 30 to 34.9 kg/m2 (42, 88, 90, 159, 173, 199-207). Multiple meta-analyses that specifically examined bariatric surgery outcomes in patients with BMI < 35 kg/m2 have been published and support clinical benefits regarding glycemic control and weight loss (36, 208-210). In patients with T2D and class-I obesity, bariatric surgery can also lead to improvements in blood pressure and dyslipidemia (36). Importantly, a significant number of patients will experience remission of T2D with maintenance of normal or near-normal blood glucose values in the absence of diabetes medications (88, 141, 173, 200, 207, 210-214).
The STAMPEDE trial randomized patients with T2D and BMI 27 to 43 kg/m2 to medical therapy or to RYGB or SG with the primary end point being A1C ≤6% (≤42 mmol/mol) on or off medications. After 1, 3, and 5 years, this outcome was met by 42%, 38%, and 29%, respectively, in the RYGB group, 37%, 24%, and 23% in the SG group, and 12%, 5%, and 5% in patients treated with medical therapy (34, 88, 141). Overall, the patients randomized to bariatric surgery maintained lower A1C with fewer diabetes medications, improved lipids, and better quality of life than the medically treated patients. Nevertheless, the STAMPEDE trial indicates that, while remission rates can be higher in the immediate years following surgery, over time, T2D tends to recur consistent with the progressive nature of the disease. In the Swedish Obese Subjects study, remission of T2D was observed to be 72% at 2 years, falling to 30% at 15 years, compared with 16% and 7%, respectively, in matched controls (31). Shorter-duration T2D is associated with a higher likelihood of remission in both mild (210) and severe (31) obesity.
Because of increasing evidence, the second Diabetes Surgery Summit Consensus Conference guidelines recommend that bariatric surgery be considered for BMI 30 to 34.9 kg/m2 in patients with T2D (210). It will be important to continue to follow these patients long term to determine the lifelong impact of bariatric surgery on metabolic status and CVD risk. A rigorous definition of “T2D remission” should be standardized and applied across studies (215), and the a priori predictors for efficacy of T2D remission will need to be better defined to optimize the benefit-risk ratio of the procedure (216, 217). Finally, with SG now the most common bariatric surgical procedure performed, future studies will need to elucidate the differential impact of multiple current surgical treatments for efficacy and safety. The ongoing DiaSurg2 trial has randomized patients with BMI 26 to 35 kg/m2 and insulin-requiring T2D to RYGB or standard medical therapy (44). The primary end point is a composite time-to-event end point, including cardiovascular death, myocardial infarction, coronary bypass, percutaneous coronary intervention, nonfatal stroke, amputation, and surgery for peripheral atherosclerotic artery disease, with follow-up of 8 years. These and other trials should help better define evidence-based utilization of bariatric surgery in patients with mild obesity.
R4. (NEW). BMI cutoffs for identifying excess adiposity and risk of cardiometabolic disease are lower for some ethnicities and should be taken into account during screening and diagnosis (85, 192, 218). Specifically, a lower BMI threshold for screening of obesity is recommended in South Asian, Southeast Asian, and East Asian adult populations. Based on the evidence that lower BMI values are correlated with risk of T2D, the ADA (81), the WHO Expert Consult Group (219), and the Working Group on Obesity in China (220) recommend that screening for diabetes should be considered for all Asian American adults who present with BMI ≥ 23 kg/m2 and that a BMI cutoff of ≥ 23 kg/m2 would be the optimal single criterion for screening all Asian ethnicities for obesity based upon correlations with cardiometabolic risk factors and increased risk of mortality (82, 220-227). Based on epidemiologic data, the WHO has proposed the following weight classifications in adult Asians: BMI < 18.5 kg/m2 indicates underweight, 18.5 to 22.9 kg/m2 normal weight, 23 to 24.9 kg/m2 overweight, 25 to 29.9 kg/m2 obesity class I, and ≥ 30 kg/m2 obesity class II (219). The prevalence of various ORCs may also vary as a function of region and ethnicity, and this should be considered in the transculturalization application of these guidelines in the evaluation of patients with obesity.
Waist circumference measurements provide additional information regarding risk of cardiometabolic disease and should be measured in all patients, especially when BMI is < 35 kg/m2. Risks conferred by waist circumference are continuous despite the use of categorical cutoff values, and, at any given BMI (above and below 35 kg/m2), risks of T2D and CVD increase progressively with additional increments in waist circumference (228). However, when the BMI exceeds 35 kg/m2, most patients will exceed categorical waist circumference cutoff values by a high BMI whether they are insulin resistant and have cardiometabolic risk factors. Thus, above a BMI of 35 kg/m2, waist circumference cutoff values become less effective in describing cardiometabolic risk. Waist circumference cutoff points for predicting CVD also exhibit ethnic variation, including a consistently lower threshold in South Asian, Southeast Asian, and East Asian adults. Therefore, ethnic-specific cutoffs as advocated in the 2009 Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention should be used. Waist circumference predicted increased risk with values starting at ≥ 84 cm for men and ≥ 74 cm for women in a large Hong Kong cohort, while a value of 85 cm for men and 80 cm for women were recommended as cutoffs for central obesity in Chinese adults, according to the Cooperative Meta-Analysis Group of the Working Group on Obesity in China (220, 229). Waist circumference estimates relative accumulation of visceral adipose tissue relevant to the ABCD model, which incorporates abnormal distribution (in addition to amount and function) of adiposity as an important metric (18).
- Are baseline and target anthropometrics (BMI, weight, excess weight, etc.) determinants of whether a bariatric procedure should be recommended?
- Are ORCs determinants of whether a bariatric procedure should be recommended?
- Should patients with qualifying indications proceed directly to a bariatric procedure or rather proceed only after a trial of more intensive lifestyle change with or without weight-loss medications?
The main purpose of any therapeutic intervention is to improve the health and quality of life of the patient. Morbidity and mortality associated with obesity arise from complications that result from increased adiposity mass, distribution, and/or function (13, 18, 230). BMI provides an indirect anthropometric measure of adipose tissue mass but alone is not sufficient to indicate the health status in patients with obesity (231). The impact of obesity on health is directly related to the risk, presence, and severity of ORCs (13, 231-234). ORCs are wide ranging (13, 231-234) and include problems related to cardiometabolic, biomechanical, and psychological processes. The amount of weight loss that is necessary to predictably prevent or treat ORCs varies as a function of the specific complication profile unique to each patient (231-234). In short, bariatric procedures optimally address health and quality of life when enough weight loss needed to prevent or treat ORCs cannot be obtained using lifestyle or medical therapy alone.
Q2. Which bariatric procedure should be offered?
R6. (2019*). Shifts in procedure preference by bariatric surgeons and their teams reflect an evolution in decision-making based on technical surgical factors, risk-benefit analysis, costs, and other logistics, as well as new surgical and nonsurgical bariatric procedures and an updated knowledge base about mechanisms of action and clinical goals in current obesity care models (Tables 6-8). Unfortunately, there are very few preoperative factors among the wealth of available biochemical and clinical information that are sufficiently predictive of actual weight loss for an individual patient after a specific bariatric procedure. To this point, Courcoulas et al. (235) analyzed data from 2006-2009 in 10 hospitals, extracted over 100 preoperative variables, and found only a few variables with statistically significant predictive power for weight loss: diabetes, kidney function, and tobacco history for RYGB, and band size for LAGB. Additionally, Robinson et al. (236) found that behavioral variables, such as increased dietary adherence and decreased grazing, were associated with greatest weight loss after bariatric surgery. Seyssell et al. (237) developed a predictive model for 5-year weight loss after RYGB and validated the tool with a French cohort of patients. Higher BMI, younger age, and male gender were the best predictors of more weight loss, and this calculator can be used to provide patients with realistic expectations about their long-term weight-loss outcomes after RYGB. The emergence of new information, technology, and clinical trial data on established and emergent procedures will hopefully provide more concrete direction in shaping clinical decision-making and the calculus for selecting specific bariatric procedures. As an example, Samczuk et al. (238) found different molecular pathways affected by SG versus RYGB in patients with obesity and T2D, which in the future can improve the highly sought precision in bariatric procedure selection.
RYGB, once the most performed bariatric procedure, was relegated to the second most performed bariatric procedure in 2015 (239). Specifically, in 2011, RYGB was the most highly performed bariatric procedure at 36.7% and SG third at 17.8% (239). By 2015, these numbers significantly changed, with SG as the dominant bariatric procedure at 53.8% and RYGB second at 23.1% (239). According to an analysis of the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) data registry, SG had approximately half of the risk-adjusted odds of mortality, serious morbidity, and leak in the first 30 days compared with LRYGB (240). The benefits of SG on weight loss were also similar in patients over age 50 years compared with younger patients (241). A novel single-incision laparoscopic SG has also been developed and has comparable mean operative times, hospital length of stay, and complication rates, but better cosmetic results, compared with conventional SG (242). However, in a 2018 report by the National Institute for Health Research, RYGB was found to be the most costly but also the most cost-effective intervention for obesity (BMI ≥ 35 kg/m2) compared with orlistat or weight-management programs, with or without very-low-calorie diets (243). Another swing in the numbers has been the steady decline in the number of LAGB from 35.4% of all bariatric procedures in 2011 to less than 5.7% in 2015 (239). There are also declines in the number of BPD/DS procedures performed, primarily due to the risks involved and decreased number of surgeons trained in this technique (239).
The most recent estimate (2016) of bariatric procedures provided by the ASMBS found that the total number of procedures performed in the United States is 216,000 (18% RYGB, 58% SG, 3.5% LAGB, 1% BPD/DS, and 14% revisions) (244). Notwithstanding the published benefits of LAGB (245), in a meta-analysis, Chang et al. (246) found that LAGB had relatively low complication rates but high re-operation rates, with SG having weight-loss effects comparable with RYGB, which had more complications. The emergence of gastroesophageal reflux as a long-term complication after SG, however, may temper some of the enthusiasm about this procedure or lead to a more tailored approach for these procedures (247).
The laparoscopic greater curvature (gastric) plication (LGP) is an alternative to the SG that is reversible and avoids gastrectomy but has less weight loss at 2 years compared with the conventional SG procedure (248, 249). However, LGP has not gained popularity in the U.S. and is still considered investigational by the ASMBS (250). In addition, when LGP is performed with LAGB (laparoscopic adjustable gastric banded plication [LAGBP]), there is greater weight loss at 36 months and less band slippage (251). In a retrospective, matched control analysis of LAGBP and SG, Cottam et al. (252) found that weight regain started at 1 year with the SG, but not with the LAGBP, which still showed weight stability.
The mini-gastric bypass, or more recently termed single- or one-anastomosis gastric bypass (OAGB), is a simple alternative to RYGB performed with one anastomosis but results in more acid and bile reflux (253, 254). In patients with very high BMI (≥ 60 kg/m2), Parmar et al. (255) found that OAGB achieved greater weight loss at 18 and 24 months compared with RYGB. Moreover, in patients with milder BMI elevations, OAGB with a longer (80 cm) biliopancreatic limb had better T2D remission rates than RYGB (256). In a meta-analysis, Wang et al. (257) found that the OAGB had a great weight reduction effect compared with RYGB. The OAGB is not recommended for patients with GERD or hiatus hernia (253). While it remains a concern, the long-term risk of bile reflux-related adenocarcinoma of the esophagus appears to be small based on the current literature (258). Currently, the OAGB is not an endorsed procedure by the ASMBS because of these and other concerns (259).
A loop (single-anastomosis) duodenal-jejunal bypass with laparoscopic sleeve gastrectomy (LDJB-LSG) has also been developed in China with specific application to patients with mild obesity (ethnicity-adjusted; BMI > 27.5 and < 32.4 kg/m2) and T2D (260). There were comparable benefits in weight loss, glycemic control, insulin resistance, β-cell function, lipids, and uric acid compared with LRYGB (260). Interestingly, the LDJB-LSG affected intestinal microbiota differently than SG alone (261).
Another type of single-anastomosis procedure has also emerged. The one-anastomosis duodenal switch (OADS, also referred to in the literature as single-anastomosis duodeno-ileal bypass with sleeve [SADI-S] or stomach intestinal pylorus-sparing [SIPS] procedure) has been developed as a primary procedure but is still under review by the ASMBS. This procedure involves creating an SG (larger volume than a primary sleeve) with duodenal transection and a loop duodenoileostomy. The length of the efferent alimentary limb (anastomosis to colon) varies from 150 to 300 cm. These procedures have been shown to be safe and as effective as a Roux-en-Y DS with a trend toward fewer nutritional deficiencies at mid-term (3 to 5 years) follow-up (262-271). When compared with LAGB and RYGB, single-anastomosis DS was most effective for weight loss in patients age 70 years and over (272). SIPS surgery has also been used to treat GERD in patients with severe obesity, with (273, 274) and without laparoscopic fundoplication (274). Due to the lack of robust longer-term follow-up, the OADS procedures have not been endorsed by the ASMBS as primary procedures.
The choice of re-operative bariatric surgery depends on the type of primary operation and the indications for re-operation. The ASMBS has developed nomenclature for re-operative bariatric surgery to better characterize this heterogeneous group of procedures (275). Re-operations that result in a new or different type of procedure are considered conversions, operations intended to resolve a complication or anatomic defect are called corrective procedures, and those that attempt to restore normal anatomy are called reversals. In addition to providing additional therapy for weight loss, re-operative procedures have been shown to improve metabolic outcomes, specifically diabetes improvement and remission rates (276, 277). In a study by Boru et al. (278), among high-volume bariatric surgery centers, only 3% of patients having an SG required re-operations.
Salama and Sabry (279) have proposed both OAGB and RYGB as a conversion option for vertical-banded gastroplasty, depending on the pouch length available. The optimal conversion of SG for GERD is RYGB, and conversions for additional weight loss after SG can either be RYGB or DS. Conversion of SG to DS results in greater weight loss than conversion to RYGB but poses a higher risk of long-term nutritional deficiencies. Conversions after LAGB to RYGB or SG can be performed in one or two stages (band removal with interval procedure). Behavioral factors, such as binge-eating, may be responsible for increased risk of poor weight outcomes after re-operation following LAGB (280). Retrospective data suggest a higher leak rate with a single-stage approach, particularly with conversion to SG (275). There are currently very little data to provide evidence-based decision-making for re-operative strategies for RYGB after weight regain. Revision of the gastric pouch and gastrojejunostomy as well as conversion to a distal bypass have been proposed with variable success rates (275).
Many of the new bariatric procedures involve endoscopic disruption of normal physiology and/or the insertion of a device, with variable weight-loss results (262, 264-271, 281, 282). Vagal nerve–blocking device therapy is an FDA-approved surgically implanted medical device that intermittently blocks vagus nerve signaling, impacting both hunger and satiety (281, 283-286). IGB are space-occupying devices inserted into the stomach. The IGB work by occupying space in the stomach, especially when the antrum is involved, thereby limiting capacity and altering gastric motility (17, 281, 287). Three of the products (ReShapeTM, Orbera®, and Obalon®) have been FDA approved for patients with a BMI 30 to 40 kg/m2, age 22 and older (for ReshapeTM: age 22 to 60 years and one comorbidity) (281). IGB have a maximal implantation time of 6 months, with variable amount of fill in the balloon(s) as per product recommendations (281). Using the Orbera® device, the early removal rate was 16.7% (median 8 weeks) associated with use of selective serotonin or serotonin-norepinephrine reuptake inhibitors, and with average weight loss of 8.5% (3 months), 11.8% (6 months), and 13.3% (9 months) and significant reduction of lipid and glycemic status markers at 6 months (288). Other balloon products (e.g., BioEnterics® and End-ball® [nonadjustable] (289, 290), Spatz Balloon® [adjustable], and Elipse Balloon® [a procedureless device that is swallowed]) are not FDA approved at this time but function similarly as other space-occupying devices within the stomach. Medications that reduce nausea and production of gastric acid are frequently used concomitantly (291-293). Common complications include abdominal discomfort, balloon deflation, and late intolerance (294). Rare complications such as gastric perforation, erosive esophagitis, and acute pancreatitis support regular follow-up and appropriate timing for device removal (292, 295-297). The FDA issued a communication to HCP, informing them of five reported deaths since 2016 that occurred unexpectedly in patients who had been treated with fluid-filled IGB, though root causes of these deaths are not yet available (24, 298).
Aspiration therapy is an endoluminal device that can eliminate gastric content through a gastrostomy (17). This “A-tube” is inserted endoscopically and has FDA approval for patients with a BMI of 35 to 55 kg/m2 (17). Mechanism of action is primarily through the postprandial elimination of 25 to 30% of the consumed meal but may also include behavioral changes (17).
Primary obesity surgery endoluminal (299) and endoscopically sutured gastroplasty (ESG) (300-303) are two endoscopic procedures that are safe and alter the anatomy of the stomach to limit the capacity for intake (304). In a single-center retrospective cohort study by Novikov et al. (302), ESG achieved 12-month weight-loss amounts (13.3% total body weight loss) between SG and LAGB but had lower morbidity rates and hospital lengths of stay than the other procedures. Other endoscopic bariatric and metabolic devices/procedures being developed include small-bowel therapy such as the duodenal-jejunal bypass liner (305-310) and duodenal mucosal resurfacing (311), as well as transoral gastroplasty, transoral endoscopic restrictive implant system, articulating circular endoscopic stapler, gastric botulinum toxin A injection, endoscopic sclerotherapy, and radiofrequency ablation (304).
Clinical decision-making regarding the selection of an appropriate bariatric procedure depends not only on a stipulated target weight loss and therefore indirect effects to manage specific ORCs but also the direct effects of the procedure on those specific complications (13, 312). Cardiometabolic risks such as dysglycemia, HTN, and dyslipidemia qualify as these strategic targets (313). Hence, a joint statement by several international diabetes organizations indicates that metabolic surgery should be recommended to treat T2D in patients with class-III obesity (BMI > 40 kg/m2) and in those with class-II obesity (BMI 35.0 to 39.9 kg/m2) when hyperglycemia is inadequately controlled by lifestyle and optimal medical therapy (29). Surgery should also be considered for patients with T2D and BMI 30.0 to 34.9 kg/m2 if hyperglycemia is inadequately controlled despite treatment with either oral or injectable medications (29).
More recent data (217) indicate procedure-specific recommendations based on the severity of T2D utilizing an individualized metabolic surgery (IMS) score and risk-benefit analysis. Based on the IMS score, which classifies T2D as mild, moderate, or severe (according to predictors of long-term remission, such as preoperative number of T2D medications, insulin use, duration of T2D, and glycemic control), SG was the preferred bariatric procedure for patients with a higher risk profile. Aminian et al. (217) recently published a calculator to predict 5-year T2D remission rates after SG based on the severity of the disease at the time of surgery. The findings were validated with data from another institution, and the study concluded that early T2D remission rates were high with either procedure (but favored RYGB); patients with moderately severe diabetes had significantly higher 5-year remission rates compared to SG, and those with severe, long-standing diabetes at the time of surgery had equally low remission rates after both procedures. While there are other factors that should be considered regarding procedure choice (NSAID use, inflammatory bowel disease, GERD, or organ transplant), this calculator is a valuable tool to be used as part of the informed consent and education process for those patients with diabetes at the time of a bariatric procedure (217). Additionally, Haskins et al. (314) reported a small increased risk in 30-day morbidity and mortality among smokers (compared with nonsmokers) after SG. RYGB was the bariatric surgery of choice for patients with GERD or Barrett’s esophagus. Sudan and Jain-Spangler found that SG and RYGB were associated with higher resolution of GERD compared with BPD/DS (177, 315). Of note, Casillas et al. (316) studied 48 patients undergoing conversion of SG to RYGB for reflux, highlighting the importance of reflux as a specific ORC in the determination of a best surgical procedure.
Further recommendations for the SG were endorsed by expert surgeons at the Fifth International Consensus Conference, including a stand-alone procedure in high-risk patients, kidney and liver transplant candidates, MetS, BMI 30 to 35 kg/m2 with associated comorbidities, inflammatory bowel disease, and the elderly (317).
There are no data available to guide definitive recommendations for referral to a regional or national center. However, bariatric surgery programs accredited through the MBSAQIP must meet criteria for patient acuity based on the accredited level of practice. At present, all centers should be available to manage any patient requiring services based on the level of accreditation. Patients beyond the scope of accreditation should be referred to a center with appropriate accreditation. Specifically, patients age ≥ 65 years, males with a BMI > 55 kg/m2 and females with a BMI > 60 kg/m2, patients with organ failure, organ transplant, or significant cardiac or pulmonary impairment, patients on a transplant list, and nonambulatory patients should be referred to an accredited comprehensive center. Patients < 18 years of age should be referred to a center accredited for adolescents (318). Improvements in overall clinical outcomes have been, at least in part, attributed to facility accreditation (319) (though Doumouras et al. found no association in a Canadian cohort), and despite longer travel times, centralization of care to these accredited facilities has actually improved access, particularly among underserved populations (320).
Decisions regarding bariatric procedures should also be based on safety concerns regarding specific organ systems. In general, the greater the inherent risk of a specific bariatric procedure, independent of the risk of not treating obesity and severity of ORCs, the less complicated procedure is selected (321). In addition, preoperative estimation of the likelihood that a patient will experience a cardiac complication at the time of noncardiac surgery can guide procedure selection and prevent postoperative morbidity and mortality. In addition to the history, physical examination, and 12-lead electrocardiogram, several risk assessment tools are available for risk stratification. These include the Revised Cardiac Risk Index (322-324) and the Gupta Myocardial Infarction (325) or Cardiac Arrest Calculator (326). The Revised Cardiac Risk Index (322-324) includes six independent prognostic factors: (1) high-risk intervention (including intra-abdominal); (2) history of coronary disease; (3) past or present heart failure; (4) stroke; (5) diabetes needing insulin; and (6) creatinine > 2.0 mg/dL. Similarly, the Gupta Myocardial (325) Infarction or Cardiac Arrest Calculator (326) (not externally validated) includes 20 patient risk factors, such as increasing age, ASA class, preoperative serum creatinine > 1.5 mg/dL, functional status, and the surgical procedure. Other dedicated organ-system assessments that impact selection of procedure include, but are not limited to, diabetes (13), behavioral health (327-337), and reproductive health (338).
Procedure selection also depends on cost, insurance coverage, and ability to pay. For the general population, bariatric surgery had a cost until postoperative years 4 to 5, when cost savings appeared, which were higher in patients with T2D (339, 340). In contrast, overall health care costs in the Brazilian system were not reduced as a result of decreased ORCs after bariatric surgery, indicating that there are likely many direct and indirect economic factors involved (341). Demonstrable drivers of costs related to bariatric surgery in the U.S. are suboptimal outcomes (342) and the rising number of malpractice claims, though these appear to simply parallel the increased number of surgical procedures performed (343). Bariatric surgery is associated with a positive effect on social transfer payments (e.g., Social Security, unemployment benefits, and welfare) but no real effect on income (344). Similarly, in the adolescent population with severe obesity, bariatric surgery initially incurred substantial costs and morbidity; however, when assessed over a 5-year period, bariatric surgery was found to be a cost-effective treatment in adolescents (345). Unfortunately, there has been inconsistent support for Medicaid coverage of bariatric surgery for adolescents with severe obesity (346), even though among middle-aged patients with Medicaid coverage, weight loss was comparable to those with Medicare or private insurance coverage (347). In 2010, the cost-effectiveness of bariatric surgery was < $25,000 per quality-adjusted life year versus no treatment and well below benchmarks of $50,000 to $100,000 (348, 349). However, in a 2013 longitudinal analysis of claims data, bariatric surgery, regardless of type, was not associated with reduced health care costs (350). In a 2015 report, inpatient mortality rates with bariatric surgery decreased 9-fold with only modest increases in cost after adjusting for inflation (lower increase than for appendectomy) (351). What is alarming, however, is a report that with 22% of medically acceptable candidates not approved for insurance reimbursement, their mortality rate increases 3-fold (352). Taken together, these data support a shift in emphasis from cost savings to relevant health-related metrics for patients, on a population scale, undergoing bariatric surgery (353).
Coverage for bariatric surgery is often lacking, even when there is a perception by employees that their wellness programs will reimburse for these procedures (353). When available, coverage for bariatric surgery under the Affordable Care Act varies from state to state (354), even though 2015 data do not show an association of coverage with increased monthly premiums (355). Unfortunately, in a retrospective study of patients having RYGB by Jensen-Otsu et al. (356), patients with Medicaid coverage, in aggregate, had longer lengths of hospital stays and higher hospital readmission rates within 30 days of discharge, compared with those having commercial insurance coverage. On the other hand, among patients having LAGB, there was no difference in postoperative weight loss between those paying out-of-pocket and those covered by private insurance (357). An assessment on the cost evaluation in patients receiving Medicare reimbursements demonstrated significantly lower payments at hospitals with low complication rates (358). With increased variation in hospital episode payments, bundled payment programs are being considered for bariatric procedures (359).
After LAGB in an Australian retrospective study, drug utilization—especially those treating T2D and CVD—is decreased and significantly contributes to cost reductions (360). However, in a large retrospective study of 19,221 LAGB procedures from 2004-2010 in the state of New York, the total revision rate was 34.2% (361). In another retrospective review among Medicare beneficiaries who underwent LAGB from 2006-2013, device-related re-operation was common, costly, and varied widely across hospital referral regions (362). Based on these and other similar findings, it has been suggested that payers should reconsider their coverage of LAGB (362).
RYGB continues to demonstrate sustained long-term weight-loss results as well as improvement and resolution of ORCs, such as GERD, CVD, degenerative joint disease, T2D, OSA, HTN, pulmonary disease, and psychiatric disease (363-366). In addition to weight loss and comorbid disease improvement/resolution, both RYGB and SG were further validated as durable bariatric surgeries with significant improvement in patient-reported outcomes based on quality-of-life scores (367).
The preference of the individual bariatric surgeon, performance of medical institutions, learning curve of the bariatric surgeon, as well as the subjective experience base of the referring physician also play significant roles in the decision regarding which procedure to select. For robotic surgery in general, an adequate number of cases deemed necessary for surgical competence was 10 to 128 cases, depending on the procedure involved and determined primarily by docking, robot, and total operative time (368). The learning curve for robot-assisted RYGB was 66 cases in a study by Starnes et al. (369). Another study of robot-assisted RYGB found 100 cases on the learning curve to be a discriminator in terms of operative time but without any differences in outcomes or complications (370). This 100-case mark was also reported in a study by Beitner et al. (371) for RYGB, in which late complication and re-operation rates were eventually improved with modification in surgical technique. In a Chinese study of patients undergoing RYGB, the learning curve was more associated with operating time and morbidity than mortality or amount of eventual weight loss (372). However, Rausa et al. (373) found that the relative superiority of LRYGB over open RYGB may be due to extended learning curves in the former. For LAGB, the learning curve is closer to 50 cases (374). For SG, the learning curve is in the same or higher range as for RYGB—100 to 200 cases—below which correlates with increased risk for a proximal leak (375-377). Guebbels et al. (378) found that bariatric surgery learning curves depend on mentorship and improve as the preceding surgeon’s skill improves. The superiority of 3D over 2D laparoscopy was observed at early and later stages in the learning curve (379). In Polish (380) and Dutch studies (381), the involvement of residents in training with an experienced teacher does not compromise complication rates or weight-reduction outcomes after bariatric surgery. On the other hand, mastery refers to having outcomes significantly better than the average surgeon, whereas competency (the learning curve figure discussed above) refers to having outcomes comparable to the average surgeon. Mastery for RYGB surgeries is approximated at 500 cases (382). Thus, the question arises of whether selection of a bariatric surgery procedure should, in some fashion, depend on availability of a surgeon with competency versus mastery for the specific procedure.
The likelihood of malpractice lawsuits was also found to correlate with the number of procedures performed and years in practice by the bariatric surgeon (383). Nevertheless, there does not appear to be correlation of hospital charges with improved bariatric surgery outcomes (384).
Doumouras et al. (385) found that surgeon volume and a teaching hospital setting (but not accreditation) predicted lower all-cause morbidity after bariatric surgery. However, Kwon et al. (386) did find a favorable association of accreditation with lower rates of bariatric re-operations and complications. But then again, Scally et al. (387) demonstrated no association of the Medicare distinction of Center of Excellence status with savings to the health care system for bariatric surgery. Furthermore, Nicholas et al. (388) found that the Center of Excellence designation had the unintended consequence of reducing bariatric surgery in nonwhite Medicare beneficiaries. However, this was refuted by a different study using the National Inpatient Sample from 2006-2011 where the Center of Excellence designation was not associated with limited access to bariatric surgery (389). These and other inconsistent studies have fueled the controversy about the need for and nature of accreditation for bariatric surgery, especially considering the subsequent elimination of the Center of Excellence accreditation requirement for Medicare reimbursement of bariatric surgery and in the context of selecting specific bariatric procedures and settings (390, 391).
Such intertwining relative risks support a nuance-based clinical decision-making approach to the selection of bariatric procedures. Despite all this available information, both scientific and vetted in the popular lay press, the lack of knowledge about bariatric procedures by patients and referring HCP remains a distinct barrier to effective decision-making (392). Hence, a critical analysis of the above factors is provided as an algorithm in Figure 1 (incorporating information in Tables 6-8) to assist with clinical decision-making for bariatric procedure selection.
Q3. How should potential candidates be managed before bariatric procedures?
R7. (2008). Decision-making concerning the use and type of bariatric procedures should be based on comprehensive health goals, meaning the prevention and management of ORCs in patients with obesity. This overarching precept is detailed in the AACE obesity care model (393).
R8. (2008). The preoperative checklist in Table 9 compiles evidence-based items that should be evaluated to mitigate operative and postoperative risks of bariatric procedure. The primary goal of checklists is to maximize safety. However, this tool can also assist with decision-making by highlighting potential variables that can influence selection of bariatric procedure. Other variables should also be considered to guide decision-making. Unfortunately, in a review of RCTs, Colquitt et al. (394) found that adverse events and re-operation rates were poorly reported with follow-up times of only 1 to 2 years, precluding any conclusions about long-term effects. Risks for readmission, which can be better integrated into decision-making, include surgical complexity, ASA class, prolonged operative time, and major postoperative complications (395). Overall risks for morbidity and mortality with bariatric procedures primarily correlate with age and BMI, but also with male gender, gastric bypass procedure, and open procedures (396, 397). Interestingly, there was no statistical association of advancing chronic kidney disease stage with 30-day postoperative complication rates (398), with good safety and efficacy in those patients on dialysis (399). SG has been identified as a preferable option in those over age 65 years (400). Various composite scoring systems have been devised for estimating risks of bariatric procedures, and further validation studies are eagerly awaited (397, 401). Various preoperative psychological instruments have also been used to predict postoperative outcomes (337, 402, 403). The use of chronic steroids is associated with mortality and serious postoperative complications after stapled bariatric procedures, with no difference between patients undergoing RYGB and patients undergoing SG (404, 405).
R9. (2008). Pre-bariatric surgery insurance requirements and correct documentation of medical necessity can be onerous, despite a lack of evidence that they correlate with improved clinical outcomes. Love et al. (406) found that surgical dropout during this process was due to a longer diet requirement (OR, 0.88; P < .0001), primary care physician letter (OR, 0.33; P < .0001), cardiology evaluation (OR, 0.22; P < .038), and advanced laboratory testing (OR, 5.75; P < .019).
R10. (2019*). The informed consent process should include the provision of appropriate educational materials. Mahoney et al. (407) found that levels of education and health literacy figure prominently in a patient’s ability to adhere with postoperative instructions and avoid hospital readmissions.
R11. (2013). The costs of bariatric procedures vary greatly and mainly depend on ORCs and other comorbidities, concurrent procedures, robotic platform, surgical complexity, and length of hospital stay (408). For example, in a 2017 study by Khorgami et al. (408), the calculated cost (median and interquartile range) for RYGB was $12,543 ($9,970 to $15,857), for SG $10,531 ($8,248 to $13,527), and for LAGB $9,219 ($7,545 to $12,106).
R12. (2013). A review from 2016 (56) suggests little impact of preoperative weight loss attempts on surgical outcomes. In a retrospective review of 1,432 patients having bariatric surgery, insurance-mandated preoperative weight-loss programs were not associated with better outcomes at 2 years (409). In another observational study, preoperative weight loss was not associated with greater postoperative weight loss, comorbidity resolution at 1 year, or lower 30- or 90-day rates of readmission (410). In fact, Keith et al. (411) found that insurance-mandated preoperative diets delay treatment and adversely affect weight outcomes. On the other hand, Deb et al. (412) also found that pre-operative weight loss did not affect long-term postoperative weight-loss outcomes. Watanabe et al. (413) even found minor beneficial effects of preoperative weight loss on postoperative complications in patients undergoing SG. Notwithstanding the potential benefits of improved preoperative health associated with weight loss on postoperative outcomes, taken together, these studies argue against weight loss as a prerequisite for bariatric surgery, since a likely adverse effect of failure is denial of a potentially life-saving procedure (i.e., denial of a timely bariatric procedure). Routine prehabilitation clinical pathways that include deep breathing exercises, CPAP as appropriate, incentive spirometry, leg exercises, sips of clear liquids up to 2 hours preoperatively, H2 blocker or proton-pump inhibitor, thromboprophylaxis, and education about perioperative protocols, in conjunction with intraoperative and postoperative ERABS protocols, are associated with improved outcomes (414).
Q4. What are the elements of medical clearance for bariatric procedures?
R13. (NEW). Lifestyle medicine is the nonpharmacological and nonsurgical management of chronic disease (and to reemphasize: obesity is a chronic disease) (415). A significant number of patients fail to meet target metrics following bariatric procedures. This is not only due to biological factors, selection pitfalls, and technical issues, but also preoperative lifestyle habits. Gilbertson et al. (416) provide evidence that supports the hypothesis that lifestyle intervention is beneficial in those patients with unhealthy lifestyles and bariatric surgery resistance. However, in a prospective, randomized intervention study (N = 143) on preoperative behavioral lifestyle using face-to-face and telephone encounters for 6 months, there were no improvements in weight loss by 24 months postoperatively (417). Nevertheless, completing the lifestyle medicine component of the preoperative checklist (Table 9) can be useful, particularly since formal lifestyle medicine training is seldom part of formal medical education, though the specific timing, content, and methodology of preoperative lifestyle intervention, beyond usual standards of care for patients with obesity, remain to be determined.
R14. (2019*). Current evidence-based glycemic control targets are provided by updated AACE/ACE (418) and ADA (419) CPGs and algorithms (420). In general, chronic hyperglycemia is associated with poor surgical outcomes (421). Achieving preoperative glycemic control within months without weight gain can be facilitated using an interprofessional diabetes team (422). Better preoperative glycemic control, with pharmacotherapy and low-calorie diets, correlates with complete T2D remission rates after RYGB (423-425). Aminian et al. (217) individualized bariatric surgery procedure selection in patients with T2D using a Metabolic Surgery Score based on T2D duration, number of preoperative T2D medications, insulin use, and glycemic control (A1C < 7% [53 mmol/mol]). If there is doubt concerning diabetes type in a preoperative evaluation, beyond history (more abrupt onset possibly with an episode of diabetic ketoacidosis with T1D), C-peptide and autoantibodies (e.g., anti–glutamic acid decarboxylase, insulin autoantibodies, insulinoma-associated-2 autoantibodies, zinc transporter 8) may be ordered to assist differentiating T1D (usually antibody-positive with very low C-peptide) from T2D (usually antibody-negative with low, normal, or elevated C-peptide) (426).
R15. (2013*). Patients evaluated for bariatric procedures have a significant number of endocrine abnormalities, with nodular goiter and autoimmune thyroiditis among the most prevalent; for instance, 18.1% had hypothyroidism (427). Obesity is associated with TSH elevation in the absence of a primary thyroid disease, with reference ranges increasing based on BMI classes: underweight (BMI < 20 kg/m2), 0.6 to 4.8 μUI/mL; normal weight and overweight (BMI 20 to 29.9 kg/m2), 0.6 to 5.5 μUI/mL; obesity (BMI 30 to 39.9 kg/m2), 0.5 to 5.9 μUI/mL; and severe obesity (BMI ≥ 40 kg/m2), 0.7 to 7.5 μUI/mL (428, 429). TSH levels are therefore not recommended as a routine screen prior to bariatric procedures, since the higher upper limit with obesity may result in considerable overdiagnosis and unnecessary lifestyle levothyroxine treatment. However, many insurance companies still require pre-operative TSH testing before bariatric procedures (1). Postoperatively, thyroid hormone replacement or supplementation requirements are variable due to decreased requirements as body mass and volume of distribution decrease, increased requirements as thyroiditis may progress in some, and variable effects such as GI absorption may worsen or actually improve (430, 431).
R16. (2019*). Evidence-based recommendations to manage lipid disorders are provided in recent AACE/ACE (432) and National Lipid Association (NLA) CPGs (433, 434), with an emphasis on bariatric surgery in another CPG by ASMBS/NLA/OMA (435, 436). Baseline preoperative abnormalities in the lipid profile can guide procedure selection. In a systematic review and meta-analysis, Christelle et al. (437) found that RYGB was superior to SG in not only improving weight loss and glycemic control, but also improving short- (1-year) and mid-term (5-year) lipid metabolism, with and without T2D. In a small (N = 38) prospective cohort trial before and after RYGB, preoperative n-3 polyunsaturated fatty acid and vitamin A levels were negatively correlated with fasting insulinemia and high-sensitivity CRP, and positively with high-density-lipoprotein cholesterol; preoperative linoleic levels were associated with postoperative weight loss (438). In a meta-analysis, Heffron et al. (439) found that mean low-density-lipoprotein cholesterol decreased by 42.5 mg/dL with BPD/DS, 24.7 mg/dL with RYGB, 8.8 mg/dL with LAGB, and 7.9 mg/dL with SG (the changes for LAGB and SG were not significantly less than those among patients in the nonsurgical control group). Interestingly, in a longitudinal study, improvements in pancreatic lipid metabolism (fat volume and fatty acid uptake) with RYGB or SG were associated with better glycemic control and β-cell function (440). Somewhat surprisingly, Lima et al. (441) found a high rate of chromium deficiency—55 of 73 (75.3%) patients tested who were awaiting bariatric surgery—and this low chromium state was associated with lower cholesterol and higher triglyceride levels. More studies are required to understand the role of chromium nutrition on insulin sensitivity, obesity, and responses to bariatric surgery.
R17. (2013*). Bariatric surgery has a significant effect on increased fertility (442). Fetal growth is positively correlated with protein supply and negatively correlated with maternal iron status. This need for monitoring increases with increasing malabsorptive procedures (443, 444). Typical recommendations for time to conception have been based primarily on nutritional concerns, with the implication that weight stability (12 to 24 months) is important. However, there are no studies showing outcome differences for conception at less than 1 year postoperatively, with one large study showing no differences in outcomes at less than 1 year (445-447). Multiple studies show an improvement in fertility and lower risk for gestational diabetes and large-for-gestational-age births following bariatric surgery. By contrast, risk for small-for-gestational-age births were increased, with possibly a small increase in premature births (445, 446). The harmful effects of various deficiencies (iron, calcium, B12, folic acid, and vitamin D) and teratogens (vitamin A) are well known. Appropriate monitoring and supplementation are recommended (445, 448).
R18. (2008*). Hormone therapy, including oral hormonal contraception, postmenopausal hormone therapy, and use of selected estrogen-receptor modulators, has been associated with an increased risk of venous thromboembolism (VTE) (449, 450). There is insufficient evidence for any recommendation regarding optimal timing of hormone therapy resumption after a bariatric procedure.
R19. (2008*). Bariatric surgery can improve both incidence of polycystic ovary syndrome (PCOS) and associated infertility as well as reduced risk of endometrial hyperplasia (338, 451).
R20. (2019*). Most rare causes of severe obesity will manifest in childhood. A recent review found 79 distinct obesity syndromes, of which 19 have been elucidated genetically (452). Prader-Willi syndrome is the most common syndromic monogenic cause (incidence 1/15,000), and MC4R defects are the most common nonsyndromic monogenic cause (2 to 4% of pediatric obesity) (453, 454). Craniopharyngiomas and resultant surgery are rare causes of hypothalamic obesity (455). A small study of eight matched patients with craniopharyngioma showed benefit from RYGB but not SG (456, 457).
R21. (2019*). The latest American College of Cardiology/American Heart Association guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery provides the evidence base for recommendations regarding preoperative noninvasive cardiac testing (458). Additional guidelines are provided by Feely et al. (459) and the European Society of Cardiology and European Society of Anaesthesiology (460).
R22. (2019*). Studies have shown prolonged hospital stays and higher complication rates after bariatric surgery in patients with OSA (461-463). Hence, routine preoperative clinical screening for OSA with confirmatory polysomnography may be considered, with further diagnostic testing and treatment of appropriate at-risk patients (461, 464-467). However, the data are generally mixed in terms of overall benefit of screening, with several studies showing no risk reduction with OSA screening or treatment (463, 466, 468-472).
R23. (2019*). Recent data support the association of smoking cigarettes with an increased risk of postoperative morbidity (473). Among 12,062 patients undergoing bariatric surgery in Western Australia, anesthetic complications were uncommon (0.5%) but accounted for 9.7% of all ICU postoperative readmissions, of which, smoking history (and not BMI) was the only prognostic factor for airway-related complications (474). All smokers must be advised to stop smoking at any time before bariatric surgery, even if it is within 6 weeks before surgery (475). Unfortunately, in a retrospective review of the NSQIP database, Haskins et al. (314) found that smoking within the year before SG was associated with increased 30-day morbidity and mortality risk, compared with nonsmokers. Structured cessation programs are more effective than general advice, which is more effective than usual care (476).
R24. (2013*). Recent position papers continue to recommend routine prophylactic measures to prevent VTE, which includes both DVT and PE, after bariatric surgery (477, 478).
R25. (2019*). Survey data in the U.K. fail to show consensus on the use of routine versus selective preoperative esophagogastroduodenal endoscopy in patients considered for bariatric surgery (479). Yet, in one notable exception in a primarily Chinese population with obesity, routine preoperative upper-GI endoscopy demonstrated significant abnormalities (480). Systematic reviews, meta-analyses, and other retrospective studies have demonstrated benefit of preoperative endoscopy in patients with GI symptoms, where results altered surgical planning in roughly 7 to 12% of patients (481-485). A retrospective study by Yormaz et al. (486) found that in patients undergoing bariatric surgery, the Gastrointestinal Symptom Rating Scale and upper-GI symptoms were independent predictive markers of abnormalities found with preoperative esophagogastroduodenal endoscopy. The correlation of preoperative endoscopic abnormalities with postoperative complications is not clear based on current evidence (486, 487).
R26. (2019*). NAFLD is common across age groups in obesity (488). While age, waist circumference, serum alanine aminotransferase, serum triglycerides, aminotransferase-to-platelet ratio, and ultrasound and transient elastography all have some predictive value, there are no reliable noninvasive presurgical predictors of disease severity or progression (489-491). Liver biopsy remains the diagnostic standard (492). Severity of liver disease as determined by MELD score (Model of End-Stage Liver Disease) correlates with short-term outcomes (493). Bariatric surgery improves multiple metabolic conditions, including insulin resistance, glucose metabolism, HTN, plasma lipids, transaminases, liver steatosis, steatohepatitis, and fibrosis (494).
R27. (2013*). Two recent studies illustrate a relationship of Helicobacter pylori with the occurrence of marginal ulcers postoperatively (495, 496). Specifically, Mocanu et al. (496) found a 10-fold increase in the rate of this complication in H. pylori–positive versus –negative patients after undergoing RYGB.
R28. (2013*). Long-term studies have shown a beneficial effect of bariatric surgery on urate levels and gout incidence (497-499).
R29. (2008*). Decreases in bone density over time are common after bariatric surgery, particularly in postmenopausal women (500-502). Abnormalities of bone metabolism, including secondary hyperparathyroidism and vitamin D deficiency are common in obesity both before and after bariatric surgery (503, 504). Current screening recommendations for bone mineral density testing vary somewhat but generally agree that postmenopausal women and women age greater than 65 years should be screened (505).
R30. (2019*). The important role of behavioral medicine in the pre-operative and continuing management of patients undergoing bariatric surgery is strengthened, particularly in the context of durable interdisciplinary team management, assessing and enhancing patient readiness for surgery, improving patient-centered care by increasing a patient’s knowledge about postoperative behavioral regimens and potential challenges, and reducing risk, liability, and clinic burdens (506). Formal domains for preoperative psychosocial evaluation are weight history, eating-disorder symptoms (night-eating syndrome, binge eating, compensatory behaviors, anorexia nervosa, etc.), psychosocial history, developmental and family history, current and past mental health treatment, cognitive functioning, personality traits and temperament, current stressors, social support, quality of life, health-related behaviors (substance abuse, smoking history, adherence, and physical activity), motivation and knowledge base (including weight-loss expectations) (336), as well as self-harm and suicide (507). Formal psychometric testing is commonly employed preoperatively and should be performed by qualified behavioral HCP providing a written report and organizing appropriate postoperative monitoring (336). Alcohol metabolism and addiction are recognized problems that occur in patients who have undergone malabsorptive bariatric surgical procedures. In a report by Acevedo et al. (508, 509), SG was similar to RYGB with respect to adverse effects on a patient’s response to alcohol ingestion. In fact, in these patients, there are faster and higher peak blood alcohol concentrations, resulting in underestimation of alcohol levels by breath analyzers (508).
R31. (2013*). Preoperative binge-eating disorder was associated with less weight loss after RYGB or LAGB, but patients still lost more weight than those receiving lifestyle modification alone (510). Postoperative engagement with behavioral therapy, psychological services, and spousal engagement are positive predictors of outcome for all patients undergoing bariatric surgery, and therefore advised (510-512). Bariatric surgery was associated with a slight increase in suicide and self-harm, but the absolute risks were still low (513).
R32. (2013*). Recent guidelines provide an updated, initial evidence-based approach to micronutrient supplementation after bariatric surgery (448). Of note, adherence to vitamin therapy after bariatric surgery is lower than self-reports and represents a potential risk to patients’ health, which needs to be promptly addressed (514-516). Iron studies including ferritin, fat-soluble vitamins other than 25-vitamin D (vitamins A, E, and K), and vitamin C levels do not need to be ordered routinely preoperatively but may be considered in patients at risk for deficiency states related to these nutrients (517-520).
In general, thiamine deficiency occurs in 15.5 to 29% of patients with obesity (521). Thiamine testing may be considered pre-operatively in light of reports describing relatively high prevalence rates of thiamine deficiency in patients awaiting bariatric surgery (16 to 47%, depending on ethnicity), early onset Wernicke encephalopathy (WE) 2 weeks after bariatric surgery instead of the more usual 3 months, and the potential prevention of WE with diligent pre-operative thiamine replacement protocols (522-524). In a single institution, a retrospective observational study of 400 patients undergoing bariatric surgery showed that 16.5% had clinical thiamine deficiency preoperatively (consistent symptomatology and either low biochemical levels or significant improvement with thiamine supplementation) and 18% after RYGB (525). However, in another study of patients after SG, the preoperative prevalence of thiamine deficiency was only 3.4%, with rates decreasing by postoperative year 2 (526). In a small (N = 22) prospective study of women undergoing LAGB, 38% had low thiamine levels (527).
R33. (2013*). All patients should have age-appropriate screening for cancer according to U.S. Preventive Services Task Force recommendations (528). Mechanistic studies implicate chronic inflammation and crosstalk between adipose tissues and cancer-prone cells (529, 530). Recent studies have demonstrated improved clinical oncologic metrics for certain malignancies (risk, biomarkers, survival, etc.) in general (531-534) and for breast (535) and colorectal (536-538) cancer in particular. In contrast, other studies have shown poorer prognosis in another cohort study of colorectal cancer by Tao et al. (539) and in endometrial (540, 541), liver (542), and pancreatic cancers (543) in patients after bariatric surgery. Esophageal carcinoma represents a unique challenge since, when diagnosed after bariatric surgery, surgical resection carries a high risk (544). Gastric carcinomas, in the gastric pouch or excluded stomach, are rare and also represent a unique clinical challenge without clear guidelines (545, 546). These findings affirm the relevance and potential benefit of preoperative screening and, when appropriate, aggressive case finding, though much more evidence is needed for more detailed recommendations. Interestingly, cancer survivors had comparable weight-loss effects after bariatric surgery to those without a history of cancer (547).
R34. (NEW). ERABS clinical pathways focus on obesity-related perioperative risks specific for the patient undergoing bariatric surgery and are based on the ERAS general recommendations (Table 10). Perioperative noninvasive ventilation is associated with decreased risk for postoperative respiratory complications (548).
Q5. How can care be optimized during and within 5 days of a bariatric procedure?
R35. (NEW). Best practice anesthetic and intraoperative techniques, as part of an overall ERABS clinical pathway, are provided in Table 10 (549). King et al. (550) found that these clinical pathways were not associated with increased postoperative day-1 discharges, but were associated with reduced perioperative opioid use, postoperative nausea, and emergency room visits within 7 days after hospital discharge. Key components of intraoperative care include: proper positioning and monitoring of patients, accounting for obesity-related changes in pharmacology, adjusting for potentially difficult tracheal intubations and airway management, and applying ventilatory strategies, including PRMs (551). Dupanovic et al. (552) identified intraoperative factors with LAGB that affected postoperative outcomes: meticulous surgical technique, least number of access ports, and multimodal analgesic approach.
Laparoscopic techniques for bariatric surgery induce a CO2 pneumoperitoneum, which adversely affects cardiopulmonary function that may already be compromised due to obesity. PRMs can improve anesthesia-related functional residual capacity reductions intra-operatively, but not postoperatively, in patients undergoing bariatric surgery (553, 554). However, PRMs can improve postoperative pain intensity and opioid requirements after SG or RYGB (555). In a study by Eichler et al. (556), intraoperative noninvasive monitoring using electrical impedance tomography (554), with increasing positive end-expiratory pressure demand during capnoperitoneum to maintain positive transpulmonary pressures throughout the respiratory cycle, was associated with improved postoperative oxygenation. In addition, intraoperative transcutaneous CO2 monitoring has been found to provide a better estimate of arterial CO2 partial pressure in patients undergoing laparoscopic bariatric surgery than end-tidal CO2 partial pressure (557). Noninvasive hemodynamic monitoring has potential advantages, especially among patients at high risk for CVD, but at present, these methods lack sufficient accuracy and require more study in the obesity and bariatric surgery settings (558).
In an unmatched, case-controlled study, the use of the analgesia nociception index was associated with decreased intraoperative use of sufentanil, but not postoperative opiate use (559). In an observational study by Vaughns et al. (560) of 26 consecutive adolescent patients undergoing bariatric surgery, the intraoperative use of dexmedetomidine, 1.62 μg/kg (0.89 to 2.032; median total dose and interquartile range), as initial bolus and then continuous infusion was associated with lower opioid requirements intraoperatively and in the first 48 hours postoperatively. These results were affirmed in a meta-analysis involving a broader range of patients having bariatric surgery (561) and a guideline implementation study demonstrating feasibility and significant cost avoidance (562). Of note, adolescents with severe obesity have increased fentanyl clearance, underscoring the need for more pharmacologic data on this population (563). The short-acting inhalation anesthetic agents sevoflurane and desflurane are safe with bariatric surgery and may be considered as alternatives for maintenance of anesthesia (564). Postoperative bleeding is a rare but serious complication, occurring in < 1% of patients, and can be prevented with a standard intraoperative protocol that increases blood pressure and reduces the pneumoperitoneum to identify possible silent bleeding sites (565). Goal-directed fluid therapy is also recommended during bariatric surgery, and the potential for excessive IV fluid administration can be mitigated using dynamic indicators, such as the Pleth Variability Index (PVI) (566).
R36. (NEW). A protocol-based approach with ERABS strategies is critical to improve the early postoperative care of patients undergoing bariatric surgery. These protocols continue to evolve and be applied to a growing number of programs (Table 10). In general, clinical “enhanced recovery” pathways focus on decreasing surgical stress and maintaining normal homeostasis as much as possible and avoiding the routine use of catheters, drains, and radiologic testing after surgery. These protocols also include focused education about the bariatric surgery process and are associated with decreased length of stay postoperatively (567). These protocols are based on experience in other specialties, such as orthopedic and colorectal surgery (568-571). Enhanced recovery can only be accomplished with an interdisciplinary strategy to manage key components of the early postoperative care plan to include multimodal pain management strategies (572), minimization of opioid use during and after surgery (573), goal-directed fluid management, and tight glycemic control. Ideally, ERABS is combined with preoperative prehabilitation and comorbidity optimization, as well as evidence-based intraoperative clinical pathways (414). Implementation of ERABS in patients decreases length of hospital stay (574-578) without increasing morbidity, readmission rates (579-584), or postdischarge resource utilization (585, 586).
ERABS may also decrease costs of care in the early postoperative period (576, 584, 587). A meta-analysis of ERABS barriers by Ahmed et al. (588), prospective cohort studies by Mannaerts et al. (589) and Blanchet et al. (590), and a retrospective study of consecutive patients by Matlok et al. (582) affirm these correlations and find ERABS generally safe and effective. Factors that delayed discharge after SG reported by Jonsson et al. (591) include preoperative opioid use, history of psychiatric illnesses, chronic kidney disease, and revisional procedures, but not ASA class, diabetes, congestive heart failure, HTN, distance to home, and insurance status. Length of hospital stay after SG was reduced by early operating start time and treated OSA, while length of stay was increased with creatinine > 1.5 mg/dL, ejection fraction < 50%, and increased operative time (591). Deneuvy et al. (592) found that in a French multicenter study, ERABS compliance was 79.6%, arguing for continued training and audits, with the elements least often applied being limb intermittent pneumatic compression during surgery (23.3%), multimodal analgesia (49.5%), and optimal perioperative fluid management (43.8%). On the other hand, ERABS may need to be deferred in patients with extremes of age (< 18 or > 60 years), poor adherence or motivation, cognitive impairment, poor social support, or location of residence at a significant distance from a hospital (593). Even though ERABS implementation is associated with improved clinical outcomes, reporting systems will need to be optimized (594).
R37. (NEW). Providing the patient with preemptive antiemetic and nonopioid analgesic medications pre- and intraoperatively as part of a multimodal pain management strategy improves postoperative pain control and decreases opioid use (572), as well as decreases postoperative nausea and vomiting (595).
R38. (2013*). Recent reviews have commented on the early postoperative dietary strategy (596, 597). Patients should be allowed to start drinking clear liquids the night of surgery. Clear liquid intake and an emphasis on oral hydration should continue the day after surgery; the patient can also be advanced to full liquids as tolerated on postoperative day 1. Each of the nutritional components of ERAS, as outlined by the European Society of Parenteral and Enteral Nutrition (598), should be implemented: avoid long periods of preoperative fasting (e.g., sips of clear liquids with carbohydrates up to 2 hours), postoperative oral feedings as soon as possible with nutrition support as needed based on early risk assessments, early recognition and correction of factors leading to catabolism and/or GI dysfunction, and early mobilization to optimize protein synthesis and muscle recovery (Table 12).
After discharge from the hospital, patients should continue drinking full liquids (stage 2) with an emphasis on protein intake and hydration. Within several days of the surgery, the patient should be tolerating at least 60 oz (1,800 cc) of fluid daily to avoid dehydration. This should continue for 10 to 14 days until an assessment can be made by the clinical team at the initial postoperative appointment regarding their intake and suitability for diet progression. If the patient is tolerating stage 2 well, they can then be advanced to a pureed diet (stage 3) approximately 2 weeks after surgery. This can be described to the patient as food that can be eaten without chewing, and the consistency and texture should progress gradually. Patients should continue in stage 3 for another week and, if intake is improving, they can advance on their own to soft foods (stage 4). Patients should be instructed to limit stage-4 foods to those that can be mashed or do not require excessive chewing. After 1 or 2 weeks on soft foods, most patients begin introducing some solid food and can progress to all solids as tolerated (stage 5), generally 4 to 6 weeks after surgery. Patients should be instructed that when solid food is introduced, only several bites will be tolerated until they adapt to their new anatomy and when the postoperative edema and inflammation have resolved. Typical patients should also avoid drinking 30 minutes before or after eating solid food. Typical daily calorie intake the first week after surgery is 400 kcal/d and progresses to 600 to 800 kcal/d by weeks 3 to 4. Several months after surgery, patients should consume 1,200 to 1,500 kcal/d, with most patients consuming approximately 1,500 to 1,800 kcal/d, 6 months postoperatively and long-term. Refer to Tables 12-14 for additional information regarding diet progression. If patients do not progress through these stages of their diet in the appropriate time periods due to nausea, vomiting, or dysphagia, careful evaluation of nutrition should be performed, and the surgeon should consider investigating potential causes (e.g., early anastomotic ulcer, stricture, and mechanical obstruction) (599).
R39. (2019*). Recommendations for initial micronutrient dosing in the early postoperative period immediately following the bariatric procedure and, if applicable, during the initial hospitalization are based on preoperative deficiency states, type of procedure performed, dietary progression protocols, and oral tolerances, with the intention to adjust in the late postoperative period based on clinical course, symptoms, and judicious biochemical testing, as outlined in subsequent recommendations (Tables 11, 13, and 14). Special attention should be made to avoid oversupplementation during this period, which could be a result of faulty a priori decision-making, various mutations/polymorphisms, altered physiology, especially decreased binding proteins, confounded or unnecessary biochemical testing, and indiscriminate/inappropriate continuation that induces other metabolic derangements (600). This includes, but is not limited to, iron (601-603), zinc (604, 605), and vitamin D (606, 607). With respect to routine vitamin D supplementation, patients who have had an SG or RYGB had comparable 12-month safety and effectiveness with early postoperative individualized dosing starting with only 800 IU/d and uptitrated based on serum levels or a fixed high dose with 2,000 IU/d (607). In this CPG, the latter approach is still recommended based on the weight of evidence with titration to target levels in the late postoperative period. In a randomized, prospective cohort study of 50 patients, there were no significant differences in micronutrient deficiencies in the early postoperative period between those undergoing LSG versus RYGB (169).
R40. (2019*). Intraoperative and postoperative fluid management in patients undergoing bariatric surgery should be goal directed (566). Utilizing continuous noninvasive measurements of fluid status, such as the PVI, stroke volume variation, or other technologies, results in less fluid administration during bariatric surgery than empiric calculations of volume requirements (566, 608) or by monitoring urine output (609). Administration of excess IV fluids can increase the rate of postoperative nausea and length of stay after surgery (596, 610). To decrease the chances of preoperative dehydration, patients should be allowed to drink clear liquids up to 2 hours prior to surgery. This should be extended to 4 hours for patients with known gastroparesis or delayed gastric emptying (611).
R41. (2019*). EN support has been used for treatment-refractory dumping syndrome after bariatric surgery (612) and leaks after SG (613). The need for EN and/or PN support in some patients with OAGB indicates the need for similar, close follow-up for nutritional problems as with other malabsorptive bariatric procedures (614). When PN support is required for patients undergoing bariatric surgery based on high nutritional risk and inadequate intestinal function, CPGs from the American Society for Parenteral and Enteral Nutrition recommend a high-nitrogen (1.2 g/kg actual or 2 to 2.5 g/kg ideal weight of amino acid), low-energy (50 to 70% estimated requirements) formulation (615). This type of formulation also avoids overfeeding in a setting where, in the absence of indirect calorimetry measurements of actual energy consumption, formulaic calculations frequently overestimate needs (616). In a randomized, controlled study of patients undergoing RYGB, preoperative oral carbohydrate loading and perioperative peripheral PN were safe but not associated with improved body composition or clinical outcomes compared with standard nutritional management (617). Refeeding syndrome is a potential complication of PN in patients who have had severe weight loss after bariatric surgery, especially after BPD/DS (618), prompting special attention to adequate micronutrition (especially phosphate, magnesium, potassium, calcium, vitamins, and trace elements) with initial limited nonprotein calories (especially dextrose).
R42. (2019*). IV insulin for tight glycemic control is associated with improved outcomes following GI and bariatric surgery (619-622). In a comprehensive review, Batterham and Cummings (623) review a broad range of mechanisms, acting in concert, that mitigate/reverse the T2D state. Within 1 week after RYGB, first-phase insulin secretion and hepatic insulin sensitivity increase, consistent with clinical findings of rapid amelioration of hyperglycemia postoperatively (624). In fact, among patients with T2D, blood glucose levels were significantly reduced by 48 hours after SG and RYGB, regardless of diabetes medication (oral, noninsulin injectables, or insulin) (625). Moreover, glycemic control in the early postoperative period is associated with higher rates of long-term T2D remission (626). Diabetes status does not appear to be associated with postoperative infection rates during the first month after bariatric surgery (627). Patients with insulin-requiring T2D prior to surgery will have up to 87% reduction in their total daily insulin requirements by postoperative day 2 (628). These more recent findings further support the practice of holding or dramatically reducing diabetes medication in the early postoperative period, to not only decrease the risk of hypoglycemia, but also avoid unnecessary medication.
R43. (2013*). ICU monitoring is recommended for those patients at high cardiopulmonary risk (629, 630). Patients with left ventricular systolic dysfunction (left ventricular ejection fraction < 50%) had a slight excess in early postoperative heart failure and myocardial infarction but no excess mortality at 1 year (631). In a systematic review and meta-analysis by Chang et al. (632), the 30-day rate for myocardial infarction was 0.37%, with a mortality rate of 0.37%. RYGB had higher rates than SG or LAGB (632). The risk for cardiac events after bariatric surgery may be increased with OSA and this risk mitigated with the use of CPAP (633), though other studies fail to demonstrate these associations (469, 470). Parenthetically, even though bariatric surgeries involving senior-level residents had more statistically significant morbidities, including postoperative cardiac events, this association is more likely related to perioperative rather than intraoperative care (634). This finding argues for greater emphasis on resident training in perioperative bariatric surgery care.
R44. (2019*). Patients who use CPAP preoperatively should have this therapy initiated as early as the postanesthesia care unit to minimize the risk of apnea, hypoxia, or other pulmonary complications (635, 636). The use of CPAP immediately after bariatric surgery is not associated with increased risk of anastomotic or suture-line leaks (637). According to guidelines, patients with OSA who have had bariatric surgery should have continuous monitoring with pulse oximetry in the early postoperative period with minimization of sedatives and opioids (638, 639). Since patients with OSA and adequate CPAP use are at low risk for cardiopulmonary complications after laparoscopic bariatric surgery, routine ICU admission in the immediate postoperative period is not necessary (470). However, there is a need for additional research to assess risk factors and impact of sleep-associated desaturation, which is not unusual in patients after bariatric surgery (640).
R45. (2019*). VTE is a leading cause of morbidity and mortality after bariatric surgery and includes both DVT and PE. Portal-splenic-mesenteric venous system thrombosis is a rare but potentially lethal VTE complication after bariatric surgery (641). Patients who experienced upper-extremity DVT after bariatric surgery also have been described (642). In a recent study by Helm et al. (643), the postoperative incidence of VTE was 0.5%, with an average time to diagnosis of 11.6 days and 80% occurring after hospital discharge. After bariatric surgery, major complications occurred prior to VTE in 22.6% of patients, with VTE likelihood directly related to the number of complications, and an unadjusted 30-day mortality increasing 13.89-fold with VTE (643).
DVT prophylaxis is recommended for every patient after bariatric surgery (477). At a minimum, sequential compression devices and early ambulation should be utilized for all patients. Chemoprophylaxis should begin prior to surgery with unfractionated or low-molecular-weight heparin and be continued throughout the hospital stay unless there is a contra-indication (477, 644). More than 80% of DVT events following bariatric surgery are diagnosed after hospital discharge (645). Therefore, the use of extended postdischarge chemoprophylaxis should be used for patients who are at high risk for DVT, such as those with a personal history of DVT, known hypercoagulable state, or limited ambulation. Risk calculators are available to guide prophylaxis regimens (645). Congestive heart failure, paraplegia, dyspnea at rest, and re-operation are associated with the highest risk of postdischarge DVT. Postoperative bleeding and subsequent transfusion after bariatric surgery are also associated with increased VTE risk, most likely due to withholding chemoprophylaxis (646).
Using risk calculators can prompt routine postdischarge chemoprophylaxis for high-risk patients (i.e., DVT risk > 0.4%) (645, 647). Additional risk factors for postoperative DVT are advanced age, BMI > 60 kg/m2, open or revisional surgery, age > 50 years, anastomotic leakage, nicotine use, past DVT/PE, venous insufficiency, hypoventilation, or thrombophilia (e.g., protein-S deficiency, which is more likely with obesity) (648, 649). Serum anti-Xa levels can be used to guide low-molecular-weight heparin dosing in the prophylactic range (650-652). Fondaparinux 5 mg once daily achieves appropriate prophylactic anti-Xa levels more often than enoxaparin 40 mg twice daily after bariatric surgery (653).
Of note, patients undergoing bariatric surgery who are chronically anticoagulated preoperatively have increased risk for postoperative complications and all-cause readmission rates (654). Whether the benefits of inferior vena cava (IVC) filter placement prior to bariatric surgery are outweighed by the risks is unclear based on the current literature; however, it is important to note that IVC filters are associated with higher rates of postoperative DVT and mortality after bariatric surgery (655-657).
R46. (NEW). PE is a leading cause of mortality after bariatric surgery, with an incidence of about 1% (632, 658), but a leading cause of death at 20.7% (659) and accounting for 40% of all deaths within 30 days postoperatively (643). Mortality rates from PE are lower after laparoscopic, compared with open, bariatric procedures (660).
R47. (2019*). Respiratory distress or failure to wean from ventilatory support should also raise suspicion for an anastomotic leak. Anastomotic or staple-line leaks can present with clinical signs of sustained resting tachycardia, hypoxia, and fever and are highly morbid events (661). There is no evidence that routine placement of a drain after bariatric surgery is beneficial. In fact, placement of a drain may increase morbidity and should only be used in select, high-risk cases (662). If a leak is suspected in a stable patient, CT imaging is a more sensitive and specific diagnostic test than an upper-GI contrast study and should be the diagnostic test of choice to evaluate all the surgical anatomy (663, 664). In the setting of worrisome clinical signs and normal imaging, laparoscopic or open operative exploration is warranted to rule out GI leak (664). Nonoperative methods of GI leak treatment after both RYGB or SG include endoscopic endoluminal self-expandable stents, clips and sutures, endoscopic and percutaneously placed drains, and biologic glue/tissue sealants (665-671). Because length of hospital stay after bariatric surgery continues to decrease with the use of ERABS, some septic complications will occur after the relatively earlier hospital discharge (672). In fact, most SG leaks occur after hospital discharge. Serum markers such as CRP and procalcitonin are sensitive and specific in predicting surgical-site infections in patients after bariatric surgery (673).
R48. (2019*). Rhabdomyolysis (defined as a postoperative serum creatinine kinase level > 1,000 U/L) is associated with longer operative times (> 230 minutes) and can be effectively treated with fluid therapy and diuretics within 24 hours of surgery (674). The development of rhabdomyolysis is also associated with increasing volumes of IV fluid after bariatric surgery, suggesting that decreasing IV fluid administration (goal-directed fluid management) may lower the risk of rhabdomyolysis (675).
Q6. How can care be optimized 5 or more days after a bariatric procedure?
R49. (2019*). Recommended follow-up intervals are generally based on expert opinion (Table 11). There are very few bariatric surgery studies reporting long-term results with sufficient follow-up of patients (only 29 of 7,371 with at least 2-year follow-up and 80% of initial cohort represented), creating bias in outcome reporting (175). There are relatively few studies on the nature of retention and attrition after bariatric surgery (676). Nevertheless, among 46,381 patients who had some follow-up within 12 months after surgery (30.6% of all patients having RYGB), complete postoperative follow-up (75.6% of the 46,381 patients) was associated with greater comorbidity improvement and remission rates, compared with incomplete follow-up (677). In a review of 79 papers (out of 872 searched), with a majority representing retrospective reviews of prospectively collected clinical data, adherence with follow-up appointments was generally poor, with up to 89% attrition and worse with lesser amounts of weight loss achieved, younger age, unemployment, and lower BMI (678). Other predictors of increased adherence with 2-year follow-up were LAGB and attendance at the 6-month appointment, while dysthymia was associated with decreased follow-up (679). Similar results were found in a 5-year French cohort of 16,620 patients (680). Long-term success after bariatric surgery also depended on adherence with physical activity, vitamin supplementation, and healthy eating patterns, the last of which was impaired in patients with poorer mood, preference for sweets, and eating disorders (678).
Since increased adherence with follow-up is associated with improved outcomes, various strategies should be implemented to minimize attrition, such as the use of telemedicine (676) and better collaboration between inpatient and outpatient teams, including those with specialization in obesity medicine (677, 681-683). Moreover, though there is little consensus on what defines an acceptable amount of postoperative weight regain, patients often express anxiety and a sense of failure with any amount of weight regain, leading to guilt, shame, and a reluctance to attend critical follow-up appointments. Hence, clarity is needed regarding weight regain. Notwithstanding the above, in a cohort study of 794 patients with 90% follow-up over 10 years, there was a 38% rate of band removal with higher rates for those age < 40 years, BMI > 50 kg/m2, women, and longer duration of time (684).
R50. (2013*). The diagnosis of hyperinsulinemic hypoglycemia can be challenging due to the variability in presenting symptoms, which can be autonomic or neuroglycopenic in nature. Hyperinsulinemic hypoglycemia has been reported after SG (685), in addition to BPD/DS and RYGB. Newer studies have found an association of hypoglycemia after bariatric surgery with weight regain (686). To confirm the diagnosis of hyperinsulinemia hypoglycemia, patients must have confirmed postprandial hypoglycemia in combination with symptoms (687). A low-carbohydrate, low-glycemic index diet with adequate protein and inclusion of heart-healthy fats along with restricting alcohol and caffeine intake recently has been shown to be an effective strategy to manage hypoglycemia after bariatric surgery (688). In fact, most patients with hypoglycemia after bariatric surgery will respond to dietary modification or pharmacologic intervention (687-692). As an example, continuous glucose monitoring was useful in a pregnant patient with dumping syndrome after RYGB and poor adherence with conventional glucose monitoring (693).
R51. (2013*). The beneficial role of physical activity (high-intensity interval training, moderate-intensity continuous training, etc.) in patients with obesity, especially during the active treatment phase, has been described previously (694-700). Patients who undergo weight loss, especially with bariatric procedures, are particularly susceptible to skeletal muscle loss or sarcopenia, which is associated with physical disability, poor quality of life, and increased mortality risk (701). Biweekly physical activity training sessions for 6 months after RYGB improved cardiometabolic risk factors and muscle strength, but patients did not maintain these benefits (compared with controls) in follow-up (702, 703). However, physical activity was able to induce and maintain improved health-related quality of life for up to 2 years after RYGB (704). In several studies, there are positive correlations between the amount of physical activity and the amount of weight loss after bariatric surgery (705-707). In one systematic review of 50 studies, there was more physically active time (e.g., step count) during the first 6 months postoperative, but the intensity was less (708). Taking this into account, patients should be counseled on physical activity preoperatively and long-term after bariatric surgery (709, 710). The use of wearable technologies and activity monitors should be also considered as they can have a positive effect on healthy physical activity behaviors in patients with obesity (711). There are many web-based resources on general recommendations for physical activity in adults (712, 713).
R52. (2019*). The simple practice of self-monitoring (e.g., daily self-weighing using smart scales) may lead to improved weight-loss results (714). However, the incorporation of more sophisticated mobile technologies using a variety of delivery methods (e.g., text-messaging, e-mail, cell phone interactions, diet tracking, and virtual reality software) shows promising results (many with RCTs) in terms of additional or alternative low-cost patient-support modalities (715-726).
R53. (2019*). In patients who have undergone SG, there is a potential increase in gastroesophageal reflux requiring long-term proton-pump inhibitor therapy (727-729), which can interfere with absorption of calcium, thus further increasing the risk of secondary hyperparathyroidism (729, 730). Additional reviews (448, 731), a cross-sectional study (732), and a prospective study (733) further delineate the effects of bariatric surgery on calcium and vitamin D status.
R54. (2008). Patients who have had bariatric surgery are at increased risk for fracture (approximately 1.2-fold) (47) due to bone loss (primarily related to malabsorptive procedures and effects on protein, calcium, vitamin D, and possibly copper and vitamin K; though bone density is generally higher in patients with obesity), abnormal bone microarchitecture (independent of bone mass and primarily related to mechanical loading, physical activity, and various hormonal and other humoral factors), and increased risk of falls (734-736). In fact, the nature of decreased bone strength, independent of bone density, is an area of intense interest.
Frederiksen et al. (737) utilized high-resolution peripheral quantitative computed tomography (HR-pQCT) to affirm microarchitecture changes after RYGB that suggests accelerated endosteal resorption and disintegration of trabecular structure. Screening guidelines for osteoporotic fracture for all patients may be guided by recommendations from the U.S. Preventive Services Task Force (505). Schafer et al. (502) found that significant bone loss after RYGB occurred in postmenopausal women as early as 6 months postoperatively and persisted through the study duration, which was only 12 months. Using the trabecular bone score as an indirect assessment of skeletal microarchitecture, women had preserved bone microarchitecture for at least 3 years after RYGB (738). In a smaller study of both genders, bone strength by HR-pQCT was preserved for a year after bariatric surgery (LAGB, RYGB, or BPD/DS) (739). However, in another small study, bone strength declined by a year after bariatric surgery (740). Bone loss after RYGB and SG was comparable (at about 8 to 9% loss in patients with T2D) (741), though loss was greater at total hip and femoral neck with RYGB (501). In a meta-analysis of 10 studies (of 1,299 screened), bone density significantly decreased in the femoral neck, but not in the lumbar spine after bariatric surgery, compared with nonsurgical controls (742).
Indices of bone marrow adipose tissue (inversely related to bone density) may serve as a potential marker of skeletal risk in patients after bariatric surgery (501, 743). Although ultrasound of the phalanges yields comparable results with DXA in patients not having bariatric surgery, results are discordant in those having bariatric surgery, most likely due to mechanical loading effects (744). In short, there are insufficient data to provide a more specific recommendation at this time, other than monitoring DXA at lumbar spine and proximal femur sites, at baseline and 2 years post bariatric surgery, with interventions based on clinical judgment (e.g., treating patients with persistent loss and increased fracture risk) (734, 745).
R55. (2013*). In a large Taiwanese database (N = 2,064), bariatric surgery (primarily with malabsorptive procedures) was associated with increased fracture risk in the first 1 to 2 postoperative years (47). In a case-matched study of 120 patients using lumbar spine and total hip DXA, RYGB was associated with greater bone loss than LAGB or SG (746). However, in another study of 66 patients, bone loss was comparable between RYGB and SG (747). Secondary hyperparathyroidism may play a significant role or be a significant marker of this bone loss process. Among 1,470 patients undergoing various bariatric surgical procedures, the overall prevalence of secondary hyperparathyroidism was 21.0% preoperatively, 35.4% at 1 year postoperatively, and 63.3% at 5 years postoperatively, with some procedural differences in these 5-year rates: OAGB (73.6%) > RYGB (56.6%) > LAGB (38.5%) > SG (41.7%) (504). Hence, every effort should be made to screen for and appropriately treat both secondary hyperparathyroidism and osteoporosis to lower fracture risk.
There are no data on the use of antiresorptive agents specifically for management of bone loss resulting from a bariatric procedure, including both bisphosphonates and denosumab (748). The use of specific bisphosphonates in patients with chronic kidney disease is reviewed by Miller et al. (749). Upper-GI adverse effects of oral bisphosphonates are discussed by Lanza et al. (750). The potential for secondary hyperparathyroidism, hypocalcemia, and vitamin D insufficiency/deficiency should be strongly considered and effectively managed when starting antiresorptive agents after a bariatric procedure (748).
R56. (2013*). The pathophysiology of calcium oxalate stone disease following bariatric surgery is related to hyperoxaluria, low urinary volume, and hypocitraturia (751).
R57. (2019*). A recent review by the ASMBS (448) reported higher prevalence rates of certain nutrient deficiencies among patients with obesity considered for bariatric surgery. For example, the prevalence of preoperative deficiencies among fat-soluble vitamins are 14% for vitamin A and 2.2% for vitamin E, but no data are available for vitamin K (448). Postoperatively within 4 years, vitamin A deficiency occurs in up to 70% after RYGB and BPD/DS, whereas vitamin E and K deficiencies are uncommon. The impact of RYGB on vitamin A undernutrition is particularly severe in pregnant women (752). Micronutrient dosing strategies are outlined in Table 14. However, caution should be exercised in the interpretation of biochemical results; for example, vitamin A levels may need to be adjusted for retinol-binding protein levels and vitamin E for cholesterol levels to avoid oversupplementation (600). Additional micronutrient deficiency prevalence rates, which are discussed in subsequent recommendations, are presented by surgical procedure performed and serve to guide decision-making about appropriate biochemical testing, therapeutic dosing for prevention of deficiencies, and therapeutic dosing to manage established deficiencies (753).
R58. (2008*). There are little data about EFA status or comprehensive strategies for the work-up of fat-soluble vitamin levels after bariatric surgery. Forbes et al. (754) found transient increases in 20:4N6 (+18%) and 22:6N3 (+11%) with decreases in 20:3N6 (−47%) and 20:5N3 (−79% and −67%) at 1 and 6 months, respectively, after RYGB, but not LAGB. The 20:5N3 reduction is most concerning, since this EFA is a precursor for anti-inflammatory eicosanoids. However, the impact of these results is mitigated by decreased postoperative intake of dietary fat, decreased body fat postoperatively, and lack of data on the clinical benefit of treatment postoperatively. Topical borage oil (755), soybean oil (756), or safflower oil (756, 757) are rich in EFAs and may be applied to the affected skin areas with EFA deficiency, though conclusive clinical trials, particularly with oral supplementation, are lacking, especially in patients after bariatric surgery. A rational approach of screening for multiple nonestablished fat-soluble vitamin deficiencies with at least one established or suspected EFA deficiency remains to be proven.
R59. (2019*). In the recent ASMBS CPG, iron deficiency was as high as 45% of patients with obesity prior to bariatric surgery and therefore justifies a preoperative aggressive case-finding approach, which may include ferritin levels (448). Key clinical features of iron deficiency prompting suspicion include fatigue, microcytic anemia, glossitis, and nail dystrophy. Postoperatively, iron status should continue to be monitored, but ferritin levels are less helpful, since they are confounded by inflammation, age, and infection (448). Moreover, postoperatively, iron deficiency is 14% after LAGB, 20 to 55% after RYGB, 8 to 62% after BPD/DS, and can occur despite routine supplementation, again justifying routine testing (448). Oral supplementation should be in divided doses, since malabsorption can be exacerbated with calcium supplements, decreased gastric acid, and phytate- or polyphenol-rich foods (448). Vitamin C can be provided with iron supplementation to both improve iron absorption and also decrease the risk of iron overload (758).
R60. (2019*). In the recent ASMBS CPG, B12 deficiency was found in 2 to 18% of patients with obesity (6 to 30% in those on proton-pump inhibitors) prior to bariatric surgery and justifies preoperative aggressive case finding with biochemical testing, specifically using methylmalonic acid (448, 759). Two to 5 years after bariatric surgery, B12 deficiency is < 20% in RYGB and 4 to 20% after SG (448). However, in a meta-analysis directly examining the two procedures, there was a decreased risk for B12 deficiency (but not anemia or iron deficiency) after SG compared with RYGB (760). Notwithstanding the paucity of information about vitamin B12 status after LAGB, global recommendations for ongoing biochemical testing and appropriate B12 supplementation in all patients undergoing bariatric surgery, especially those on folic acid supplementation, may be reasonable, since there is virtually no risk from B12 dosing.
R61. (2013). In the recent ASMBS CPG, folate deficiency was found in as many as 45% of patients with obesity prior to bariatric surgery and justifies aggressive case finding preoperatively with biochemical testing, specifically using sensitive markers, such as red-blood-cell folate and homocysteine (methylmalonic acid is normal with folate deficiency and normal B12 status) (448). Up to 65% of patients after bariatric surgery have a folate deficiency, in part due to poor consumption of folate-rich foods (e.g., various beans, lentils, peas, and other vegetables and fruits) and possible multivitamin nonadherence, again justifying ongoing biochemical monitoring, especially in female patients of childbearing age (448). There remain concerns about masking B12 deficiencies (and therefore starting B12 supplementation) on higher doses of folic acid (≥ 1 mg/d) that require further research, especially after bariatric surgery (761, 762).
R62. (2013). About 10 to 12% of patients with obesity have anemia before bariatric surgery, 33 to 49% of patients have anemia within 2 years after bariatric surgery, and this postoperative prevalence is 17% after SG and 45 to 50% after the malabsorptive procedures RYGB and BPD/DS (763, 764). Though iron deficiency is the most common culprit, folate and vitamin B12 deficiencies are also highly associated with anemia. Though less common, additional micronutrient deficiencies can contribute to anemia after malabsorptive bariatric surgery, namely, vitamins A, B1, D, E, and K, and zinc, selenium, and copper (764-766). Whether a nutritional anemia work-up should be expanded to checking these less common biochemical markers, and supplementing if positive, depends on clinical judgment based on other specific signs/symptoms of deficiency. The association of low protein levels with anemia may be causative in chronic disorders (767) but more of an indirect marker of poor nutrition and other contributory factors after bariatric surgery.
R63. (2013). Clinically significant selenium deficiency is associated with myopathy, cardiomyopathy, arrhythmia, impaired immunity, hypothyroidism, loss of skin/hair pigmentation, and encephalopathy (768). Massoure et al. (769) reported heart failure in a patient 9 months after RYGB that resolved with 2 μg/kg/d × 3 months oral selenium with furosemide and an angiotensin-converting enzyme inhibitor. Among 437 patients having LAGB or SG, selenium deficiency (below normal range 0.75 to 1.85 μmol/L) occurred in 2.3% of patients pre-operatively (3.2% in another, smaller study) (518), and then, while taking a multivitamin-mineral supplement, in 14.9% patients at 3 months postoperatively, 13.8% at 6 months, 13.1% at 12 months, 15.4% at 18 months, 11.4% at 24 months, and 14.3% at 36 months (765). In another study, selenium intake and markers of deficiency were most evident at 3 months after RYGB, but not LAGB, prompting recommendations for routine increases in high selenium foods and use of routine multivitamin supplements with more than 55 μg/d of selenium (768). In a more recent report, Shoar et al. (770) found about 50% of patients undergoing SADI-S had a selenium deficiency.
R64. (2019*). At 5 years postoperatively, patients with low zinc levels after RYGB and BPD/DS are 21.15% and 44.94%, respectively (771). The amount of routine daily zinc supplementation after bariatric surgery depends on the specific procedure, ranging from 8 to 11 mg (100% of usual multivitamin-multimineral supplement zinc content) after SG or LAGB, to 8 to 22 mg (100 to 200% of usual multivitamin-multimineral supplement zinc content) after RYGB, to 16 to 22 mg (200% of usual multivitamin-multimineral supplement zinc content) after BPD/DS (448). Moreover, to avoid copper undernutrition with chronic zinc supplementation, zinc dosing should be in the range of no more than 8 to 15 mg per mg of copper supplemented (448).
R65. (2019*). Copper is primarily absorbed in the duodenum, proximal jejunum, and stomach, so surgeries affecting this functional anatomy can potentially induce a low copper state. At 5 years postoperatively, patients with low copper levels after RYGB and BPD/DS were 13.48% and only 1.92%, respectively (771). This compares with patients undergoing Roux-en-Y reconstruction for gastric cancer in which copper deficiency was relatively infrequent (5.9%) and symptoms rare (772). In the same study, copper levels among those having RYGB or BPD/DS were lower with younger age, shorter follow-up (< 3 years), and male gender (772). The amount of copper supplementation varies depending on the surgical procedure performed, with greater amounts required for patients after RYGB and BPD/DS and is guided by serum copper levels (448). Initial supplementation dosing ranges from 3 to 8 mg/day of oral copper as gluconate or sulfate to 2 to 4 mg/day intravenously, and then titrated to normal levels and amelioration of signs/symptoms (448).
R66. (2019*). In a study by Nath et al. (525), 16.5% of patients after RYGB had clinical thiamine deficiency defined by the presence of consistent clinical symptoms and either low whole-blood thiamine levels or significant improvement after thiamine supplementation. Thiamine is the first vitamin depleted in patients who experience chronic nausea/vomiting or food intolerance (521). Among those with clinical thiamine deficiency, 70% had cardiac, 59% had peripheral neurologic, 14% had GI, and 5% had neuropsychiatric symptoms. Abnormal intestinal microbiota is thought to be a contributory factor to low thiamine levels after RYGB, and levels improved with antibiotics (773). Early/aggressive supplementation of thiamine in at-risk patients (those with chronic nausea/vomiting, decreased intake by mouth) can avert the adverse effects of clinically significant thiamine deficiency. Of note, there is increased urinary thiamine excretion with both T1D (76% decreased thiamine levels) and T2D (75% decreased thiamine levels) (774). On the other hand, Aaseth et al. (775) found that thiamine levels after RYGB were relatively constant up to 5 years postoperatively. Interestingly, elevated thiamine levels were found in 43% of patients already on micronutrient supplementation up to 12 months after BPD/DS in a study by Homan et al. (776). Additional information on thiamine deficiency and supplementation can be found in the 2008 and 2013 versions of these guidelines (1, 54).
WE has been reported after purely restrictive procedures (e.g., LAGB, SG, and IGB) and may in large part reflect preexisting thiamine undernutrition; routine assessment of thiamine status in any patient after bariatric surgery with any early or suggestive features of WE is recommended (777-779). For example, in patients after bariatric surgery, fundoscopic exam can detect the early findings of a severe thiamine deficiency at risk for WE: retinal hemorrhage, optic disc edema, and peripapillary telangiectasia (780). An unusual presentation initially diagnosed as an ischemic stroke was described by Blum et al. (781) in a patient 9 months after SG, ultimately diagnosed with WE. There are also ethnicity differences in prevalence rates of thiamine deficiency, with up to 33% in Latinos preoperatively, where the total (all ethnicities) rate was only 1.8% (732). Updated physiology, recommendations, and discussion for thiamine supplementation are provided in the ASMBS guidelines (448) and a review by Frank (782). Although evidence is limited, if IV access is not available in the acute setting, then intramuscular thiamine dosing may be considered (783).
R67. (NEW). Many commercial dietary supplement products are adulterated with compounds that are not included in the manufacturer’s labelling. These products can have intrinsic toxicity; mitigate or intensify the desired clinical action; interact with certain foods, other supplements, or specific medications; or have unknown but potentially harmful effects (784). The best principle is for HCP and patients to discuss all supplements at each encounter. United States Pharmacopeia products, supplements that have been used in published clinical trials, or other brands that the prescribing HCP has a positive (safe and effective) experience with are preferred.
R68. (2013*). In a prospective, single-center cohort study of 65 patients after SG, there was a 6% reduction in lipid-lowering medication use at 1 month and 24% at 6 months (785). The pathophysiology of bariatric surgery on lipids is complex, with salutary effects on lipid metabolism postoperatively, but also downstream effects of lipids on micronutrient status and effects of micronutrients on lipid status (435, 436, 786). These networked effects among obesity, bariatric surgical disruption of GI physiology, lipid status, micronutrient status, and CVD risk will need further elucidation and research.
R69. (2019*). In a meta-analysis, 32 of 57 clinical studies reported improvement of HTN in 32,628 of 51,241 patients, and 46 of these studies reported resolution of HTN in 24,902 of 49,844 patients after bariatric surgery (97). In another analysis of 23 studies with a pooled group of 1,022 patients, bariatric surgery was cardioprotective and induced a decrease in left ventricular mass, left-atrium diameter, and improvement of left-ventricular diastolic function, but without changes in left-ventricular ejection fraction (787). Renal function also improves after bariatric surgery in those patients with HTN (788). In a prospective, single-center cohort study of 65 patients after SG, there was a 12% reduction in antihypertensive medication use at 1 month and 25% at 6 months (785). One more study of 183 consecutive patients undergoing SG showed that 50% of the patients reduced blood pressure medications and 34% discontinued the medications postoperatively (789). Overall, there are reductions in CVD risk, events, and mortality after bariatric surgery (94, 790). Decreased blood pressure can occur postoperatively even before appreciable weight loss, particularly in patients with orthostatic intolerance and possible dysautonomia (791).
R70. (NEW). The ongoing need for medications for T2D depends on the specific bariatric surgical procedure and needs to be monitored postoperatively. In a retrospective review of 400 patients in the Bariatric Outcomes Longitudinal Database, the use of oral hypoglycemic agents or insulin decreased after bariatric surgery by 18.8% and 4.2%, respectively (792). In a prospective, single-center cohort study of 65 patients having SG, there was a 50% reduction in diabetes medications (785). Among 183 patients after SG (with 58.4% 2-year median loss of excess body weight), 78.9% and 15.8% of those with T2D had their diabetes medications discontinued or reduced, respectively (789). In a retrospective study of 79 patients undergoing LAGB and followed for 10 years, diabetes control, as well as blood pressure, lipid profile, and quality of life improved, but without significant changes in diabetes medication and with a high rate of revisional surgery (793).
R71. (NEW). Thyroid dosing is generally decreased after bariatric surgery due to weight loss, but some case studies have reported increased dosing with significant malabsorption (794). Several case reports have demonstrated the benefit of liquid forms of levothyroxine in postoperative patients with hypothyroidism, significant malabsorption, and difficulty normalizing elevated TSH levels (795). Liquid forms may also be indicated in those patients with swallowing difficulties after bariatric surgery (796). The use of softgel levothyroxine may also be considered in patients with established or suspected malabsorption of tablet forms (796, 797).
R72. (2019*). In a retrospective review of patients with RYGB or BPD/DS, a CT is the most appropriate imaging tool to help identify an intestinal obstruction or internal hernia (798). In some cases, conclusive findings are missed on imaging, and diagnostic laparoscopy should be considered if symptoms persist. Severe abdominal pain after SG may be the result of mesenteric venous thrombosis, which is associated with shorter courses of VTE prophylaxis and best diagnosed with contrast-enhanced CT (641, 799). In a multi-institutional, matched, case-controlled study using a U.S. database from 2008-2012 (8,980 patients in the study group and 43,059 controls), there were 15 cases of inflammatory bowel disease in those with a prior history of bariatric surgery (OR, 1.93; 95% confidence interval [CI] 1.34 to 2.79) (800).
R73. (NEW). In a retrospective study of 919 patients undergoing SG, 13% had preexisting GERD, and 3% developed de novo GERD, with the majority responding to proton-pump inhibitors; however, there was 1 patient with de novo and 3 patients with preexisting GERD requiring conversion to RYGB (727).
R74. (2019*). Although short-term postoperative use of NSAIDs for patients after bariatric surgery is standard practice, long-term use generally should be avoided. In a retrospective review of 1,001 patients who had RYGB, NSAID and tobacco use significantly increased the risk of marginal ulceration, and upper endoscopy is useful to exclude or detect and then dilate strictures in patients who have had RYGB (801). Proton-pump inhibitor use was protective in these patients exposed to NSAIDs (801). In a retrospective cohort study of 13,082 patients having colorectal or bariatric surgery by Hakkareinen et al. (802), NSAID use was associated with an increased rate of anastomotic leak. Simply providing letters or written notification to avoid or discontinue use of NSAIDs after RYGB (and other bariatric procedures by extension) is ineffective (803).
R75. (2019*). Upper-GI endoscopy in the early postoperative period after RYGB is safe (485, 804). The use of GI endoscopy in patients who have had bariatric surgery is supported by the study by Wilson et al. (801). Interestingly, recent data from an RCT demonstrate the utility of intraoperative endoscopy to detect technical defect–related leaks using the air-leak test (805).
R76. (NEW). In a systematic review of 41 studies involving 16,987 patients having RYGB, marginal ulcers, diagnosed by upper endoscopy, occurred in 0.6 to 25% and were associated with pouch size and position, smoking, alcohol consumption, and NSAID use (806). In a retrospective cohort study (807) and a meta-analysis of 7 prospective cohort studies involving 2,917 (2,114 analyzed) patients (808), prophylactic administration of a proton-pump inhibitor for 90 days postoperatively was superior to 30 days in the prevention of symptomatic marginal ulcers. However, since most marginal ulcers occur within the first 12 months following surgery, extension of proton-pump inhibitor therapy for the first postoperative year should be considered in patients at high risk as a preventive measure (801).
R77. (2013*). A meta-analysis of 175 studies (many were single-center retrospective reviews) on patients with inadequate weight loss after bariatric surgery demonstrated improved weight loss and reduction of comorbidities with revisional surgery (though complication rates were higher with re-operative compared with primary surgery) (275). In a 1:1 comparison case-matched analysis of primary versus revisional RYGB, comorbidity resolution and total weight loss were similar, with weight loss after revisional surgery less than after primary surgery. Revisional surgery was found to be safe (809). Among 1,300 patients having SG, conversion to RYGB was associated with a mean loss of excess weight of 61.3% after 1 year (810). Based on retrospective analysis of two cohorts, endoscopic gastrojejunostomy revision also has demonstrated greater effectiveness than medical management for weight regain after RYGB (811). Band-to-bypass conversional surgery for inadequate weight loss, symptoms, clinical goals, and/or comorbidities is effective, but due to the complex nature of the procedure, it is associated with morbidity (812). There are inadequate data for a formal recommendation about band-to-bypass conversional surgery. In a retrospective review of 1,273 patients, gastro-gastric fistula occurred in 106% of those who had RYGB, generally due to gastric ischemia, fistula, or ulceration, and the majority presented with weight regain (80%) and pain (73.3%), where surgical revision was based on the anatomy: low fistula with gastric resection and gastrojejunal anastomotic revision, or high fistula with sleeve of the pouch and sleeve resection of the remnant stomach (813). Revisional surgery has also been performed to improve glycemic control in bariatric surgery patients with persistent T2D, with subsequent T2D improvement in 65 to 100% of patients (277).
R78. (2019*). Evaluation with upper-GI contrast study is the primary imaging modality to detect band slippage, esophageal dilation, and in some patients, erosion (814, 815).
R79. (2019*). Rapid weight loss is the primary risk factor for gallstones, detected by abdominal ultrasound, after SG or RYGB (816). In general, cholecystectomy should be reserved for patients with symptomatic biliary disease, as the risk of needing a postoperative cholecystectomy is 6 to 10% (817). In asymptomatic patients with known gallstones and a history of RYGB or BPD/DS, prophylactic cholecystectomy may be considered to avoid choledocholithiasis, since traditional endoscopic retrograde cholangiopancreatography can no longer be performed in these patients (818). Since the aggregate complication risk of cholecystectomy is lower when performed prior, compared with during or after RYGB, the appropriate use of preoperative cholecystectomy and optimization of preventive measures postoperatively are critical (819). In a retrospective review of a prospectively collected database, 500 mg of ursodeoxycholic acid daily for 1 year efficiently prevented gallstones after SG, with twice daily dosing effective for RYGB (820). A meta-analysis of eight studies (retrospective, prospective cohort, and randomized controlled) with 816 patients by Magouliotis et al. (821) supported the role of 500 to 600 mg/day of ursodeoxycholic acid for 6 months after bariatric surgery. A more definitive, randomized, double-blind multicenter trial (N = 900 patients with SG or RYGB) assessed the efficacy of 900 mg/day of ursodeoxycholic acid for 6 months on symptomatic gallstones by 24 months (822).
R80. (2013*). Of note, SIBO is fairly common (15 to 17%) preoperatively in patients who had RYGB (N = 378), rises to 40% after RYGB (but not LAGB), and may be associated with a lower overall weight loss (823, 824). Thiamine deficiency is associated with SIBO after RYGB (49% of patients) due to bacterial thiaminase production in the setting of compromised thiamine transporter-1 and -2 with shortened biliopancreatic limb, relatively low intakes, and small reserves, especially with obesity, while also leading to gut dysmotility (e.g., constipation) (773, 825). SIBO is also associated with severe hepatic steatosis in patients with obesity (824).
R81. (2008*). Timing of repair of abdominal wall hernias is debatable, with insufficient evidence for a recommendation; strategy would depend on the hernia size, location, and type (826).
R82. (2013*). Body contouring may be associated with weight-loss benefits following bariatric surgery, including an increase in total weight loss and an improvement in long-term weight-loss maintenance (827, 828). Currently, an estimated 6 to 41% of patients undergo body contouring after bariatric surgery, with the large amount of variability likely due to poor access to care due to limited insurance coverage (827, 829, 830). When plastic surgery practice surveys and insurance coverage requirements were analyzed by Dreifuss and Rubin (831), there were discrepancies noted regarding the criteria for panniculectomies, arguing for greater input by surgeons in the development of coverage guidelines. Correcting underlying nutritional deficiencies is important in decreasing the frequency of complications, which can occur with body-contouring surgery (832). For example, since iron-deficiency anemias, which may be found in patients after bariatric surgery, could complicate a body-contouring procedure, the use of IV iron therapy may be needed (833). While the overall complication rate of body contouring after bariatric surgery is high, the majority of such complications are considered minor (834). In a retrospective, multiple regression analysis of 205 patients having body-contouring surgery after bariatric surgery, no main risk factors were identified (835).
Q7. What are the criteria for hospital admission after a bariatric procedure?
R83. (2013). There has been a notable shift in case type since 2011, with significantly increased numbers of SG (58.1% in 2016) and revisional procedures (13.9% in 2016), with SG now the most commonly performed bariatric surgery, and a decrease in RYGB (18.7% in 2016 compared with 37.5% in 2012) and a significant decline in LAGB (3.4% in 2016 compared with 35.4% in 2011) procedures (239). There has been an interval reduction in average length of stay and hospital readmission rate. Accreditation of centers and utilization of ERABS protocols are associated with shorter lengths of stay (584, 836). However, in this case, a shorter length of stay does not appear to be associated with increased readmission rates (584). Readmission rates within 30 days were evaluated in 130,007 patients undergoing primary bariatric surgery for a total of 4.4%. Specifically, LAGB had the lowest rate of 1.4%, followed by SG 2.8%, and RYGB 4.9% (837). The most common cause for readmission was nausea, vomiting, fluid, electrolyte, and nutritional depletion (35.4%), followed by abdominal pain (13.5%), anastomotic leak (6.4%), and bleeding (5.8%), accounting for more than 61% of readmissions (837). When compared with LAGB, SG and RYGB had significantly higher rates of readmission (SG: OR, 1.89; 95% CI, 1.52 to 2.33 and RYGB: OR, 3.06; 95% CI, 2.46 to 3.81) (837). Similar trends were noted in another study, with readmission rates highest for LRYGB at 11.6%, followed by SG with 7.6%, and LAGB with 4.5% (838). Readmissions are highest within 30 days. Readmissions that occur at greater than 30 days are more frequently associated with RYGB than SG and LAGB (839).
R84. (2008). Risk factors for readmission are multifactorial and include longer index hospital length of stay, procedure choice, prolonged index operation, and complication during index hospitalization. Complication during index hospitalization is associated with greater need for readmission that requires intervention such as reoperation or endoscopy (839, 840). RYGB is associated with increased long-term (> 30 days) readmissions, compared with SG and LAGB (26, 839, 840). Race and insurance status were also risk factors for readmission in other studies (26). Preoperative education, planning, and postoperative care coordination with early follow-up can reduce preventable emergency room visits and readmissions for mild dehydration, nausea, or dietary intolerance issues (838-841). Morton et al. (842) showed a reduction in 30-day readmission rates from 8% to 2.5% over 18 months by implementation of a readmission bundle and ongoing vigilance to readmission.
R85. (2008). A recent systematic review identified 35 articles encompassing a total of 100 patients undergoing reversal of RYGB. Malnutrition was the most common indication for reversal (12.3%), followed by severe dumping syndrome (9.4%), postprandial hypoglycemia (8.5%), and excessive weight loss (8.5%) (843). Protein malnutrition and excessive weight loss remain the most common causes of reversal after BPD/DS (844).
Disclosure
Chair of the Task Force
Jeffrey I. Mechanick, MD, MACE: Abbott Nutrition, honoraria for lectures and program development.
Co-Chairs of the Task Force
Caroline Apovian, MD: Orexigen, GI Dynamics, Takeda, Nutrisystem, Zafgen, Sanofi-Aventis, Novo Nordisk, Scientific Intake, Xeno Biosciences, Rhythm Pharmaceuticals, Eisai, EnteroMedica, Bariatrix Nutrition, consultant; Gelesis and Science-Smart LLC, stock options; Aspire Bariatrics, Myos, the Vela Foundation, the Dr. Robert C. and Veronica Atkins Foundation, Coherence Lab, Energesis, Gelesis, Orexigen, GI Dynamics, Takeda, Novo Nordisk, PCORI, research grant support.
Stacy Brethauer, MD: Medtronic, speaker.
W. Timothy Garvey, MD, FACE: Merck, Novo Nordisk, American Medical Group Association, BOYDSense, Sanofi, Gilead, Amgen, Abbott Nutrition, National Diabetes and Obesity Research Institute, diaTribe Foundation, consultant; IONIS, Novartis, Bristol-Myers-Squibb, Pfizer, Merck, Lilly, stock; Sanofi, Novo Nordisk, Pfizer, research grant support.
Aaron M. Joffe, DO, FCCM: American Society of Anesthesiologists, speaker.
Robert F. Kushner, MD: Novo Nordisk, Retrofit, Weight Watchers, consultant.
Richard Lindquist, MD, FAASP: Seca scales, Metagenics/Bariatric Advantage, Livongo/Retrofit, consultant; Novo Nordisk Orexigen, speaker.
Rachel Pessah-Pollack, MD, FACE: Boehringer Ingelheim, Eli Lilly, Radius, consultant and advisor.
Richard D. Urman, MD, MBA, CPE: 3M, Sandoz, consulting fees; Merck, Medtronic, research grant support.
Julie Kim, MD; Jennifer Seger, MD: Report no potential conflicts of interest.
Task Force Members
Shanu Kothari, MD, FACS, FASMBS: Ethicon, Lexington Medical, consultant; Gore Medical, speaker.
Michael V. Seger, MD, FACS, FASMBS: Obalon Therapeutics, consultant and speaker.
Christopher D. Still, DO, FACN, FACP: Novo Nordisk, speaker; Ethicon-Endosurgery, research grant support.
M. Kathleen Figaro, MD, MS, FACE: Novo Nordisk, Boehringer Ingelheim, speaker.
Stephanie Adams, PhD; John B. Cleek, MD; Karen Flanders, MSN, CNP, CBN; Jayleen Grams, MD, PhD; Daniel L. Hurley, MD, FACE; Riccardo Correa, MD, FACE: Report no potential conflicts of interest.
Reviewers
Michael A. Bush, MD, FACE: AACE Past President; CA-AACE Past Clinical Chief, Division of Endocrinology, Sinai Medical Center; Associate Clinical Professor, Geffen School of Medicine, UCLA, Beverly Hills, CA. Dr. Bush has received speaker honoraria from Eli Lilly, Novo Nordisk, Sanofi, Boehringer Ingleheim, Janssen, and AstraZeneca.
Scott Isaacs, MD, FACP, FACE: Adjunct Assistant Professor of Medicine, Emory University School of Medicine, Atlanta, GA. Dr. Isaacs reports no potential conflicts of interest.
Ann M. Rogers, MD, FACS: Director, Penn State Surgical Weight Loss Program; Professor of Surgery, Vice Chair for Leadership and Professional Development, Penn State Milton S. Hershey Medical Center, Hershey, PA. Dr. Rogers reports no potential conflicts of interest.
Dace L. Trence, MD, MACE: Professor of Medicine; Director, Endocrine Fellowship Program; University of Washington, Seattle, WA. Dr. Trence reports stock ownership of Medtronic and Sanofi.
Disclaimer
The American Association of Clinical Endocrinologists, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists medical guidelines for clinical practice are systematically developed statements to assist health care professionals in medical decision-making for specific clinical conditions. Most of the content herein is based on clinical evidence. In areas of uncertainty, or when clarification is required, expert opinion and professional judgment were applied. These guidelines are a working document that reflects the state of the field at the time of publication. Because rapid changes in this area are expected, periodic revisions are inevitable. We encourage medical professionals to use this information in conjunction with their best clinical judgment. The presented recommendations may not be appropriate in all situations. Any decision by practitioners to apply these guidelines must be made considering local resources and individual patient circumstances.





