Reaching the Tipping Point: Identification of Thresholds at which Visceral Adipose Tissue May Steeply Increase in Youth
Abstract
Objective
This study aimed to determine whether children and adolescents demonstrate, similarly to adults, a threshold of total percent body fat (%BF) above which the slope of visceral adipose tissue (VAT) rises.
Methods
This cross-sectional study included 557 youth, aged 8 to 18 years, with a wide range of BMI values. Dual-energy x-ray absorptiometry was used to determine body composition (including VAT), and fasting blood was collected for measurement of lipids, glucose, insulin, and biomarkers. Segmented linear regression analysis identified the threshold for %BF unadjusted and adjusted for Tanner stage. Linear regression with robust variance estimation compared associations of risk factors and thresholds.
Results
Thresholds of %BF were identified by sex (males = 33%, females = 38%), age (< 12 years = 34%; ≥ 12 years = 30%), and race (White/non-Hispanic = 31%; all other races/Hispanic = 38%) above which the slope of VAT was significantly steeper (all P < 0 .001). The percentage of total body fat stored as VAT was higher above versus below these thresholds (all P < 0.001). Above threshold, but not below it, VAT was associated with triglycerides/high-density lipoprotein ratio, insulin, adiponectin, and blood pressure.
Conclusions
The thresholds should be confirmed in longitudinal studies, and they may be useful in identifying youth at increased cardiometabolic risk in need of close clinical monitoring and/or intensive intervention to reduce excess adiposity.
Study Importance
What is already known?
- Visceral adipose tissue (VAT) is a primary driver of increased cardiometabolic risk.
- In adults, sex-specific thresholds of total percent body fat (%BF) have been identified above which levels of VAT steeply increase; whether similar thresholds exist in children and adolescents remains unclear.
What does this study add?
- We identify sex-specific %BF thresholds at which VAT levels appear to steeply increase in children and adolescents.
- Cardiometabolic risk factors, adipokines, and inflammation are more closely associated with VAT above vs. below the threshold, suggesting these cut points may have clinical utility in identifying individuals at increased cardiometabolic risk and in need of more intensive intervention.
Introduction
Visceral adipose tissue (VAT) is a highly metabolically active fat depot thought to be a primary driver of cardiometabolic risk factors such as hypertension, dyslipidemia, insulin resistance, inflammation, and oxidative stress in children and adolescents (1-3). In contrast, subcutaneous adipose tissue has been shown to be protective (4-6), suggesting that the ratio of these fat depots may in large part influence cardiometabolic health. In line with this theoretical construct, we previously reported sex-specific percent body fat (%BF) thresholds in adults, above which there was a significant change in the slope of the relationship between VAT and total adiposity (7, 8). Specifically, individuals above these thresholds had higher levels of VAT and a higher ratio of visceral to subcutaneous fat compared with those below the thresholds. Moreover, VAT was more highly correlated with cardiometabolic risk factors among individuals above versus below the thresholds (8).
A relevant hypothesis has suggested that subcutaneous fat depots may have a limited capacity for expansion such that additional fat accumulation exceeding a specific threshold may be associated with “spillover” into other ectopic depots, including the visceral region (9-11). In this scenario, constraints on subcutaneous fat expandability in the presence of weight gain would be biologically disadvantageous and would potentially accelerate the development of cardiometabolic risk factors. Though some studies have reported nonlinear associations of BMI and waist circumference with VAT in children and adolescents (12, 13), this “threshold hypothesis” has been incompletely investigated in the pediatric population, especially in youth with high levels of BMI consistent with severe obesity. However, the impetus to do so is supported by our previous observations showing that youth with VAT at or above the cohort mean had stronger associations of VAT with cardiometabolic risk factors compared with those below (14). The existence of such thresholds may have clinical significance in terms of screening and weight management practices and may provide insight into appropriate weight loss goals for pediatric patients with adiposity levels above the cut points.
Therefore, the purpose of this study was to determine whether %BF thresholds exist in children and adolescents at which the slope of VAT significantly increases. Additionally, we sought to characterize the associations of VAT with cardiometabolic risk factors, adipokines, inflammation, and oxidative stress above and below the thresholds, if identified. We hypothesized that, similar to adults, children and adolescents would demonstrate %BF thresholds above which the slope of VAT would be steeper and levels of VAT much higher and more strongly associated with cardiometabolic risk factors.
Methods
Study design and participants
This cross-sectional study included children and adolescents aged 8 to < 18 years old with a wide range of BMI values recruited from the greater Minneapolis and St. Paul, Minnesota, area. For this analysis, data were combined from four different studies that utilized identical data collection methods and techniques: (1) a cross-sectional study evaluating cardiovascular health (15), (2) baseline data from participants in a trial of topiramate for weight loss (16), (3) baseline data from participants in an ongoing trial of exenatide for weight loss, and (4) baseline data from participants in an ongoing trial of financial incentives for weight loss. Participants were categorized according to BMI as having normal weight (< 85th BMI percentile), overweight (85th BMI percentile to <95th BMI percentile), class I obesity (≥ 95th BMI percentile to < 120% of the 95th BMI percentile), class II severe obesity (≥ 120% of the 95th BMI percentile to < 140% of the 95th BMI percentile), or class III severe obesity (≥ 140% of the 95th BMI percentile) (17, 18). The respective study protocols were approved by the University of Minnesota Institutional Review Board, and consent/assent was obtained from parents or guardians or from the participant.
Measurement of clinical variables
All testing was performed in the morning after participants had been fasting for a minimum of 12 hours. Pubertal development stage (Tanner stages 1-5) was determined by a pediatrician or a trained registered nurse. Height and weight were determined with participants wearing light clothes and without shoes using a wall-mounted stadiometer and an electronic scale, respectively. BMI was calculated as the body weight in kilograms divided by the height in meters squared. BMI percentiles were determined using age- and gender-based definitions from the United States Centers for Disease Control and Prevention. Seated blood pressure was obtained on the right arm after 5 minutes of quiet rest, using an automatic sphygmomanometer and an appropriately fitted cuff. Three measurements were taken, and the average of the final two was used. Blood was drawn for measurement of lipids, glucose, and insulin using standard procedures (analyzed by Fairview Diagnostics Laboratories, Fairview-University Medical Center, Minneapolis, Minnesota, a laboratory certified by the Centers for Disease Control and Prevention). On a subset of participants (N = 336), high-molecular-weight adiponectin (R&D Systems), C-reactive protein (Alpco), and oxidized low-density lipoprotein cholesterol (Mercodia) were measured by enzyme-linked immunosorbent assay (ELISA) in the University of Minnesota Cytokine Reference Laboratory (licensed by Clinical Laboratory Improvement Amendments).
Body composition and VAT quantification
Total body composition was measured using dual-energy x-ray absorptiometry (DXA) (iDXA, GE Healthcare) and analyzed using its enCORE software (platform version 16.2). Participants were scanned using standard imaging and positioning protocols while fasted >12 hours. Estimates of abdominal visceral and subcutaneous fat were obtained using the method described previously (19). Briefly, the DXA estimation method was developed by modeling the two similar regions of interest, DXA- and computed tomography (CT)-measured VAT volumes. In adults, the method showed strong association with CT for males (r2 = 0.949) and females (r2 = 0.957), respectively, and the 95% confidence interval mean difference was −96.0 to −16.3 cm3 (19). The Bland-Altman bias was +67 cm3 in females and +43 cm3 in males (19). Our group has demonstrated a high degree of correlation between single-slice measures of VAT assessed by CT and DXA in children and adolescents (20). DXA-derived visceral fat was strongly associated with cardiometabolic risk factors (to a level similar to that of CT-measured VAT), offering evidence supporting the clinical validity of the DXA method for quantifying visceral fat in youth (20). All scans were reviewed by the same technician.
Statistical analysis
Descriptive characteristics were calculated using the mean (SD) or N (%) within each BMI category for continuous and categorical variables, respectively. The relationship between %BF and VAT within sex and additionally by age group (8 to <12 years old, 12 to <18 years old) and race/ethnicity classification (White/non-Hispanic; all other races/Hispanic) was evaluated with segmented linear regression without additional covariates in the first model (model 1) and adjusted for Tanner stage in the second model (model 2) to identify a potential change in slope indicating a threshold or cut point for a shift in VAT accumulation with increasing adiposity that may be associated with metabolic health status. Davies’ test was used to test whether the slopes above and below the breakpoint were significantly different. The same analysis was performed using multiples of the 95th BMI percentile (instead of %BF) to explore whether thresholds could be identified with a more clinically relevant metric. Percent VAT (%VAT) and the ratio of VAT to subcutaneous fat in individuals above versus below the threshold were compared using generalized estimated equations with independence working correlation structure and robust variance estimation for confidence intervals and P values. We present associations of %VAT with cardiometabolic risk factors as outcomes in individuals above versus below the threshold using an interaction of %VAT and an indicator for the given threshold, and their interaction. %VAT was used in the models with cardiometabolic risk factors to control for total fat mass differences without introducing multicollinearity between total fat mass and VAT mass in individuals above the threshold. All analyses were conducted in R version 3.5.1.
Results
Participant characteristics
A total of 557 participants (mean age 13.8 [2.7] years; 260 [46.7%] males, 297 [53.3%] females) were included in this study. As expected, levels of body fat were higher and cardiometabolic risk factors were more adverse with increasing BMI category (Table 1).
| Covariate | Normal weight (N = 142) | Overweight (N = 29) | Class I obesity (N = 80) | Class II obesity (N = 169) | Class III obesity (N = 137) |
|---|---|---|---|---|---|
| Male | 80 (56.3%) | 13 (44.8%) | 34 (42.5%) | 64 (37.9%) | 69 (50.4%) |
| Race | |||||
| American Indian/Alaskan Native | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (1.2%) | 1 (0.7%) |
| Asian | 1 (0.7%) | 1 (3.4%) | 2 (2.5%) | 3 (1.8%) | 1 (0.7%) |
| African American/Black | 12 (8.5%) | 3 (10.3%) | 8 (10.0%) | 10 (5.9%) | 22 (16.1%) |
| Hawaiian/Pacific Islander | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) |
| Multiple races selected | 6 (4.2%) | 2 (6.9%) | 9 (11.2%) | 14 (8.3%) | 19 (13.9%) |
| Other | 2 (1.4%) | 0 (0.0%) | 2 (2.5%) | 1 (0.6%) | 1 (0.7%) |
| Caucasian/White | 121 (85.2%) | 23 (79.3%) | 59 (73.8%) | 139 (82.2%) | 92 (67.2%) |
| Hispanic | 8 (5.6%)2 | 2 (6.9%) | 10 (12.5%) | 23 (13.6%)2 | 17 (12.4%)3 |
| White, non-Hispanic | 116 (81.7%) | 21 (72.4%) | 51 (63.8%) | 123 (72.8%) | 79 (57.7%) |
| Tanner stage | |||||
| 1 | 54 (38.0%) | 5 (17.2%) | 22 (27.5%) | 12 (7.1%) | 4 (2.9%) |
| 2-4 | 67 (47.2%) | 16 (55.2%) | 41 (51.2%) | 68 (40.2%) | 67 (48.9%) |
| 5 | 12 (8.5%)9 | 7 (24.1%)1 | 14 (17.5%)3 | 89 (52.7%) | 61 (44.5%)5 |
| ≥ 12 years old | 81 (57.0%) | 20 (69.0%) | 45 (56.2%) | 144 (85.2%) | 126 (92.0%) |
| Age (y) | 12.5 (2.57) | 13.3 (2.61) | 12.6 (2.74) | 14.7 (2.45) | 14.8 (2.2) |
| Height (cm) | 153 (15.0) | 159 (12.3) | 156 (14.1) | 165 (11.3) | 169 (10.6) |
| Weight (kg) | 44.4 (13.4) | 61.5 (13.2) | 69.5 (18.3) | 97.4 (18.2) | 124 (23.3) |
| BMI (kg/m2) | 18.4 (2.47) | 24.0 (2.22) | 28.1 (3.77) | 35.3 (3.63) | 43.3 (5.29) |
| BMI percentile | 48.0 (23.0) | 90.7 (3.13) | 97.5 (0.94) | 99.0 (0.27) | 99.6 (0.18) |
| Percentage of 95th BMI percentile | 73.7 (6.74) | 92.9 (4.58) | 112 (5.82) | 130 (5.55) | 160 (16.5) |
| Trunk fat (kg) | 3.92 (2.03) | 8.92 (3.56) | 13.4 (4.56) | 22.5 (5.22)5 | 32.1 (7.59)9 |
| Total fat (kg) | 10.7 (4.11) | 20.0 (5.6) | 28.5 (8.53) | 43.9 (9.65)6 | 61.3 (12.8)11 |
| Total tissue fat (%) | 25.2 (5.95) | 34.5 (7.56) | 42.3 (5.31) | 47.2 (4.96)5 | 51.1 (4.15)9 |
| Visceral fat mass (g) | 80 (47) | 250 (238) | 486 (270)2 | 1041 (467)36 | 1729 (683)31 |
| Visceral fat (%) | 0.84 (0.53)2 | 1.14 (0.81) | 1.66 (0.68)2 | 2.39 (0.9)38 | 2.8 (0.81)33 |
| VAT/SC ratio | 0.01 (0.01)2 | 0.01 (0.01) | 0.02 (0.01)2 | 0.02 (0.01)38 | 0.03 (0.01)33 |
| Glucose (mg/dL) | 77.3 (9.0)3 | 79.9 (9.7) | 80.7 (8.94)1 | 78.7 (10.4)5 | 79.8 (8.52)4 |
| Insulin (mU/L) | 4.24 (2.79)3 | 7.73 (4.98)1 | 10.8 (6.18)3 | 18.2 (11.4)9 | 24.6 (14.5)6 |
| HDL cholesterol (mg/dL) | 59.8 (14.4)3 | 51.0 (14.7) | 47.3 (11.1)1 | 43.8 (11.6)5 | 40.5 (8.48)4 |
| Triglycerides (mg/dL) | 71.2 (27.8)4 | 97.5 (41.8) | 115 (55.2)1 | 118 (59.7)5 | 122 (48.4)4 |
| Triglycerides/HDL ratio | 1.29 (0.68)4 | 2.13 (1.2) | 2.7 (1.84)1 | 2.98 (1.9)5 | 3.24 (1.71)4 |
| LDL cholesterol (mg/dL) | 80.5 (23.2)3 | 90.5 (23.6) | 95.7 (23.9)1 | 93.1 (30.0)6 | 94.2 (26.8)4 |
| Total cholesterol (mg/dL) | 154 (26.7)3 | 161 (26.7) | 166 (28.0)1 | 160 (32.2)5 | 159 (31.3)4 |
| Oxidized LDL (U/L) | 41.6 (19.5)10 | 47.8 (22.7)1 | 56.0 (35.0)8 | 64.8 (42.5)111 | 59.8 (30.7)91 |
| HMW adiponectin (μg/mL) | 5.5 (3.5)10 | 4.2 (2.6)1 | 3.4 (2.0)8 | 2.5 (1.3)111 | 2.3 (1.8)91 |
| CRP (mg/L) | 1.5 (3.2)10 | 4.4 (9.1)1 | 7.4 (10.7)8 | 7.6 (9.0)111 | 12.0 (9.9)91 |
| SBP (mmHg) | 106 (9.62) | 110 (9.11) | 114 (11.3) | 119 (10.5)1 | 126 (11.1)2 |
| SBP percentile | 44.6 (24.7) | 51.3 (27.7) | 64.6 (25.6) | 68.4 (26.4)1 | 79.8 (20.8)2 |
| DBP (mmHg) | 56.7 (8.61) | 58.6 (6.85) | 58.8 (8.96) | 65.5 (8.31)1 | 69.3 (9.14)2 |
| DBP percentile | 32.1 (21.1) | 32.6 (19.4) | 37.1 (22.3) | 49.9 (23.1)1 | 59.0 (24.7)2 |
- Data presented as mean (SD) or n (%). Superscripts denote number missing observation.
- CRP, C-reactive protein; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HMW, high molecular weight; LDL, low-density lipoprotein; SBP, systolic blood pressure; VAT/SC, visceral adipose tissue/subcutaneous fat.
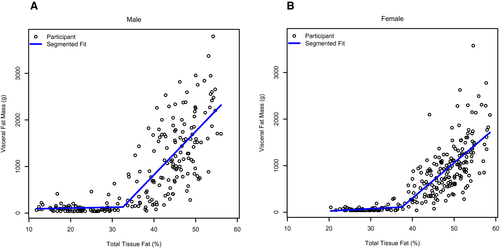
Identification of visceral fat breakpoint
Figure 1 shows the relationship between %BF and VAT in males (panel A) and females (panel B). Table 2 shows results of the segmented regression analysis identifying the various breakpoints for %BF and multiples of the 95th BMI percentile on VAT. Thresholds of %BF were identified by sex (males = 32.6%, females = 37.5%), age (<12 years = 34.0%, ≥12 years = 29.6%), and race (White/non-Hispanic = 30.9%; all other races/Hispanic = 37.7%). The slopes of the relationship between %BF and VAT below and above the breakpoints were significantly different by sex, age grouping, and race/ethnicity, with steeper slopes above versus below the respective breakpoints. Among males, for every 1% unit difference in %BF above threshold, VAT was higher by 93.1 g. Among females, for every 1% unit difference in %BF above threshold, VAT was higher by 75.8 g. Breakpoints for multiples of the 95th BMI percentile were observed at approximately 0.91 in males (equating to values between the 87th and 90th BMI percentile), approximately 0.90 in females (equating to the values between the 87th and 90th BMI percentile), approximately 0.83 in younger children (equating to values between the 70th and 80th BMI percentile), and approximately 0.85 in older children (equating to values between the 79th and 86th BMI percentile). The slopes of the relationship between multiples of the 95th BMI percentile and VAT below and above the breakpoints were significantly different by sex, age grouping, and race/ethnicity, with steeper slopes above versus below the respective breakpoints.
| Group | Measure | %BF | Multiples of 95th BMI percentile | ||
|---|---|---|---|---|---|
| Estimate (95% CI) | P | Estimate (95% CI) | P | ||
| Males | BP | 32.6 (29.1 to 36.0) | – | 0.91 (0.81 to 1.01) | – |
| Slope below BP | 1.8 (−19.1 to 22.6) | 0.869 | 67.1 (−1,299.8 to 1,433.9) | 0.923 | |
| Slope above BP | 93.1 (78.0 to 108.2) | < 0.001 | 2,677.1 (2,400.4 to 2,953.8) | < 0.001 | |
| Slope difference | 91.3 (65.6 to 117.0) | < 0.001 | 2,610.1 (1,215.5 to 4,004.7) | < 0.001 | |
| Females | BP | 37.5 (34.1 to 40.9) | – | 0.90 (0.76 to 1.04) | – |
| Slope below BP | 4.8 (−17.3 to 27.0) | 0.667 | 375.8 (−634.9 to 1,386.4) | 0.465 | |
| Slope above BP | 75.8 (64.7 to 86.9) | < 0.001 | 1,894.5 (1,654.3 to 2,134.7) | < 0.001 | |
| Slope difference | 71.0 (46.2 to 95.7) | < 0.001 | 1,518.8 (480.0 to 2,557.5) | 0.005 | |
| ≥ 12 years old | BP | 29.6 (25.4 to 33.8) | – | 0.85 (0.75 to 0.96) | – |
| Slope below BP | −2.9 (−28.9 to 23.2) | 0.829 | 186.1 (−1,175.3 to 1,547.4) | 0.788 | |
| Slope above BP | 65.6 (56.8 to 74.4) | < 0.001 | 2,231.6 (2,018.0 to 2,445.2) | < 0.001 | |
| Slope difference | 68.5 (41.0 to 95.9) | < 0.001 | 2,045.5 (667.6 to 3,423.5) | 0.003 | |
| < 12 years old | BP | 34.0 (30.5 to 37.5) | – | 0.83 (0.72 to 0.94) | – |
| Slope below BP | −1.9 (−14.1 to 10.2) | 0.753 | −3.7 (−874.0 to 866.7) | 0.993 | |
| Slope above BP | 41.0 (33.6 to 48.5) | < 0.001 | 1,131.3 (930.8 to 1,331.8) | < 0.001 | |
| Slope difference | 43.0 (28.7 to 57.2) | < 0.001 | 1,135.0 (241.8 to 2,028.1) | 0.046 | |
| White, non-Hispanic | BP | 30.9 (27.4 to 34.4) | – | 0.92 (0.84 to 1.00) | – |
| Slope below BP | −1.6 (−21.3 to 18.0) | 0.870 | 222.0 (−590.3 to 1,034.2) | 0.591 | |
| Slope above BP | 64.2 (55.1 to 73.3) | < 0.001 | 2,554.3 (2,325.4 to 2,783.2) | < 0.001 | |
| Slope difference | 65.8 (44.2 to 87.4) | < 0.001 | 2,332.4 (1,488.5 to 3,176.2) | < 0.001 | |
| Non-White or Hispanic | BP | 37.7 (31.8 to 43.7) | – | 1.00 (0.75 to 1.25) | – |
| Slope below BP | 10.3 (−20.5 to 41.1) | 0.509 | 411.9 (−1,220.1 to 2,043.9) | 0.619 | |
| Slope above BP | 83.9 (63.3 to 104.4) | < 0.001 | 2,184.4 (1,814.6 to 2,554.2) | < 0.001 | |
| Slope difference | 73.6 (36.5 to 110.6) | < 0.001 | 1,772.5 (99.1,3 to 445.8) | 0.034 | |
- %BF, percent body fat; BP, breakpoint.
Association of visceral fat with cardiometabolic risk factors above and below threshold
Table 3 shows the relationships of %VAT with cardiometabolic risk factors above and below the various %BF thresholds for males and females. %VAT was significantly associated with most cardiometabolic risk factors among individuals above the thresholds but was not associated with risk factors among individuals below the thresholds. With few exceptions, these relationships were consistent across the other subgroups (by age and race/ethnicity) using the %BF threshold and the multiples of the 95th BMI percentile thresholds (data not shown).
| Group | Outcome | Below %BF breakpoint | Above %BF breakpoint | ||
|---|---|---|---|---|---|
| Difference per VAT percentage (95% CI) | P | Difference per VAT percentage (95% CI) | P | ||
| Males | HDL cholesterol (mg/dL) | 3.9 (−1.3 to 9.0) | 0.142 | −5.6 (−7.2 to −4.0) | < 0.001 |
| Triglycerides (mg/dL) | 2.1 (−6.4 to 10.7) | 0.628 | 20.4 (12.1 to 28.6) | < 0.001 | |
| Triglycerides/HDL ratio | 0.0 (−0.3 to 0.3) | 0.909 | 0.9 (0.6 to 1.2) | < 0.001 | |
| Insulin (mU/L) | −0.1 (−1.1 to 0.8) | 0.781 | 6.1 (4.3 to 8.0) | < 0.001 | |
| Oxidized LDL (U/L) | 4.3 (−3.1 to 11.7) | 0.250 | −0.7 (−7.4 to 6.0) | 0.835 | |
| HMW adiponectin (µg/mL) | −0.66 (−1.46 to 0.14) | 0.107 | −1.12 (−1.54 to −0.71) | < 0.001 | |
| CRP (mg/L) | 0.41 (−0.80 to 1.62) | 0.506 | 2.53 (0.79 to 4.28) | 0.004 | |
| SBP (mmHg) | 0.6 (−3.2 to 4.4) | 0.767 | 6.5 (4.6 to 8.4) | < 0.001 | |
| DBP (mmHg) | 0.4 (−2.4 to 3.2) | 0.783 | 4.4 (3.0 to 5.8) | < 0.001 | |
| Females | HDL cholesterol (mg/dL) | −6.7 (−19.1 to 5.8) | 0.293 | −2.2 (−3.9 to −0.5) | 0.011 |
| Triglycerides (mg/dL) | 8.2 (−35.1 to 51.5) | 0.711 | 9.8 (−0.1 to 19.7) | 0.052 | |
| Triglycerides/HDL ratio | 0.2 (−0.8 to 1.3) | 0.687 | 0.4 (0.1 to 0.7) | 0.018 | |
| Insulin (mU/L) | −0.2 (−2.8 to 2.3) | 0.871 | 5.6 (3.3 to 7.9) | < 0.001 | |
| Oxidized LDL (U/L) | 8.6 (−16.7 to 33.8) | 0.507 | 5.9 (−2.1 to 14.0) | 0.147 | |
| HMW adiponectin (µg/mL) | 2.15 (−1.05 to 5.34.0) | 0.188 | −0.75 (−1.11 to −0.39) | < 0.001 | |
| CRP (mg/L) | 0.84 (−1.74 to 3.4) | 0.524 | 1.96 (−0.14 to 4.06) | 0.067 | |
| SBP (mmHg) | −6.2 (−13.1 to 0.7) | 0.078 | 2.9 (1.0 to 4.8) | 0.003 | |
| DBP (mmHg) | −4.8 (−10.6 to 1.0) | 0.105 | 1.8 (0.4 to 3.3) | 0.012 | |
- %BF, percent body fat; CRP, C-reactive protein; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HMW, high molecular weight; LDL, low-density lipoprotein; SBP, systolic blood pressure; VAT, visceral adipose tissue.
Discussion
In this study, we identified breakpoints in %BF above which levels of VAT appeared to exponentially increase in children and adolescents. To our knowledge, this is one of the first reports of the existence of such thresholds in the pediatric population, yet it is important to note that similar thresholds have been reported in adults, with an almost identical cut point in women versus girls but a lower cut point in men versus boys (7, 8). Initial observations of potential nonlinear relationships of BMI (12) and waist circumference (13) with VAT in children and adolescents have been previously reported. Our results are in agreement with these studies and extend the findings within the context of a much larger sample with a wider range of BMI/adiposity levels and a higher proportion of participants within the severe obesity category. Based on these findings, we hypothesize that children and adolescents may experience different patterns of body fat deposition, depending upon age, sex, and race, when a certain threshold of total body adiposity is reached. Below these %BF thresholds, levels of VAT appeared to be extremely low, reflecting a healthier pattern of body fat distribution. Though speculative, owing to the cross-sectional nature of our study, the threshold hypothesis is supported by evidence from overfeeding studies conducted in adults, which have demonstrated that increases in VAT are associated with dysfunctional subcutaneous adipose tissue expandability (9, 21). Though not directly addressed in the current study and requiring further investigation, it seems reasonable to speculate that a possible physiological explanation for our observation is that the subcutaneous fat depot may have an upper limit of expansion, and when exceeded, additional fat storage is preferentially shunted to the visceral region.
The thresholds observed in our study, particularly the cut points identified by BMI percentile, may be useful in the clinical setting to aid in risk stratification. Indeed, these thresholds may help identify children and adolescents most likely to experience steep increases in VAT levels with linear weight gain and consequently develop an adverse cardiometabolic risk factor profile. Interestingly, we found that all of the various breakpoints (by sex and age) fell within BMI percentile ranges below the current classification of obesity (≥ 95th BMI percentile). Although our findings in isolation do not lend to challenging the widely accepted 95th BMI percentile cutoff for obesity, our results suggest that the inflection point associated with worsening of the cardiometabolic risk factor profile may fall below the obesity cut point. Perhaps even youth below the 95th BMI percentile should be monitored closely for continued weight gain and development of cardiometabolic risk factors.
In the context of the current study, cardiometabolic risk factors were much more strongly associated with VAT among individuals above versus below the respective thresholds, underscoring the primary role of visceral fat in driving cardiometabolic risk as early as the first 2 decades of life. These results may also explain why pediatric studies have been somewhat mixed in terms of the relative strength of associations of total body fat, subcutaneous fat, and VAT with cardiometabolic risk factors (1, 3, 14, 20, 22, 23). In fact, the composition of a particular sample of participants, in terms of the percentages of individuals above and below threshold, may be the primary factor driving these relationships. The current data suggest that the range and distribution of adiposity level of the cohort studied may influence the relative strength of these relationships such that samples with higher mean %BF will demonstrate higher correlations of VAT with cardiometabolic risk factors since a higher percentage will be above the thresholds.
The strengths of this study include the large sample size, inclusion of participants with a wide range of BMI values (normal weight to severe obesity), and the use of reliable and standardized methods for measuring body composition and cardiometabolic risk factors. Limitations include the relative lack of racial diversity, which somewhat hampered our ability to investigate differences in body fat distribution relationships across several race categories, the potential risk of sample bias owing to the combining of multiple study cohorts, and the fact that our measurement of visceral fat was based on DXA rather than magnetic resonance imaging or CT (traditionally considered the gold-standard methods). Despite being labeled by some as only able to measure trunk fat, the iDXA technology can estimate visceral fat; however, the evidence base supporting its use is less established compared with magnetic resonance imaging or CT. However, it should be noted that the DXA-derived visceral fat method has been validated in adults, and evidence points to its validity in children and adolescents (20). Finally, and perhaps most importantly, the study was cross-sectional in nature. Therefore, our findings should be viewed as hypothesis generating and will need to be confirmed in future longitudinal studies that are designed to track temporal changes in body fat deposition patterns and associations with alterations in cardiometabolic risk and, ideally, clinical end points.
In conclusion, this study identified evidence of breakpoints in %BF above which levels of VAT appear to steeply rise in children and adolescents, suggesting that VAT accumulation is not linear throughout the body fat and BMI continuum. Moreover, we identified several cardiometabolic risk factors that were elevated only among individuals above the thresholds, as well as observed that cardiometabolic risk factors were only associated with VAT above the breakpoints. Although these findings should be considered preliminary, the results raise the possibility of the existence of a total-body-fat threshold at which metabolic health diverges into the unhealthy realm primarily driven by an increase in relative VAT deposition favoring adipocyte dysfunction. We hypothesize that subcutaneous fat depots exhibit inherent biological limitations in their ability to expand (i.e., a ceiling effect), and when this threshold is exceeded, additional storage of fat begins to be preferentially stored in the visceral region, ultimately leading to metabolic and cardiovascular decompensation. Additional studies will be needed to further investigate this hypothesis as well as characterize the pathophysiological mechanisms associated with altered patterns of fat deposition. Greater insight into this potentially important phenomenon may help guide clinical screening, monitoring, and prevention practices for obesity-associated comorbidities as well as inform decisions about optimal timing and intensity of obesity treatments during childhood and adolescence.
Acknowledgments
The authors would like to thank the individuals who participated in the study. We are grateful for the expert study coordination provided by Ms. Annie Sheldon, Ms. Erin Hurley, Ms. Cameron Naughton, Mr. Neil Hultgren, Ms. Patti Laqua, and Ms. Kristin Garcia.





