Adipose Tissue Macrophage Phenotypes and Characteristics: The Key to Insulin Resistance in Obesity and Metabolic Disorders
Abstract
Obesity is one of the most serious global health problems, with an incidence that increases yearly and coincides with the development of a variety of associated comorbidities (e.g., type 2 diabetes, nonalcoholic fatty liver disease, some immune-related disorders). Although many studies have investigated the pathogenesis of overweight and obesity, multiple regulatory factors underlying the onset of obesity-related metabolic disorders remain elusive. Macrophages contribute to modulation of obesity-related inflammation and insulin resistance (IR); adipose tissue macrophages are particularly important in this context. Based on newly identified links between the chemokine system and obesity, macrophage polarization has become an essential target of new therapies for obesity-related IR. The findings of multiple studies imply that variations in gut microbiota and its metabolites might contribute to the regulation of obesity and related metabolic disorders. Recently, several novel antidiabetic drugs, applied as treatment for weight loss, were shown to be effective for obesity-induced IR and other comorbidities. The present review will discuss the properties and functions of macrophages in adipose tissue under conditions of obesity from three perspectives: the chemokine system, the gut microbiota, and antidiabetic drug application. It is proposed that macrophages might be a key therapeutic target for obesity-induced complications.
Study Importance
What is already known?
- Macrophages contribute to modulation of obesity-related inflammation and insulin resistance (IR).
- Several antidiabetic drugs are proven to be effective for weight loss in obesity.
- Gut microbiota plays an important role in the pathogenesis of obesity-induced IR.
What does this review add?
- We review the latest progress on the properties of macrophages and their functions in regulating obesity-induced IR.
- We summarize the impact of several novel antidiabetic drugs (e.g., DPP-4 inhibitors, SGLT2 inhibitors, GLP-1 receptor agonists) on macrophage polarization and obesity-induced IR.
- We discuss the link between gut microbiota and its metabolites and macrophages in obesity-induced IR.
Introduction
Obesity is closely associated with the development of numerous comorbid conditions, including insulin resistance (IR) and type 2 diabetes (T2D), and it serves as an important trigger for a variety of devastating diseases (e.g., nonalcoholic fatty liver disease, cardiovascular diseases, several cancers) (1, 2). In patients with obesity, these metabolic maladjustments are accompanied by IR, which may underlie the links between obesity and chronic metabolic disorders. Additionally, obesity can be seen as a primary indicator of chronic low-grade inflammation because patients with obesity exhibit reductions in the levels of inflammatory cytokines following weight loss (3, 4). It is well recognized that adipose tissue regulates metabolism by releasing nonesterified fatty acids and glycerol, as well as hormones (e.g., leptin, adiponectin) that are known to be elevated in obesity. Moreover, the infiltration of inflammatory cells into adipose tissue results in the increased secretion of multiple proinflammatory cytokines and chemokines (4), such as interleukin (IL)-6, tumor necrosis factor-α (TNF-α), IL-1β, and monocyte chemoattractant protein-1 (MCP-1), which are all typically involved in IR (5). Chemokines serve as chemoattractants for leukocyte chemotaxis into inflammatory tissues. For example, various chemokine–chemokine receptor interactions play important roles in the development of inflammation in adipose tissue and obesity-associated IR.
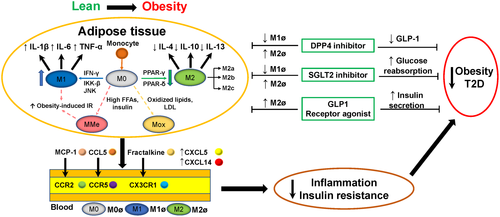
Another typical characteristic of obesity is the recruitment and infiltration of immune cells, primarily macrophages and T cells, into adipose tissue. Notably, adipose tissue macrophages (ATMs) are essential in the pathogenesis of obesity and related metabolic dysfunction, both in genetic and diet-induced overweight rodents and patients with obesity. Based on differences in activation and resulting functions, macrophages are divided into classically activated macrophages (M1) and alternatively activated macrophages (M2) (6); both M1 and M2 macrophages are closely associated with the development of IR. M1 macrophages are stimulated by interferon-γ, which promotes a harsh proinflammatory response through increased secretion of proinflammatory cytokines (i.e., TNF-α, IL-6, and IL-1β). Conversely, M2 macrophages mostly produce an anti-inflammatory environment by releasing anti-inflammatory cytokines (e.g., IL-10, IL-4) that contribute to sustain IR. Thus, it is generally understood that ATMs change from the M2 polarized state to the M1 state during the development of obesity, thereby leading to chronic inflammation and IR (6, 7). Recently, Russo and Lumeng (8) suggested that, in a state of obesity, activated ATMs may not resemble M1 or M2 macrophages. In contrast to previous traditional studies, these authors suggested that M0 macrophages, which originate from bone-marrow-derived monocytes or yolk-sac progenitors, differentiate into M1 and M2 macrophages based on a variety of stimuli (Figure 1). Moreover, M2 macrophages can be further classified into three main variants: M2a, M2b, and M2c, which are each elicited by different cytokines and stimuli and have different anti-inflammatory capabilities (8).
In the past decade, the gut microbiota has received extensive attention because of its involvement in the development of obesity-related complications (9). This finding was initially observed in germ-free mice that were fed with a high-fat diet (HFD); these mice showed significantly lower body fat and did not develop IR. It was recently suggested that altered gut microbiota causes intestinal barrier dysfunction and endotoxemia, thereby inducing inflammation via recruitment and activation of immune cells to further promote the development of obesity (9, 10). In addition, some bacterial products, such as short-chain fatty acids (SCFAs) and tryptophan (Trp) catabolites, play important roles in the pathophysiology of obesity.
The pathogenesis of T2D is closely associated with obesity, mainly in relation to IR and the distribution of body fat (11). Several antidiabetic drugs that modify macrophage phenotypes have traditionally been used for the treatment of obesity and IR, including metformin, thiazolidinediones, and α-glucosidase inhibitors. However, several novel antidiabetic drugs have recently been recognized as potential strategies for the treatment of obesity-associated inflammation and IR. These novel drugs include dipeptidyl peptidase 4 (DPP-4) inhibitors, sodium-dependent glucose transport-2 (SGLT2) inhibitors, and glucagon-like peptide-1 (GLP-1) receptor agonists, the effects of which were partly dependent on ATM infiltration and polarization.
Thus, the present review will summarize macrophage phenotypes and characteristics with particular emphasis on the role of ATMs in the development of obesity-associated IR. We also briefly clarify the effects of chemokines and discuss novel regulatory mechanisms involving gut microbiota and a variety of microbial metabolites in obesity and related disorders. Furthermore, several novel antidiabetic drugs that treat obesity-induced IR via the regulation of macrophage recruitment and polarization will be reviewed.
Macrophage Phenotypes and Characteristics
Effects of ATMs on obesity-induced IR
Macrophages are cells involved in the innate immune system that respond to acute infections and maintain tissue homeostasis by secreting specific chemokines and cytokines (12). A variety of studies have shown that tissue macrophages can regulate insulin sensitivity in various target tissues such as adipose tissue (e.g., ATM), liver (e.g., Kupffer cells [KCs]), and brain (e.g., microglia). ATMs can infiltrate adipose tissue through a C–C motif chemokine receptor 2 (CCR2)-dependent approach and then secrete a series of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6. Moreover, MCP-1 is secreted by hypertrophic adipocytes; this cytokine accelerates the infiltration of monocytes into adipose tissue, and then stimulates differentiation of monocytes into macrophages, which is a primary trigger for inflammatory activity via secretion of proinflammatory cytokines.
The activation and release of M1 macrophages via specific signaling pathways are historically understood as the major contributor to the pathogenesis underlying obesity-induced IR. M1 macrophages secrete cytokines to activate inflammatory pathways located in insulin target cells, which results in the activation of Jun N-terminal kinase, inhibitor of κB kinase β, and other serine kinases (8, 13). In addition, peroxisome proliferator-activated receptor (PPAR) belongs to a family of nuclear proteins that regulate adipocyte gene expression and functions. Thus far, PPAR-γ is known to promote monocyte differentiation into an M2 phenotype with anti-inflammatory effects (14). Another enzyme expressed by M2 macrophages, PPAR-δ, has been shown to prevent the development of obesity-associated IR in HFD-fed mice (15) (Figure 1).
However, in a state of obesity, ATMs adopt other metabolically activated (MMe) phenotypes that are produced by saturated free fatty acids (FFAs) and high insulin levels in insulin-resistant adipocytes (16). Coats et al. (17) identified a specific regulatory mechanism for MMe macrophages during ATM inflammation and IR in obesity that is distinct from the classically activated M1/M2 paradigm. In an in vivo study of HFD-fed mice, these authors identified nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (NOX2) in MMe macrophages as a central factor involved in regulating the development of obesity-induced inflammation and IR. In that study, Nox2−/− mice exhibited reduced ATM inflammation and improved IR in adipose tissues in the early state of diet-induced obesity (DIO), whereas control mice exhibited severe hepatic steatosis and IR after 16 weeks of an HFD. The authors proposed that these changes were likely to be due to the ability of MMe macrophages to perform flexible functions (i.e., they have both pro- and anti-inflammatory characteristics) that primarily include the regulation of ATM inflammation and the clearance of dead adipocytes during prolonged obesity. Although the findings of that study were mainly based on a mouse model of DIO, MMe macrophages are also a novel ATM paradigm for the treatment of obesity-induced inflammation and IR.
In a state of obesity, related metabolic symptoms (e.g., changes in oxidized phospholipid [OxPL] and low-density lipoprotein [LDL] levels) facilitate increased numbers of metabolically oxidized (Mox) macrophages, which were initially identified as a novel macrophage phenotype in response to the development of atherosclerotic lesions (18). During obesity, ATM-mediated increases in oxidative stress are regarded as the major contributor to chronic low-grade inflammation in adipose tissue. Recently, one study has suggested that the shift from truncated OxPL to full-length OxPL enhances the antioxidant effects of Mox phenotypes in ATM and subsequently activates M1/M2 macrophages to regulate the development of obesity-associated IR (19). Therefore, the Mox phenotype in ATM could be a potential perspective from which to understand more fully the link between ATM and obesity-associated IR.
Pathophysiological and therapeutic connections between macrophages and IR
Because of their phenotypic flexibility, macrophages are now regarded as ideal therapeutic targets for metabolic diseases (12). Potential macrophage-specific therapeutics could focus on inhibiting ATM-related inflammation, which would primarily include a decrease in M1-like inflammatory macrophages or, conversely, an increase in M2-like inflammatory macrophages to ameliorate systematic inflammation. One caveat that should be noted is that the inhibition of inflammation sometimes has little or no effect on improving IR. For example, studies assessing the neutralizing antibodies of proinflammatory factors found no amelioration of insulin sensitivity in patients with T2D (20). Nevertheless, in the early state of ATM expansion, mild inflammation might be beneficial and therefore the precise timing of treatment should be carefully considered as well (21).
Macrophage inflammation has also been targeted for the treatment of other metabolic disorders, including nonalcoholic fatty liver disease and atherosclerosis. The modulation of hepatic macrophage polarization and/or inhibition of inflammatory monocyte recruitment to the liver can be an effective therapeutic strategy for hepatic inflammation and fibrosis in experimental mouse models (22). More recently, it was reported that the OxPL-treated macrophage phenotype, which is known as Mox, specifically responds to the development of atherosclerotic plaques (18). Currently, macrophage-targeted therapeutic strategies are specifically provided according to different macrophage receptors and functions (reviewed in Peterson et al.) (12). Once the approach for gaining intracellular access to different receptor-mediated macrophages (e.g., F4/80, CD11b, CD68) is better understood and therapeutic agents can be delivered to them, the modulation of macrophage numbers to suppress tissue inflammation and metabolic disorders (commonly accomplished by depletion, proliferation, and gene silencing) may be more effective (23). Additionally, several novel methods of delivery, including nanoparticles, liposomes, and glucan microparticles, can target macrophages more specifically and accurately. Ultimately, these novel methods of delivery coupled with approaches for targeting different macrophage phenotypes and subsets should be assessed in future research to ensure specific targeting for different metabolic diseases.
Relationships Between hemokine Systems and Macrophage-Mediated IR
Overview of chemokine-chemokine receptor signaling pathways
Chemokines are defined as chemotactic cytokines that regulate the migratory patterns and positioning of immune cells, such as T cells, monocytes, and neutrophils. Chemokines are classified into four elementary groups by structure: CXC, CC, C, and CX3C, in which X is any amino acid residue. Initially, chemokines are expressed in response to tissue inflammation or infection primarily via interactions with seven-transmembrane G-protein-coupled receptors, which are classified as class C receptors (XCR), CC receptors (CCR), CXC receptors (CXCR), and CX3C receptors (CX3CR) (3). These receptors subsequently induce the migration of immune cells toward the site of inflammation or injury (e.g., leukocyte movement), which is recognized as a major feature of tissue inflammation. In addition to the roles that chemokines play in the trafficking of immune cells, their interactions with specific chemokine receptors can induce multiple cellular responses, such as multiplication, activation, and differentiation, in a variety of metabolic diseases. Notably, obesity-induced macrophage infiltration and polarization are regarded as pivotal triggers in the pathogenesis of IR, and to date, many chemokines have been shown to play essential roles in the development of obesity-related metabolic diseases. In fact, the contributions of two common chemokine systems, MCP-1/CCR2 and Chemokine (C-C motif) ligand 5 (CCL5)/CCR5, to the development of obesity have been reviewed in detail by previous studies. For example, treatment with a dual CCR2/CCR5 antagonist significantly ameliorates HFD-induced metabolic disorders, including IR and inflammation in adipose tissue, which mainly occurs through the regulation of ATM recruitment and polarization (24). To elucidate further the link between chemokine-chemokine receptor signaling and obesity, we discuss several novel chemokine-chemokine receptor axes that may be involved in obesity-induced metabolic disorders via the regulation of macrophage infiltration and polarization.
C–X3–C motif ligand 1/CX3CR1 signaling
C–X3–C motif ligand 1 (CX3CL1), which accumulates in atherosclerotic plaques, is prominently upregulated in human adipose tissue in a state of obesity as well as in evoked adipose inflammation (25). Additionally, chronic CX3CL1 treatment effectively improves glucose tolerance and insulin secretion (26), which suggests that CX3CL1 signaling is involved in ATM recruitment and adipose tissue inflammation. Furthermore, deficiencies of CX3CR1 in KCs augment inflammatory properties, and CX3CL1 induces the alternative activation of KCs via CX3CR1 (27). CX3CR1/CX3CL1 interactions preferentially mediate the arrest and migration of inflammatory cells (28), and the activation of CX3CR1+ macrophages in the gut increases the production of inflammatory cytokines, which is indicative of the pivotal roles that CX3CR1+ gut-resident macrophages play in intestinal homeostasis (29, 30). Although investigations of CX3XR1GFP/GFP mice, which are deficient in CX3CR1, have produced conflicting data, this model has been associated with decreases in adiposity and adipose inflammation (31). It is possible that these discrepancies may be caused by the different genetic backgrounds of the mice used in the studies. Taken together, these data indicate that the CX3CL1/CX3CR1 system is involved in the pathogeneses of obesity and IR via the recruitment and polarization of macrophages. Furthermore, pharmacological therapies that target the CX3CL1/CX3CR1 system might be a novel candidate for the treatment of obesity and its related metabolic disorders.
Novel chemokine and chemokine receptors in macrophages
Novel chemokine systems have been identified that may also be involved in ATM infiltration, inflammation, and IR during obesity. For example, CXCL5 and CXCL14 and their corresponding receptors are dramatically upregulated in the visceral fat of patients with extreme obesity, where they participate in the development of IR (32, 33). High CXCL5 expression is found in white adipose tissue (WAT) in mice, whereas it is primarily observed in macrophages in human WAT. CXCL5 appears to play an important role in the promotion of obesity-induced IR. Deficiencies of the CXCL5 receptor, CXCR2, are associated with markedly normalized insulin secretion and prevent the development of DIO and IR in CCR2−/− mice (32). Similarly, CXCL14 serves as an effective macrophage chemoattractant in WAT and participates in the development of obesity-induced IR (33). CXCL14 targets tissue macrophages and is significantly increased in the WAT of HFD-fed mice, whereas HFD-fed CXCL14−/− mice exhibit an amelioration of IR as well as reduced macrophage infiltration into WAT (33). Additional unidentified combinations of chemokine systems may modulate ATM infiltration and obesity-associated inflammation or IR.
Gut Microbiota and Related Metabolites in ATMs and Obesity-Related Metabolic Diseases
Gut microorganisms as driving force in pathogenesis of obesity-induced IR
Recent studies have demonstrated that changes in gut microorganisms are central to the development of obesity. Interestingly, the gut microbiota is considerably altered in patients with obesity compared with normal controls, which is particularly applicable in terms of bacterial richness and function in patients with obesity-related metabolic disorders and IR (34). Likewise, germ-free mice initially show a marked resistance to HFD-induced obesity and IR, regardless of increased food intake, and these obesity-related phenotypes can be delivered through intestinal microbial transplantation (9, 35). Another recent study found that a diet consisting of fewer dietary resistant starches could improve insulin sensitivity mainly through the regulation of bile acids and the ATM immune system rather than the gut microbiota (36). Therefore, the present review will discuss the primary link between the gut microbiota and obesity-induced IR and/or related metabolic disorders, especially through ATM-dependent mechanisms.
Lipopolysaccharides: the link between gut microbiota and adipose tissue IR during obesity
Several studies have shown that a defective intestinal barrier, particularly because of bacterial-derived lipopolysaccharide (LPS; i.e., endotoxins), is a triggering factor in the onset of obesity-related inflammation and associated comorbidities (37). For example, the administration of LPS for 1 month to mice that were fed normal chow caused obesity, and this change resulted in ATM infiltration, inflammation, and IR (38). Another aspect of this relationship involves the secretion of proinflammatory cytokines (e.g., TNF-α, IL-6, MCP-1, which activates nuclear factor-κB), particularly when LPS activates the CD14/toll-like receptor 4 (TLR4) complex in immune cells and macrophages. LPS exposure enhances the multiplication of pre-adipocytes, which can differentiate into macrophages under a state of obesity, through a CD14-dependent mechanism (39). Interestingly, CD14-deficient mice are protected from HFD-induced metabolic disorder, which supports the importance of TLR4 and CD14 signaling in obesity and IR (40) (Figure 2A).
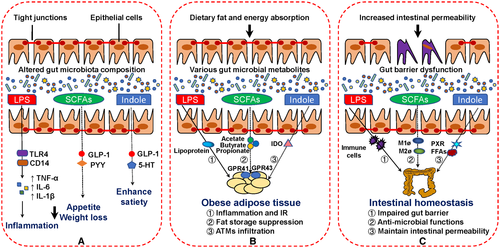
Moreover, intestinal epithelial cells are regarded as a physical barrier that separates the noxious luminal environment from the underlying lamina propria and deeper intestinal layers (37). Impairments in the intestinal epithelial tight junction (TJ) elicit and promote inflammatory responses by allowing the paracellular permeation of luminal antigens (37). Indeed, LPS is actively transported into cells with high levels of lipids in the lumen of the gut, which in turn contributes to dysfunctional intestinal permeability. When LPS binds to lipoproteins via an interaction with either scavenger receptor of class B type I or CD36, this complex can be absorbed by large adipocytes, macrophages, or other tissue cells, and the resulting increases in lipogenesis can aggravate inflammation and IR. Furthermore, LPS delivery into tissue cells might be correlated with macrophage polarization from M2 to M1 in the adipose tissue of mice fed an HFD (41). Therefore, it is essential to maintain the integrity of intestinal epithelial cells to prevent extracellular substances from invading the intestinal system.
SCFAs as mediators of obesity-induced metabolic syndrome
The gut microbiota produces a variety of metabolites, including SCFAs, bile acids, and a mass of amino acids. The main bacterial fermentation products of dietary fiber are SCFAs such as acetate, propionate, and butyrate. SCFAs are vital meditators of fat accumulation in adipocytes via receptor activation through G-protein-coupled receptor (GPR)43 and GPR41 (42), which are expressed primarily on adipocytes and immune cells (Figure 2A). Several studies have suggested that complexes of GPR43 and SCFAs (mainly propionate and butyrate) promote leptin secretion in adipocytes and peptide YY (PYY) secretion in enteroendocrine cells (43), which would increase levels of plasma GLP-1 and PYY to inhibit appetite. Moreover, GPR43 deficiencies partly alleviate HFD-induced obesity and glucose tolerance and result in reduced ATM infiltration in WAT, which may be essential for the amelioration of obesity-associated IR in mice (44).
SCFAs are also essential for the maintenance of intestinal barrier functions (Figure 2B). It is widely recognized that dietary fiber consumption can partly ameliorate obesity as well as obesity-related metabolic disorders (45). Furthermore, this capability may be related to the increased production of SCFAs, such as butyrate, that maintain the functions of the colonic epithelium by inducing apoptosis. Multiple studies have reported that butyrate plays a key role in the maintenance and repair of intestinal permeability. For example, the administration of sodium butyrate effectively improves the expressions of colonic mucin and TJ proteins, which strengthens the intestinal barrier and reduces LPS leakage to decrease ATM infiltration indirectly and ameliorate IR. Similarly, in a common HFD-induced mouse model, butyrate reduces intestinal barrier dysfunction through the increased expression of claudin-1 (46) in conjunction with a modulating mechanism that might be associated with epigenetic regulation. As an alternative to supplementing butyrate into the diet, a well-tolerated probiotic with butyrogenic bacterial strains exerts similar ameliorative effects on obesity-related IR (47). Collectively, SCFAs protect against LPS-induced dysfunction of the intestinal barrier and indirectly improve LPS-induced ATM recruitment and IR during obesity.
SCFAs also participate in the intestinal immune system via the modulation of monocyte and macrophage activities (Figure 2C). According to Julie et al. (48), macrophages exhibit elevated antimicrobial activity in the presence of butyrate. Another study found that SCFA-activated GPR43 significantly facilitates M2 macrophage marker gene expression to inhibit fat accumulation (49); changes in proinflammatory M1 macrophage levels were not observed in that study. Furthermore, the long-term administration of inulin-propionate ester effectively reduces the accumulation of intra-abdominal adipose tissue and improves insulin sensitivity (50). In a co-culture system with adipocytes and macrophages, butyrate supplementation significantly decreases the secretion of inflammatory cytokines such as TNF-α, MCP-1, and IL-6, which indicates that butyrate inhibits the delivery of inflammatory signaling during the adipocyte inflammatory response induced by ATM infiltration (51).
Similarly, an intraperitoneal injection of butyrate to db/db mice markedly reduces the expression of inflammatory factors in subcutaneous adipose tissue (SAT) and improves obesity-induced IR in conjunction with the decreased expression of inflammatory ATM marker genes (52). Although these data cannot demonstrate that SCFAs directly mediate the M1/M2 macrophage ratio to modulate the development of IR and inflammation in adipose tissue, SCFAs could be a potential target for furthering current understanding of the relationship between the gut microbiota and obesity-induced IR in adipose tissue.
Roles of Trp catabolites in obesity-induced metabolic complications
In addition to SCFAs, various crucial gut microbiota metabolites, such as microbial Trp catabolites, are known to play a central role in the microbiota–host cross talk in human health and metabolic disorders (Figure 2). To date, numerous intestinal microbiota have been shown to produce a variety of Trp catabolites, including indole, indolen-3-ethanol, indolelactic acid, tryptamine (TA), indolepropionic acid (IPA), skatole, indoleacrylic acid, indoleacetic acid, and indolealdehyde; of these, indole is the most abundant Trp catabolite in the human gut.
Indole and its derivatives are considered to be vital contributors to intestinal homeostasis and systemic metabolism. Bansal et al. (53) was the first to demonstrate the beneficial roles of indole in intestinal barrier function in terms of the enhancement of the stability of intestinal epithelial cells/TJs and by regulating the pathogenesis of intestinal inflammation in the colon. Later, several studies of HFD-fed mice indicated that indole, IPA, and indoleacrylic acid maintain intestinal permeability and the inflammation response by combining with the xenobiotic sensor, pregnane X receptor (54, 55). Additionally, indole and IPA modulate GLP-1 secretion from enteroendocrine L cells and improve insulin sensitivity by altering ATM infiltration (56, 57). Additionally, the in vitro applications of indole-3-acetate and TA significantly decrease indicators of inflammation in cultured macrophages, and more importantly, pretreatment with indole-3-acetate and TA effectively inhibits the migration of proinflammatory cytokines to increase the release of FFAs that subsequently activate macrophages (58). Similarly, indole alleviates liver inflammation that partly depends on KCs (59). Indole also modulates the gut–brain axis, primarily via 5-hydroxytryptamine signaling (54), and peripheral 5-hydroxytryptamine levels in the gut can enhance satiety to inhibit the development of obesity.
During the process of Trp catabolism in the gut, indoleamine 2,3-dioxygenase (IDO) plays an essential role by regulating the release of proinflammatory cytokines in macrophages and is also involved in the pathogenesis of IR in a state of obesity (60). HFD-fed IDO−/− mice show an amelioration in obesity-induced metabolic disorders with less macrophage infiltration in adipose tissue as well as decreased IR and adipose tissue inflammation (61). Because IDO catalyzes Trp catabolites from indole-related derivatives to kynurenine production, which facilities the development of macrophage infiltration and IR, IDO inhibitors could be a potential therapeutic approach for the treatment of obesity and obesity-related metabolic diseases. However, further study will be required to focus on the specific mechanisms underlying the altered macrophage characteristics between Trp metabolism and gut-mediated metabolic health.
Novel Antidiabetic Drugs Provide New Therapeutic Perspectives in Obesity-Related IR via Macrophage Activation
Major findings of commonly used antidiabetic drugs in obesity-induced IR
Several common antidiabetic drugs have been shown to exert a positive effect on the prevention of obesity and related disorders. Biguanides, mainly metformin, constitute the most widely used clinical pharmacotherapy for patients with T2D (62); metformin has been shown to ameliorate chronic low-grade inflammation in obesity by modulating ATM infiltration and polarization (63). Thiazolidinediones (a type of PPAR-γ agonist) have been shown to restore peripheral insulin sensitivity and to increase glucose production and lipolysis in patients with T2D (64). Some thiazolidinediones, such as lobeglitazone, have been shown to contribute to amelioration of inflammation and IR in rodents with a reduced M1/M2 ratio in adipose tissue. α-glucosidase inhibitors can postpone intestinal absorption and digestion of nonabsorbable complex carbohydrates into absorbable monosaccharides (64); thus, these constitute an attractive therapeutic approach for the treatment of obesity and related complications. In addition to the normalization of blood glucose by targeting different aspects of the immune system, the present review will highlight the specific roles of three novel antidiabetic drugs in obesity-related IR and metabolic disorders (Table 1). Although the detailed mechanisms remain unclear, ATMs might be altered by the administration of these drugs (Figure 1).
| Class of drug | Marketed drugs | Key/main findings | Reference |
|---|---|---|---|
| DPP-4 inhibitors | Linagliptin | Linagliptin partly decreased inflammation and IR in DIO mice by regulating M1/M2 macrophage status | (66) |
| Sitagliptin | Sitagliptin decreased inflammatory cytokines and M1 macrophage level in ob/ob mice | (68) | |
| Vildagliptin | Vildagliptin could inhibit the expression of TLR-4 and proinflammatory cytokines in murine macrophage cells | (69) | |
| Saxagliptin | Saxagliptin had a modest effect on adipose tissue inflammation in patients with overweight and obesity | (71) | |
| SGLT2 inhibitors | Empagliflozin | Empagliflozin decreased obesity-induced inflammation and IR via M2 macrophage polarization in WAT and liver in HFD-induced-obesity mice | (74) |
| Ipragliflozin | Ipragliflozin ameliorated obesity-related inflammation and IR with altered macrophage phenotypes | (76, 77) | |
| Canaglifozin | Canaglifozin decreased excess fat accumulation and HFD-induced inflammation to modify obesity in mice | (78, 79) | |
| Tofogliflozin | Tofogliflozin could improve IR and inflammation of adipose tissue in HFD-fed mice | (80) | |
| GLP-1 receptor agonists | Liraglutide | Liraglutide ameliorated HFD-induced IR and macrophage accumulation | (85) |
| Dulaglutide | Dulaglutide could lower blood glucose levels; function of neuroprotection via modulating immune cell infiltration shown | (86) | |
| Taspoglutide | Once weekly taspoglutide treatment had a beneficial impact on weight loss | (87) | |
| Semaglutide | Semaglutide could improve glycemic control and body weight in T2D patients | (88) |
- DIO, diet-induced obesity; DPP-4: dipeptidyl peptidase 4; GLP-1: glucagon-like peptide-1; HFD, high-fat diet; IR, insulin resistance; SGLT2, sodium-glucose cotransporter 2; T2D, type 2 diabetes; TLR-4: toll-like receptor 4; WAT, white adipose tissue.
DPP-4 inhibitors in macrophage-associated inflammation and IR
DPP-4, a 766-amino acid serine protease, plays a role in the development of inflammation through degradation of a variety of chemokine and peptide hormones involved in the immune system (65). Traditionally, DPP-4 inhibitors can normalize blood glucose levels by attenuating the levels of incretin peptides, GLP-1, and glucose-dependent insulinotropic polypeptide (65, 66). In addition to investigations of the degradation of GLP-1, earlier data suggest that DPP-4 could induce IR within adipose tissue and skeletal muscle in patients with obesity. For example, DPP-4 is defined as a novel adipokine with high expression levels in the visceral adipose tissue and exhibits positive correlations with the development of obesity-induced IR and inflammation (67). However, it has also been reported that DPP-4 is mainly expressed by macrophages rather than adipocytes (66). Therefore, future studies that clarify the cellular source of DPP-4 might provide new insights into the role of DPP-4 in macrophages.
Several DPP-4 inhibitors (e.g., linagliptin, sitagliptin, vildagliptin, anagliptin, saxagliptin) have been increasingly regarded as clinically effective pharmacotherapies for obesity and obesity-related IR, especially with regard to the regulation of ATM recruitment and polarization (64). Zhuge et al. (66) demonstrated that linagliptin partially attenuates obesity-induced low-grade inflammation and IR in DIO mice by reducing M1-polarized macrophage migration while improving M2-dominant macrophages in both WAT and the liver. Similarly, sitagliptin suppresses inflammation through regulation of M1/M2 macrophage status and improvement of obesity-induced hepatic IR and steatosis in leptin-deficient (ob/ob) mice (68). Vildagliptin inhibits the expressions of TLR4 and proinflammatory cytokines in mouse macrophages (69). Collectively, the available data suggest that DPP-4 has a considerable effect on obesity-induced inflammation and IR, primarily through regulation of M1/M2 macrophage polarization; moreover, the data highlight the potential clinical utility of DPP-4 inhibitors, particularly in treatment of macrophage-mediated metabolic disorders.
However, the silencing of DPP-4 in hepatocytes reduces visceral adipose tissue inflammation and IR via a different targeting pathway than that of sitagliptin (70). Moreover, a placebo-controlled study showed that saxagliptin had a modest effect on the reduction of adipose tissue inflammation in patients with overweight and obesity (71). Recently, it was shown that oxidized LDL upregulates DPP-4 expression on macrophages via specific pathways (e.g., TLR4/TIR-domain-containing adapter-inducing interferon-β (TRIF)/CD36), which redefines the role of DPP-4 in the pathogenesis of obesity or atherosclerosis (72). Similarly, excessive levels of oxidized LDL contribute to the upregulation of Mox macrophages in adipose tissue, expression of which is associated with an insulin-resistant state during obesity (8). Additionally, DPP-4 inhibitors suppress the expressions of proinflammatory cytokines in adipose tissue, as reviewed above. Notably, the MMe macrophage phenotype is regarded as an important source of inflammatory factors in a state of obesity. Taken together, these data suggest that DPP-4-induced obesity-related IR and inflammation may partly depend on changes of the MMe and Mox macrophage phenotypes. Therefore, further studies evaluating other DPP-4 inhibitors will be necessary to clarify the pathways important for the treatment of obesity and obesity-related complications.
SGLT2 inhibition in macrophage polarization
Sodium-glucose cotransporters are generally thought to comprise SGLT1 and -2, which are located primarily in the kidney and specialize in the cotransport of sodium and glucose across different cell types. SGLT2 is defined as a low-affinity, high-capacity transporter specifically expressed in renal proximal tubules (73). In recent years, several SGLT2 inhibitors (e.g., empagliflozin, ipragliflozin, dapagliflozin) have been identified as novel therapies for stabilizing glycemic level and ameliorating T2D symptoms through inhibition of glucose reabsorption following its filtration through the renal glomerulus (64).
Interestingly, with the exception of apparent reductions in blood glucose level, SGLT2 inhibitors exhibit an anti-obesity impact on patients and animals with T2D by targeting macrophages. For example, empagliflozin ameliorates energy expenditure and chronic inflammation in mice with HFD-induced obesity (74). First, empagliflozin administration reduced weight largely through dose-dependent reduction of the accumulation of visceral and subcutaneous fat in DIO mice; urine volume and urinary glucose excretion (UGE) were increased in treated mice (74, 75). Second, empagliflozin ameliorated HFD-induced IR and reduced inflammation in WAT of DIO mice. In addition, an M2-dominant phenotypic shift was present in ATMs, and T-cell accumulation was reduced in the liver and WAT, upon administration of empagliflozin.
Moreover, other SGLT2 inhibitors exert considerable effects on macrophages and IR. One study revealed that ipragliflozin ameliorates obesity-associated inflammation and IR in epididymal fat, with reduced accumulation of proinflammatory M1 macrophages (76). Similarly, ipragliflozin treatment induces healthy adipose tissue expansion and decreases the M1-like/M2-like ratio of ATMs in HFD-fed mice in conjunction with improvements in hyperglycemia and hyperinsulinemia (77). Xu et al. (78) were the first to demonstrate that canagliflozin treatment markedly suppresses the inflammatory response in LPS-induced mouse and human immune cells via reductions in intracellular glycolysis and enhancements in autophagy. Moreover, reductions in obesity-induced IR and excess fat accumulation are observed when canagliflozin or tofogliflozin are administered (79, 80). Excess lipid accumulation partially increases ATM recruitment, which is thought to play a vital role in the clearance of dead adipocytes and the balance of saturated FFA release (8). Meanwhile, saturated FFAs facilitate the production of MMe macrophages in adipose tissue, as mentioned above. Therefore, the lipid accumulation induced by SGLT2 inhibitors may indirectly mediate FFA-induced MMe macrophage activity to inhibit the release of proinflammatory cytokines and improve obesity-related IR.
GLP-1 receptor agonists in macrophages and IR
GLP-1 is secreted by intestinal epithelial L cells in response to food intake. Traditionally, the major functions of GLP-1 are to facilitate insulin secretion and inhibit glucagon release to balance postprandial glucose levels (81). Because of its actions regulating glucose-dependent insulin secretion and controlling food intake by modulating satiety, GLP-1 is currently considered to be an effective therapy for T2D and obesity patients.
Earlier rodent studies showed that GLP-1 attenuates macrophage infiltration in adipose tissue, which ameliorates obesity-induced IR in leptin-deficient (ob/ob) mice. This action is associated with an apparent reduction in classically activated M1 macrophage marker genes but not changes in the marker genes of M2 macrophages (82). Similarly, decreases in the expressions of inflammatory cytokines are observed in 3T3-L1 adipocytes and ATM in vitro. Moreover, a study of female patients with obesity demonstrated that GLP-1 certainly plays an anti-inflammatory role in the development of inflammation in adipose tissue and contributes to the increased accumulation of activated proinflammatory CD14+ or CD16+ monocyte/macrophages (83). Another recent study using the murine monocyte/macrophage cell line RAW264.7 reported that GLP-1 contributes to macrophage polarization toward M2 as well as the inhibition of the M1 macrophage polarization/inflammatory response (84). Taken together, these studies suggest that GLP-1 directly improves IR and inflammation in humans and animals by reducing ATM infiltration and inhibiting the inflammatory pathway in adipocytes. Furthermore, an obesity-induced high-insulin or high-glucose state also results in an increased number of MMe macrophage phenotypes in adipocytes (8). Although few studies have investigated the possible mechanisms underlying this phenomenon, GLP-1 may indirectly improve obesity-associated IR through the regulation of alternative MMe macrophages in adipose tissue.
In a rat model of HFD-induced IR, liraglutide treatment improves IR and ameliorates the development of fatty liver via the activation of adenosine 5′ monophosphate-activated protein kinase (85). Some new GLP-1 receptor agonists (RAs) (e.g., dulaglutide, taspoglutide, semaglutide) have shown efficacy in lowering fasting and postprandial glucose levels, improving β-cell function, and promoting weight loss in patients with T2D and obesity (86-88). However, while clinical studies have shown that GLP-1 receptor agonists are effective in the alleviation of T2D-related syndromes, the specific mechanisms by which they reduce obesity-induced inflammation, IR, and/or other autoreactive disorders remain unclear, especially with respect to macrophage recruitment and polarization. Therefore, further studies will be required to clarify the specific mechanisms of action associated with different GLP-1 RAs for regulating ATM recruitment and activity during the treatment of obesity-related complications.
Conclusions and Future Perspectives
In the case of progressive obesity, ATMs are recognized as a key driver in the development of obesity and IR. Currently, increasing attention is being directed toward treatment and prevention strategies for weight loss and obesity-induced metabolic disorders, and the targeting of ATMs is thought to be a novel therapeutic approach because of their tremendous degree of plasticity. Although many studies have demonstrated the various ATM phenotypes and functions during obesity, the ATM-targeted treatments for obesity-related comorbidities that occur via different activation mechanisms have yet to be fully investigated. The present review discussed three potential mechanisms for ATM activation during obesity: (i) chemokine-chemokine receptor systems improve obesity and IR via alternative methods of ATM infiltration and polarization; (ii) the gut microbiota and its metabolites secrete intestinal hormones and mediate the release of inflammatory cytokines that regulate ATM recruitment and polarization, which ameliorates obesity and ATM inflammation; and (iii) several novel antidiabetic drugs specifically inhibit and/or promote regulatory factors to improve obesity-induced IR and metabolic disorders via regulation of the functions of different ATM phenotypes in adipose tissue. However, many questions remain regarding the mechanisms by which chemokines regulate macrophage polarization, the specific antidiabetic drugs that can be applied for clinical treatment and their side effects, and the precise roles of gut microbial metabolites in the pathogenesis of obesity. Further studies investigating the relationships between various regulatory factors and ATM polarization are expected to provide novel strategies for the treatment and prevention of obesity-induced metabolic disorders.





