Endogenous Omega-3 Polyunsaturated Fatty Acids Reduce the Number and Differentiation of White Adipocyte Progenitors in Mice
Abstract
Objectives
Reducing the increased number of white adipocyte progenitors (WAP) is considered a novel approach to controlling obesity. The role of omega-3 polyunsaturated fatty acids (PUFA) in regulating the WAP resident population is unclear. The objective of this study was to investigate the effect of omega-3 PUFA on the niche composition of adipose-derived stem cells.
Methods
Stromal vascular cell fraction (SVF) was collected from subcutaneous fat of wild-type (WT) and transgenic mice carrying a fat-1 gene from Caenorhabditis elegans (Fat-1 mice), which are capable of synthesizing omega-3 PUFA and have much higher tissue levels of omega-3 PUFA relative to WT mice. The isolated SVF cells were cultured and used for the examination of adipocyte differentiation, adipogenic markers, fatty acid composition, and WAP numbers.
Results
SVF isolated from Fat-1 mice (Fat-1-SVF) exhibited markedly fewer differentiated adipocytes with smaller cell size and less lipid content than that of WT mice (WT-SVF). Accordingly, adipogenesis-related genes and the white adipocyte surface marker ASC-1 were downregulated in Fat-1-SVF relative to WT-SVF. Furthermore, WAP numbers and adipose tissue macrophages were lower in Fat-1-SVF than WT-SVF.
Conclusions
Omega-3 PUFA can both limit the WAP resident population and suppress their differentiation to white adipocytes, suggesting a new mechanism for the antiobesity effect of omega-3 PUFA.
Study Importance
What is already known?
- Omega-3 fatty acids are known to have antiobesity effects in mice.
- Mechanisms shown for these effects in mice include reducing chronic inflammation, increasing thermogenesis, modulating the gut microbiome, and improving lipid metabolism.
What does this study add?
- This study demonstrates a new mechanism - omega-3 fatty acids suppress adipogenesis by reducing the number and differentiation of white adipocyte progenitors in mice.
Introduction
Obesity is a complex health issue in which appetite control and energy metabolism are dysregulated. White adipose tissues, including visceral fat and subcutaneous fat, expand because of an excess intake of energy resulting in obesity (1). Visceral adiposity increases primarily through hypertrophy and is accompanied by chronic inflammation, leading to high risks of obesity-related complications. Meanwhile, subcutaneous adiposity, which increases mostly by hyperplasia, is not correlated with risk factors for obesity and insulin resistance (1). Understanding fat distribution and controlling white fat mass accumulation are important for treating obesity.
Adipose tissue comprises adipocytes and nonadipocyte cells, which are referred to as the stromal vascular cell fraction (SVF). SVF contains heterogenous cell types, including immune cells, endothelial cells, preadipocytes, and adipose-derived stem cells (ADSC). In adults, there is an annual turnover of white adipocytes of approximately 10%, and ADSC are the main contributor to adipose tissue expansion through hypertrophy and hyperplasia (1, 2). ADSC function similarly to multipotent mesenchymal stem cells (MSC) of the bone marrow and are capable of differentiating into a variety of cell lineages. White adipocyte progenitors (WAP), a subpopulation of ADSC, can be isolated from subcutaneous fat. WAP were first characterized in humans by the cell markers CD31− and CD34+ in SVF of adipose tissue and were prone to be adipogenic (3). Approximately 100% of WAP are now identified based on the absence of hematopoietic (CD45−) and endothelial negative (CD31−) surface markers, as well as enriched expression of MSC markers such as CD29+, CD34+, stem cell antigen-1+, and platelet-derived growth factor receptor α (PDGFRα+), during white adipocyte differentiation (4). Because mature white adipocytes are postmitotic, WAP contributing to de novo adipogenesis are necessary for fat mass reconstitution (5). Deletion of WAP not only suppresses adipogenesis but restores thermogenic capacity (6). Therefore, characterizing WAP may provide new approaches to the management of obesity and its related pathogenesis.
Even though it is widely accepted that excessive intake of saturated fat is associated with a high incidence of diet-induced metabolic syndromes, one of the most drastic changes in Western diets since the 1900s has been much higher consumption of omega-6 polyunsaturated fatty acids (omega-6 PUFA) relative to omega-3 PUFA (7). This change in diet resulted in a large increase of omega-6:omega-3 ratios from 1:1 to a range of 20:1-30:1, as well as the ensuing omega-3 PUFA deficiency that is related to the progression of chronic metabolic disorders (7). Additionally, omega-3 PUFA have the potential to halt adipogenesis (8) and promote the differentiation of MSC into osteoblasts as opposed to adipocytes (9). Many studies have reported differential effects of omega-6 and omega-3 PUFA on terminally differentiated mature adipocytes and obesity-related syndromes, but the effect of omega-3 PUFA on WAP during early onset of obesity is unknown. In this study, we aimed to compare the adipogenic capacity of SVF and the quantity of WAP between aged-matched wild-type (WT) and transgenic Fat-1 mice. These transgenic mice carry a copy of the Caenorhabditis elegans fat-1 gene, encoding an omega-3 fatty acid desaturase that converts omega-6 PUFA to omega-3 PUFA, and have an enhanced level of omega-3 PUFA and a balanced ratio of omega-6 to omega-3 PUFA in all tissues (10). Thus, WT and Fat-1 mouse littermates can be fed with an identical diet to generate distinct PUFA profiles (i.e. the tissue levels of omega-3 PUFA are higher in Fat-1 mice relative to WT mice) (10), allowing for studies to be conducted without dietary confounding factors.
Methods
Animals
Transgenic Fat-1 mice were generated as previously described (10) and showed a significant phenotype in terms of low tissue n-6/n-3 ratio. Animals were maintained on a 10% corn oil diet (TestDiet, Quakertown, Pennsylvania) ad libitum and housed with a 12-hour light/dark cycle, under specific pathogen-free conditions. The age-matched WT and Fat-1 mice were subjected to experiment of SVF isolation. The animal study was approved by Institutional Animal Care and Use Committee in Massachusetts General Hospital.
Cell isolation and culture
The isolation of SVF was used by the modified methods (11). Inguinal adipose tissue was dissected from individual mice and minced tissue was digested in collagenase D (Roche, Indiana) in sterile Hank’s Balanced salt solution (HBSS containing 5 mM D-glucose, 1.6 mM calcium chloride, 2.2 mM magnesium chloride, and 0.83 mM zinc chloride). The solution was incubated in a water bath at 37°C and vigorously shaked by hand every 6 minutes until 90% of minced tissue was digested. Then 10% FBS Dulbecco’s modified eagle medium (DMEM) was added to stop the digestion and filtered through a 70-µm cell strainer (BD Bioscience, California). SVF was separated by centrifugation at 1100 rpm for 5 minutes and the lipid layer was removed. The cell pellet was resuspended with ACK lysis buffer (ThermoFisher Scientific, New York) and incubated for 3 minutes. After centrifugation, cells were plated in 10% FBS DMEM. For white adipogenesis induction, cells grown to confluence were cultured in differentiation medium containing 5 µg/mL of insulin, 2 µg/mL of dexamethasone, 0.5 mM IBMX, and 0.5 µM rosiglitazone for 2 days. The medium was changed to the medium containing 10% FBS DMEM, 5 µg/mL of insulin, and 0.5 µM rosiglitazone for another 2 days. The cells were then maintained in 10% FBS DMEM until completed adipocyte differentiation. The lipid droplets in adipocytes were stained with Oil Red O (ORO) or a lipid-specific fluorescence dye (a gift from Dr. Chongzhao Ran in MGH), and photographed. ORO dye was extracted by isopropanol and measured at the wavelength of 490 nm using Epoch microplate spectrophotometer (Biotek, Vermont). Unless specified, all chemicals were purchased from Sigma Aldrich, St. Louis, Missouri.
Gene expression
Total mRNAs from cells/tissues were extracted with TRIzol® (Invitrogen, Grand Island, New York). The cDNA was synthesized by iScriptTM system (Bio-Rad, Hercules, California) and reverse transcription reaction was performed using PTC-100 programmable thermal controller (MJ Research Inc., Waltham, Massachusetts). A real-time PCR was performed using an iTaqTM universal STBR green Supermix (Bio-Rad) in an Mx3005P qPCR thermocycler (Agilent Technologies, Santa Clara, California). The primer sequences were designed using NCBI primer-BLAST library (Supporting Information Table S1). All values were normalized by PPIA expression and further analyzed using the ΔΔCT method.
Fatty acid analysis
Lipids were methylated in 1:1 mixture of hexane and 14% boron trifluoride/methanol (Sigma-Aldrich) at 100°C for 1 hour. Fatty acid methyl esters were analyzed by a fully automated 6890N Network Gas Chromatography (GC) equipped with a flame-ionization detector (Agilent Technologies). Individual fatty acid was determined by retention time compared with a reference standard, GLC461 (Nu-Chek Prep., Waterville, Minnesota).
Flow cytometry
SVF was isolated from inguinal adipose tissue as described, and cell sorting was previously described (11). Antibodies such as CD45, CD31, CD34, CD29, Scal1, and CD140a (BioLegend, San Diego, California) were diluted in PBS with 2% FBS (staining buffer). SVF (5x105 cells) were resuspended in 100 µL of antibody staining solution, and sequentially placed on ice in the dark for at least 30 minutes. Then 500 µL of staining buffer was added to wash and SVF was centrifuged at 2000 rpm for 5 minutes. Prior to analyzing the samples, fluorescent-minus-one (FMO) control was used to adjust the compensation setting and this scenario provided the positive signal correctly. Cell sorting was applied on LSR II flow cytometer (BD Biosciences, Franklin Lakes, New Jersey).
Statistical analysis
All values are shown as mean ± SEM. The differences between two groups were evaluated by Student t test using GraphPad Prism software. Means were considered statistically significant at P < 0.05.
Results
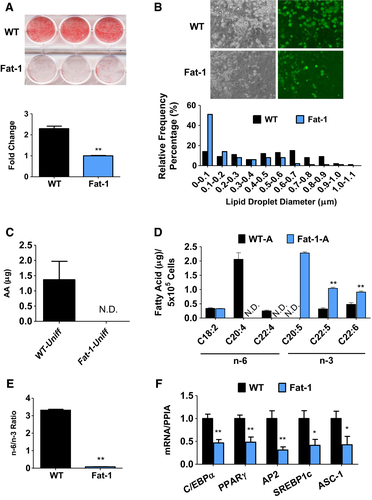
SVF, collected directly from adipose tissue, has been widely used to study human and animal adipogenesis (2); this ex vivo model is closely associated with WAP expansion. SVF cells isolated from subcutaneous fat of WT and Fat-1 mice (referred as WT-SVF and Fat-1-SVF, respectively) with the same cell number for each group were cultured and used for comparison in the quantity and differentiation of WAP between Fat-1 and WT mice. Relative to WT-SVF, Fat-1-SVF exhibited a significantly lower level of lipid accumulation as observed by Oil Red O (ORO) staining (Figure 1A). Greater adipocyte size and numbers were observed in WT-SVF compared with Fat-1-SVF (Figure 1B). Accordingly, adipogenesis-related genes such as PPARγ, C/EBPα, AP2, and SREBP-1c, as well as white adipocyte surface marker ASC-1 (11), were increased in WT-SVF relative to Fat-1-SVF (Figure 1F). Analysis of total lipid content extracted from undifferentiated and differentiated SVF cells exhibited distinct fatty acid profiles between WT-SVF and Fat-1-SVF using gas chromatography. Prior to differentiation, higher amounts of omega-6 PUFA (mainly arachidonic acid) were detected in WT-SVF compared with Fat-1-SVF, while omega-3 PUFA were rarely detectable in both SVFs (Figure 1C; Supporting Information Table S2). After 8 days of adipocyte differentiation, significant amounts of omega-3 PUFA (including eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid) with very little of omega-6 PUFA were found in Fat-1-SVF, whereas the opposite was observed in WT-SVF (Figure 1D; Supporting Information Table S2). This resulted in a nearly 35-fold lower ratio of omega-6 to omega-3 PUFA in Fat-1-SVF (0.08) compared with WT-SVF (3.31) (Figure 1E; Supporting Information Table S2). These data demonstrate that SVF of Fat-1 transgenic mice with high levels of omega-3 PUFA has a low potency to generate white adipocytes.
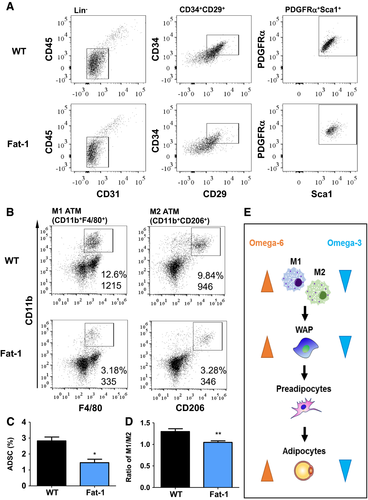
Flow cytometry analysis of SVF revealed a significant difference in the WAP subpopulation between groups. Relative to the Fat-1-SVF group, WT-SVF exhibited a higher percentage of hematopoietic Lin-negative stem cell populations (CD31− and CD45−) (Figure 2). Among these non-Lin-committed cell populations, WT-SVF had an increased percentage of MSC markers (CD29+ and CD34+), contributing to a nearly two fold increase in the WAP population (WAP, stem cell antigen-1+, and PDGFRα+) (Figure 2A and 2C). Additionally, we determined the profile of adipose tissue macrophages (ATM) in SVF. ATM comprises two major subsets, M1 ATM, whose number is associated with obesity (1), and M2 ATM, which alters adipose tissue remodeling by the clearance of dead adipocytes and by the promotion of adipocyte progenitor proliferation, leading to hyperplasia in early obesity development (12). Our results showed that WT-SVF had higher numbers of both M1 (CD11b+ and F4/80+) and M2 (CD11b+ and CD206+) macrophages with a higher ratio of M1 to M2 in WT-SVF (1.30) compared with Fat-1-SVF (1.05) (Figure 2B and 2D). Overall, our results suggest that a high tissue level of omega-3 PUFA with a low omega-6:omega-3 PUFA ratio (as seen in Fat-1-SVF) creates an unfavorable microenvironment for hyperplasia through limiting WAP population and differentiation (Figure 2E).
Discussion
Even though it is generally accepted that omega-3 PUFA or a balanced ratio of omega-6 to omega-3 is important for prevention and management of obesity (7), most of the research has focused on how to alleviate obese microenvironments such as chronic low-grade inflammation. Previous reports have shown that the defined number of adipocytes is established during adolescence, and an increased threshold of adipocyte number has the potential to raise adipose tissue expansion in adulthood (1). However, little is known about the influence of omega-3 PUFA on the status of WAP. The key finding reported here is that omega-3 PUFA suppress both the size of the WAP pool in adipose tissue and WAP differentiation, leading to fewer white adipocytes. This possibly contributes to reduced abdominal adipose tissue weight and fewer adipocyte numbers in Fat-1 mice relative to WT mice, as previously observed (13). Although more studies are needed, this finding has clinical implications for the use of omega-3 PUFA as a new strategy for obesity prevention through blocking early hyperplasia.
A high-fat diet (HFD) promotes progenitor proliferation and adipogenesis in adipose tissue in rodents prior to a significant increase of body weight (14, 15). Our results showed that the relative percentage of M1 macrophages in WT-SVF was about 12% of total SVF compared with a previous study in which nearly 40% was observed when WT mice had a significant body weight gain relative to Fat-1 mice in an HFD-induced obesity model (16). Without HFD challenge, no body weight difference between WT and Fat-1 mice at the age of 9 to 12 weeks was observed in this study, thus indicating that the mice used for WAP identification were not yet in an obese state; most likely, their adipose tissue was prone to hyperplasia after HFD feeding.
Lipid rafts act as microdomains in plasma membranes; they are involved in a wide range of activities, including signal transduction, determining cellular biophysical properties, and determining cell fate and function. PDGFRα directly activates phosphatidylinositol 3-kinase/AKT serine/threonine kinase 2 (PI3K/Akt2) in lipid rafts during hyperplasia, and this regulation is required for WAP proliferation (17). Our observation that there was less PDGFRα expression in Fat-1-SVF relative to WT-SVF suggests that omega-3 PUFA may limit WAP differentiation through, partially, suppression of PDGFRα expression. The composition of lipid rafts is influenced by dietary fat, and omega-3 PUFA (docosahexaenoic acid) has been shown to be incorporated into the plasma membrane, contributing to neuron stem cell renewal (18) and anti-inflammation in adipocytes (19). These findings together with our results provide a possible explanation for why omega-3 PUFA constitute a cell-specific microdomain of the plasma membrane favoring osteoblast differentiation versus adipocytes in MSC (9). Moreover, previous studies have reported potential adverse effects of inhibiting adipogenesis on metabolism (20). However, this is not the case for the effects of omega-3 PUFA (7, 16). Herein, omega-3 PUFA reduced the WAP resident population rather than completely abolished WAP numbers and their differentiation to white adipocytes. Additionally, the literature has shown that omega-3 PUFA can attenuate obesity, increase insulin sensitivity, and improve diabetes-associated metabolic syndromes through inhibition of lipogenesis, suppression of inflammation, etc. (7, 16). It is obvious that omega-3 PUFA can inhibit adipogenesis without causing metabolic dysfunction.
Conclusion
Omega-3 PUFA may have potential to limit WAP pool size in adipose tissue and suppress WAP differentiation to white adipocytes. These findings not only uncover a new mechanism for the antiobesity effect of omega-3 PUFA but also highlight the potential clinical utilities of omega-3 PUFA in the management of obesity.





