Bariatric Surgery Alters microRNA Content of Circulating Exosomes in Patients with Obesity
Abstract
Objective
Exosomal microRNAs (miRNAs) are potential biomarkers for obesity, in which they regulate biological processes. Bariatric surgery has health benefits for patients with obesity; however, the mechanisms of these benefits are not clear. This study attempted to identify the exosomal miRNA signature associated with obesity and how it changed after bariatric surgery.
Methods
Healthy volunteers (HVs) and nondiabetic patients with obesity were prospectively enrolled in the study. The study assessed the serum exosomal miRNA profiles of HVs and patients with obesity using RNA sequencing. To evaluate the effects of bariatric surgery, the study also analyzed exosomal miRNAs in patients 6 months after surgery.
Results
RNA sequencing revealed differential expression of 72 exosomal miRNAs in patients with obesity compared with HVs and differential expression of 41 miRNAs in post- versus presurgery blood. Among the differentially expressed miRNAs, the study identified nine surgery-responsive miRNAs that were highly expressed in patients before surgery compared with HVs. Biological pathway analysis of the nine miRNAs indicated that they are likely involved in WNT, neurotrophin, and insulin signaling; the insulin receptor signaling cascade; and focal adhesion.
Conclusions
Patients with obesity have a distinct exosomal miRNA expression profile compared with HVs. In addition, weight loss after surgery alters the exosomal miRNA profile of patients with obesity.
Introduction
Obesity has emerged as a leading health issue in the past few decades (1). Obesity plays a major role in the pathogenesis of chronic diseases such as type 2 diabetes mellitus (DM), hypertension, cancer, heart disease, stroke, obstructive sleep apnea, and chronic kidney disease (2).
MicroRNAs (miRNAs) are single-stranded, noncoding RNAs that are highly conserved across species. Several studies have demonstrated the deregulation of circulating miRNAs in obesity (3-9). The miRNAs circulating in bodily fluids are transported by heterogeneous carriers, including exosomes, microparticles, and apoptotic blebs, and exist in a vesicle-free form that is associated with RNA-binding proteins (10-12). Exosomes actively shed endocytic vesicles that contain and transport proteins and nucleic acids, such as mRNAs and miRNAs, between cells (13, 14). Circulating exosomal miRNAs derived from adipocytes may regulate metabolism and mRNA translation in other cells or tissues (15-17). Exosomes carrying specific materials are also promising therapeutic tools for the treatment of various diseases (13, 18). Thus, exosomal miRNAs could be valuable biomarkers for obesity and potential therapeutic targets. A few studies have used microarray or real-time quantitative PCR (qPCR) assays to examine exosomal or extracellular vesicular miRNAs in obesity (19, 20). Because of the limitations of these technologies, unknown miRNAs and other RNA species are undetectable (21). The very low background levels in RNA sequencing (RNA-seq) allow it to accurately map genomes of interest with a large dynamic range of expression levels and high reproducibility (22). Therefore, this technique could facilitate the early diagnosis of comorbidities and identification of optimal therapies for obesity (23).
Bariatric surgery has recently emerged as a potentially useful treatment for morbid obesity; it can prevent cardiovascular events, diabetes, and diabetes-related complications (24-26). Few studies have investigated the beneficial mechanisms, with respect to miRNAs, behind bariatric surgery (3, 19, 20, 27). In addition, the exosome-mediated effects of bariatric surgery have not been well established.
In the current study, we compared the exosomal miRNA profiles of patients with obesity and healthy volunteers (HVs) using RNA-seq to identify obesity-specific exosomal miRNAs. To elucidate the mechanisms behind the beneficial effects of bariatric surgery, we also investigated exosomal miRNA content in patients after bariatric surgery and compared it with exosomal miRNA content in patients with obesity prior to surgery and HVs.
Methods
Participants
The study was approved by the Soonchunhyang University Seoul Hospital Institutional Review Board (number 2015-11-020). We obtained written informed consent from each participant. We prospectively recruited HVs (n = 18) and patients with morbid obesity (n = 16) between January 2016 and August 2017. The participants ranged from 30 to 59 years of age. Patients with BMI ≥ 35 kg/m2 or ≥ 30 kg/m2 and comorbidities related to obesity, including inadequately controlled obstructive sleep apnea and obesity-related arthropathy, were classified as having obesity. Patients with diabetes, decreased estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration equation < 60 mL/min/1.73 m2), or resistant hypertension were excluded. The patients with obesity subsequently underwent bariatric surgery. At 6 months after surgery, the patients returned for follow-up and data collection.
Serum exosomal RNA isolation and assessment
Exosomes were isolated from serum using ExoQuick isolation agent (System Bioscience, Palo Alto, California) according to the manufacturer’s guidelines. Serum samples (1,000 μL) were centrifuged at 3,000g for 15 minutes to remove cells and cell debris. We mixed the supernatants with ExoQuick reagent and incubated the mixture for 30 minutes at 4°C. After incubation, the samples were centrifuged at 1,500g for 30 minutes. After centrifugation, the supernatants were aspirated. The remaining pellets contained exosomes. The exosomes in the pellets were resuspended in 100 to 200 μL of sterile phosphate-buffered saline (PBS). RNA was extracted from the exosomes using the miRNeasy Mini Kit (Qiagen, Hilden, Germany). Exosome suspensions (200 μL) were mixed with QIAzol lysis buffer (1 mL) (Qiagen), and the mixtures were processed according to the manufacturer’s guidelines. The RNA was eluted in RNase-free water (20 μL) (Qiagen). The purified RNA was analyzed using an Agilent 2100 Bioanalyzer with an RNA Pico Chip and Small RNA Chip to examine the size distribution of the exosomal RNAs (Agilent Technologies, Santa Clara, California) (Supporting Information Figure S1).
Complementary DNA library preparation and small-RNA-seq
The samples were processed to produce exosomal RNA (10 ng) as input for each library. Small RNA libraries were constructed using SMARTer smRNA-Seq Kit for Illumina (Takara Bio, Shiga, Japan) according to the manufacturer’s guidelines. We generated sequencing libraries by polyadenylation, complementary DNA (cDNA) synthesis, and polymerase chain reaction (PCR) amplification.
The libraries were gel-purified and then validated by assessing size, purity, and concentration on the Agilent Bioanalyzer. The libraries were quantified by qPCR according to the qPCR Quantification Protocol Guide (KAPA Library Quantification Kits for Illumina Sequencing Platforms). We assessed the quality of the libraries using the D1000 ScreenTape System (Agilent Technologies, Waldbronn, Germany). Equimolar amounts of the libraries were pooled and sequenced on an Illumina HiSeq 2500 instrument (Illumina, San Diego, California) to generate 101 base reads. Image decomposition and quality value calculations were performed using the modules of the Illumina pipeline. All procedures for next-generation sequencing analysis were performed by Macrogen (Seoul, Korea).
Analysis of RNA-seq data
We performed sequence alignment and detected known and novel miRNAs using the miRDeep2 software algorithm (Berlin Institute for Medical Systems Biology at the Max-Delbruck-Center for Molecular Medicine, Berlin-Buch, Germany). Prior to aligning the sequences, we retrieved the Homo sapiens reference genome release hg19 from the University of California Santa Cruz Genome Browser, which we indexed using Bowtie (1.1.2; Johns Hopkins University, Baltimore, Maryland), a program for aligning experimental and reference sequences. The reads were then aligned to mature and precursor H sapiens miRNAs obtained from miRBase 21 (http://www.mirbase.org/).
Proportions of miRNAs and other RNAs
The uniquely clustered reads were sequentially aligned to the reference genome using miRBase 21 and the noncoding RNA database Rfam 9.1 (http://rfam.xfam.org/) to identify known miRNAs and other types of RNAs, respectively.
Analysis of differential miRNA expression
The raw data (the reads for each miRNA) were normalized by relative log expression using DESeq2 (Genome Biology Unit, European Molecular Biology Laboratory, Heidelberg, Germany). For preprocessing, miRNAs absent from more than 50% of all samples were excluded, leaving mature miRNAs to be analyzed. We added 1 to the normalized read count of the filtered miRNAs to facilitate log2 transformation to draw the correlation plot. For each miRNA, the baseMean and log fold change were calculated between the case and control. We conducted a statistical hypothesis test for the comparison of the two groups using the negative binomial Wald test in DESeq2. Differentially expressed miRNAs between the two groups were determined by assessing miRNAs with |fold change| ≥ 2 and false discovery rate–adjusted P value < 0.05. We also performed hierarchical clustering analysis using complete linkage and Euclidean distance as measures of similarity to display the expression patterns of the differentially expressed miRNAs that satisfy the criteria |fold change| ≥ 2 and false discovery rate–adjusted P value < 0.05. All data analysis and visualization of the differentially expressed genes were conducted using R 3.3.1 (www.r-project.org).
Identification of miRNA target genes and their molecular pathways
We uploaded the miRNAs that were differentially regulated in the healthy, presurgery, and postsurgery groups into popular prediction programs, such as DIANA-miRPath (http://diana.imis.athena-innovation.gr/DianaTools/index.php) and miRSystem (http://mirsystem.cgm.ntu.edu.tw/), for further analyses. The DIANA-miRPath version 3.0 database used DIANA-microT-CDS (DIANA-Lab, Biomedical Sciences Research Center Alexander Fleming, Greece) and TargetScan 6.2 (Howard Hughes Medical Institute, Chevy Chase, Maryland) to analyze miRNA–gene interactions. The database schema incorporates the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and the Gene Ontology (GO) and GO slim annotations. The gene and miRNA annotations were derived from Ensembl (https://useast.ensembl.org/index.html) and miRBase, respectively. miRSystem used seven algorithms for predicting miRNA targets (DIANA-microT, miRanda, miRBridge, PicTar, PITA, RNA22, and TargetScan) and two experimentally validated databases of miRNA target genes (TarBase and miRecords). Five pathway databases, GO, KEGG, BioCarta, Pathway Interaction Database, and Reactome, were used to annotate the biological functions and canonical pathways of the target genes.
Statistical analysis
Continuous variables with a normal distribution are expressed as mean ± standard deviation; variables without a normal distribution are expressed as the median with interquartile range. The Mann–Whitney U test was used to analyze the statistical significance of differences between continuous variables, and the χ2 test was used for categorical variables to compare baseline characteristics between HVs and patients with obesity. We performed paired t tests to study the differences before and after surgery in patients.
Results
Clinical characteristics
The mean age of the participants was 35.2 ± 9.0 years. The clinical characteristics of the participants in the baseline cross are shown in Table 1. Among them, two patients underwent sleeve gastrectomy and the rest underwent Roux-en-Y gastric bypass surgery. After bariatric surgery, the average BMI of the patients was 21% lower than before surgery (Table 2).
| HVs (n = 18) | Patients with obesity (n = 16) | P value | |
|---|---|---|---|
| Age (y) | 38.6 ± 7.9 | 31.3 ± 8.76 | 0.014 |
| Female, n (%) | 13 (72.2) | 9 (56.3) | 0.331 |
| Height (cm) | 163.4 ± 7.7 | 169.06 ± 9.7 | 0.049 |
| Weight (kg) | 57.9 ± 5.7 | 115.4 ± 24.9 | 0.000 |
| BMI (kg/m2) | 21.6 ± 1.4 | 39.9 ± 4.6 | 0.000 |
| sBP (mm Hg) | 106.1 ± 8.5 | 127.5 ± 12.3 | 0.000 |
| dBP (mm Hg) | 72.2 ± 8.0 | 76.2 ± 9.5 | 0.228 |
| LDL (mg/dL) | 112.7 ± 23.3 | 152.3 ± 44.4 | 0.004 |
| T-chol (mg/dL) | 183.89 ± 24.1 | 222.1 ± 46.4 | 0.007 |
| HDL (mg/dL) | 65.3 ± 14.5 | 47.3 ± 9.7 | 0.000 |
| TG (mg/dL) | 105.4 ± 82.8 | 208.5 ± 120.9 | 0.002 |
| Creatinine (mg/dL) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.541 |
| eGFR (mL/min) | 107.1 ± 10.9 | 115.6 ± 11.0 | 0.031 |
| Albumin (g/dL) | 4.7 ± 0.2 | 4.5 ± 0.2 | 0.019 |
| Glucose (mg/dL) | 97.0 ± 8.5 | 108.0 ± 16.4 | 0.018 |
| AST (IU/L) | 18.8 ± 5.2 | 44.2 ± 33.9 | 0.006 |
| ALT (IU/L) | 18.6 ± 10.6 | 74.7 ± 70.1 | 0.001 |
| Uric acid (mg/dL) | 4.7 ± 1.4 | 6.2 ± 1.8 | 0.035 |
| Urine protein (mg/d) | 62.2 ± 17.3 | 96.2 ± 73.4 | 0.081 |
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; dBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HV, healthy volunteer; LDL, low-density lipoprotein; sBP, systolic blood pressure; T-chol, total cholesterol; TG, triglyceride.
| Variable | Before surgery (n = 12) | After surgery (n = 12) | P value |
|---|---|---|---|
| Weight (kg) | 117.65 ± 26.84 | 91.92 ± 23.15 | < 0.001 |
| BMI (kg/m2) | 39.73 ± 5.06 | 31.00 ± 4.68 | < 0.001 |
| Systolic BP (mm Hg) | 125.83 ± 11.65 | 127.08 ± 19.49 | 0.804 |
| Diastolic BP (mm Hg) | 75.83 ± 9.96 | 76.08 ± 12.32 | 0.955 |
| AST (U/L) | 43.00 ± 24.60 | 22.42 ± 5.84 | 0.010 |
| ALT (U/L) | 69.0 ± 25.30 | 21.0 ± 7.62 | 0.012 |
| Total cholesterol (mg/dL) | 212.75 ± 42.54 | 179.50 ± 23.96 | 0.002 |
| HDL cholesterol (mg/dL) | 47.08 ± 11.16 | 56.92 ± 13.52 | 0.001 |
| LDL cholesterol (mg/dL) | 143.75 ± 42.49 | 115.17 ± 23.34 | 0.005 |
| Triglyceride (mg/dL) | 148.5 [90.5-268.0] | 106.0 [74.0-141.0] | 0.028 |
| Glucose (mg/dL) | 103.5 [100.2-114.5] | 95.00 [90.2-101.2] | 0.008 |
- BP, blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Small RNA composition
On average, we obtained 30 million raw reads (ranging from 18 to 80 million). To examine the diversity of the exosomal RNA species, we applied an iterative strategy by sequentially mapping to each of the RNA reference databases. Next-generation sequencing identified exosomal small RNAs, including miRNAs, small nuclear RNAs, small nucleolar RNAs, and transfer RNAs (tRNAs). The ratio of small RNAs, excluding tRNAs, in exosomes from HVs differed from that in patients with obesity. However, the ratio of tRNA components in exosomes post surgery increased compared with pre surgery in patients with obesity (Supporting Information Table S1).
Profiling of exosomal miRNA expression in HVs and patients with obesity
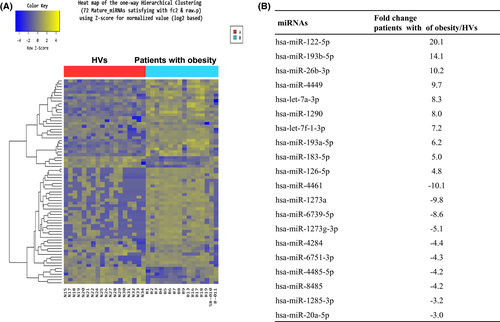
Based on RNA-seq, we identified 57 upregulated miRNAs and 15 downregulated miRNAs in the patients compared with the HVs. Figure 1A shows the expression pattern of the exosomal miRNAs in the groups and Figure 1B summarizes the 10 miRNAs that were more highly upregulated and downregulated in patients with obesity compared with HVs.
Effects of bariatric surgery
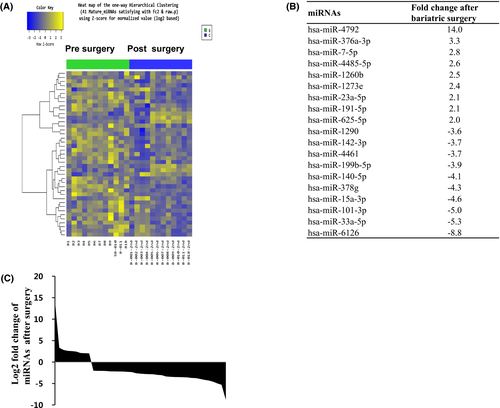
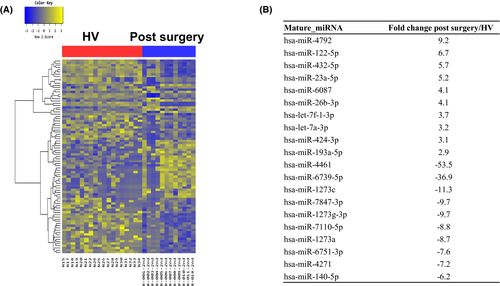
Based on RNA-seq, the expression of nine miRNAs was significantly higher, whereas expression of 32 miRNAs was significantly lower, in the postsurgical compared with the presurgical samples (Figure 2). The data set from the patients who underwent surgery differed from the presurgery and HV data sets (Figure 3). The expression of nine of the miRNAs that were upregulated in the patients compared with HVs prior to surgery decreased after bariatric surgery (Table 3). Among the nine miRNAs, miR-424-5p pre surgery correlated with weight loss after surgery (r = 0.687, P = 0.014). We used miRSystem to identify the enriched biological pathways for the nine candidate miRNAs. Using a threshold of P < 0.01, we identified canonical pathways that were enriched in the surgery-responsive data set. Table 4 shows the canonical pathways most likely targeted by the miRNAs based on their miRSystem scores. This last filter was used to screen out ubiquitous cancer-specific pathways.
| Mature miRNA | Fold change in obesity | Fold change after surgery |
|---|---|---|
| hsa-miR-1246 | 2.77 | −2.60 |
| hsa-miR-1290 | 5.94 | −3.57 |
| hsa-miR-193b-5p | 6.76 | −2.37 |
| hsa-miR-378c | 3.50 | −3.35 |
| hsa-miR-378d | 2.16 | −2.98 |
| hsa-miR-378g | 4.24 | −4.30 |
| hsa-miR-424-5p | 2.30 | −3.50 |
| hsa-miR-4449 | 6.60 | −2.70 |
| hsa-miR-6126 | 2.46 | −8.80 |
| Term | Total genes in pathway | Union targets in pathway | Score |
|---|---|---|---|
| WNT signaling pathway | 150 | 29 | 1.109 |
| Neurotrophin signaling pathway | 127 | 24 | 0.91 |
| Insulin signaling pathway | 137 | 25 | 0.909 |
| IRS cascade | 86 | 19 | 0.863 |
| IRS-mediated signaling | 81 | 18 | 0.826 |
| IRS-related events | 81 | 18 | 0.826 |
| Focal adhesion | 199 | 29 | 0.792 |
| PDGFR-beta signaling pathway | 126 | 22 | 0.771 |
| ERBB1 downstream signaling | 106 | 20 | 0.771 |
| PI3K cascade | 70 | 16 | 0.763 |
| Signaling by insulin receptor | 109 | 20 | 0.748 |
| mTOR signaling pathway | 68 | 15 | 0.697 |
| Signaling by NGF | 221 | 29 | 0.686 |
| MAPK signaling pathway | 272 | 33 | 0.683 |
| CDC42 signaling events | 70 | 15 | 0.678 |
| SHP2 signaling | 54 | 13 | 0.664 |
| L1CAM interaction | 94 | 17 | 0.638 |
| Adipocytokine signaling pathway | 68 | 14 | 0.614 |
| T cell receptor signaling pathway | 108 | 18 | 0.614 |
| Regulation of actin cytoskeleton | 213 | 27 | 0.613 |
- IRS, insulin receptor substrate; PDGFR, platelet-derived growth factor receptor; ERBB1, EGFR-epidermal growth factor receptor; PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; NGF, nerve growth factor; MAPK, mitogen-activated protein kinase; CDC42, cell division control protein 42 homolog; SHP2, PTPN11-tyrosine-protein phosphatase non-receptor type 11; LICAM, neural cell adhesion molecule L1.
Discussion
In this study, we identified bariatric surgery–responsive, obesity-specific exosomal miRNAs in nondiabetic patients with obesity. We found that bariatric surgery altered the circulating exosomal miRNA profile of patients with obesity; in particular, more miRNAs were downregulated than upregulated after surgery. However, 6 months after surgery, the exosomal miRNA profile of patients with obesity still differed from that of HVs. Our study also revealed that the ratios of different types of exosomal small RNAs differed in patients with obesity and healthy individuals.
Obesity involves more than the gain of adipose tissue but is not currently associated with specific miRNAs (3, 6). The outcome of bariatric surgery does not depend on one or some miRNAs’ alteration. It is more likely that the exosomal miRNAs’ milieu changes after surgery. However, the identification of new RNAs will increase our knowledge of obesity and metabolic disease. The obesity-related exosomal miRNAs identified in our study are novel compared with previously reported circulating miRNAs (3, 4, 19, 27). Vesicular and nonvesicular miRNAs in bodily fluids are modulated in a disease-specific fashion (12). Furthermore, the selective transfer of cargo via the exosomal pathway is emerging as an essential pathway for intercellular communication in both health and disease (28). Therefore, we isolated exosomes from the serum, thereby collecting a more homogenous sample than if we had isolated nonspecific circulating miRNAs. Exosomes contain functional proteins and nucleic acids that may be useful for diagnostic and therapeutic purposes. In addition, exosomes provide stability under various storage conditions, ensuring that storage does not influence the quality or distribution of the recovered miRNAs (29). Therefore, exosomal miRNAs are more valuable biomarkers of disease than nonspecific circulating miRNAs. Unfortunately, because of the lack of standardized protocols for the isolation and analysis of exosomes (30) and the heterogeneity of many exosome preparations (31), the use of exosomal miRNAs for research and diagnostic purposes is challenging. In our study, we successfully conducted RNA-seq of exosomal miRNAs using commercial kits. This protocol, which does not require laborious ultracentrifugation or large volumes of serum, may be practical in the clinical setting.
Adipose tissue is known to exert some of its systemic effects through the secretion of adipokines via exosomes and extracellular vesicles, which create sites of chronic low-grade inflammation in the setting of obesity (2, 15, 16). Adipose tissue–specific Dicer deficiency reduces the amounts of circulating miRNAs in exosomes. Therefore, adipose tissue has been hypothesized to be a main source of exosomal miRNAs (17). The most obvious effect of bariatric surgery is the reduction in adipose tissue mass in the first year after surgery (32). Decreased exosomal miRNA levels after bariatric surgery support the hypothesis that adipose tissue is a main source of exosomal miRNAs. Adipose-derived exosomal miRNAs regulate gene expression and metabolism in other tissues (15-17). Thus, the beneficial effects of bariatric surgery may be associated with changes in exosomal miRNAs after surgery. Although bariatric surgery improved the metabolic parameters of the patients in our cohort, their BMI remained high 6 months after surgery. The relatively short follow-up may explain why the exosomal miRNA profile of the surgical patients differed from that of the HVs. Bariatric surgery changed 41 exosomal miRNAs. However, 93 postsurgical exosomal miRNAs differed from those of HVs. These miRNA changes might represent the process of phenotypic change.
The identification of specific obesity-related miRNAs would aid the development of diagnostic and therapeutic agents for obesity-related complications. We found that exosomal miR-122-5p is associated with obesity. Circulating miR-122-5p is associated with fatty liver and lipoprotein metabolism (33) and is being considered as a marker for acute myocardial infarction (34). Antagonizing miR-122 levels in vivo decreases cholesterol levels and increases hepatic fatty acid oxidation (35, 36). The close associations between obesity, lipid disorder, and myocardial infraction may point to the function of miR-122-5p. Circulating miR-122 levels drop after bariatric surgery in rats and humans (3, 27). However, we did not detect a significant decrease in exosomal miR-122 after surgery. In our study, we found elevated levels of exosomal miR-27a in patients with obesity compared with HVs. Circulating miR-27a has been shown to be elevated in metabolic syndrome and type 2 DM (6). Exposure to high concentrations of glucose upregulated miR-27a in mesangial cells, and specific inhibition of this miRNA prevented renal fibrosis in diabetic nephropathy (37). miR-27a may have functional relevance for the long-established obesity–metabolic syndrome–DM connection.
Among the nine obesity-related, surgery-responsive miRNAs, miR-193b has been suggested as a biomarker of prediabetes (38). In our study, the patients could be considered to have prediabetes based on their fasting glucose levels. The serum abundance of miR-193b has been shown to reflect the effects of lifestyle therapy (38). Although we did not investigate the effect in this study, the changes in exosomal miRNAs could represent therapy responses in obesity. miR-1290 was abundantly expressed in patients with nonalcoholic fatty liver disease (39). In addition, miR-378c is more highly expressed in inflamed than in noninflamed adipose tissue (40, 41). miR-378c has been suggested to be a novel mediator of the development of insulin resistance in obesity. Many bariatric patients have reported poor weight loss (42). The obesity-related, surgery-responsive exosomal miRNAs could serve as biomarkers for the response of nondiabetic patients to bariatric surgery. Especially high expression of miR-424-5p pre surgery correlated with weight change post surgery. Therefore, this could be an indicator of the outcome of bariatric surgery for patients with obesity.
We detected lower expression of miR-423-5p in the exosomes of patients with obesity than in those of HVs. It also was found at lower levels in the plasma of patients with obesity than in healthy individuals (3). In patients with obesity, the activation of miR-423-5p in the liver may play an important role in the progression of type 2 DM and nonalcoholic fatty liver disease (43). However, the role of the circulating miRNAs in obesity has still not been elucidated. Surprisingly, the other differentially regulated miRNAs we identified have no reported associations with obesity or obesity-related disorders (44). Thus, the exosomal miRNA profile of patients with obesity differs from their circulating miRNA profile.
The expression of several circulating miRNAs is altered after bariatric surgery. However, we identified miRNAs that are upregulated by obesity and downregulated after surgery. Analyzing these miRNAs with miRSystem and DIANA-miRPath offered more specific insight into bariatric surgery–responsive biological pathways. A prior study of African American bariatric surgical patients also identified canonical pathways that were associated with changes in the expression of adipocyte-secreted exosomal miRNAs (19). The insulin receptor, WNT, and mitogen-activated protein kinase (MAPK) signaling pathways were among the top canonical pathways identified in their study and ours. However, our results included more insulin-related pathways. The differing results could arise from different experimental methods, as the researchers examined adipocyte-derived exosomal miRNAs using magnetic particles and the GeneChip assay in the previous study. As bariatric surgery may affect not only adipose tissue but also other organs, our strategy of examining serum exosomes offers a more comprehensive characterization of bariatric surgery–induced changes. Furthermore, RNA-seq appears to be a very promising technique for the identification of novel miRNA biomarkers (45, 46). Using small-RNA-seq, we identified obesity-related exosomal small RNAs. These RNAs are also involved in regulating the translation of target RNAs. Currently, their roles in obesity are unclear. Although it was beyond the scope of this study, their pathophysiological roles and clinical implications must be investigated. These exosomal small RNAs could also be novel biomarkers for obesity and obesity-related diseases.
Our study has some limitations. First, our cohort was relatively small; large follow-up studies are needed to confirm our results. Second, we could not directly compare nonexosomal miRNAs and exosomal miRNAs. Thus, we were unable to characterize the complete obesity-related circulating miRNA profile. Third, we isolated exosomal RNA using commercial kits. Isolation via other methods may yield different results. Finally, we were unable to determine the functional roles of the miRNAs in obesity.
Conclusion
Our study uncovered serum exosomal miRNAs that are associated with obesity and identified bariatric surgery–induced changes in exosomal miRNA content. Our findings provide a basis for miRNA regulation to treat obesity and obesity-related complications and highlight the possibility of developing exosomal RNA–based biomarkers for obesity. Nonetheless, more extensive investigation is imperative to address the potential of exosomal RNA as a novel pharmacological target for obesity and obesity-related disease intervention.
Acknowledgments
We thank Mr. Charles Virurawong for his help with editing this manuscript.





