Diet-Induced Paternal Obesity Impairs Cognitive Function in Offspring by Mediating Epigenetic Modifications in Spermatozoa
Abstract
Objective
This study aimed to determine the effects of diet-induced paternal obesity on cognitive function in mice offspring.
Methods
Male mice (F0) were randomized to receive either a control diet (10 kcal% fat) or a high-fat diet (HFD; 60 kcal% fat) for 10 weeks before being mated with normal females to generate F1 offspring. Male F1 offspring were mated with normal females to generate F2 offspring. Behavioral tests were used to assess cognitive functions in F1 and F2 offspring. Reduced representation bisulfite sequencing was used to the explore mechanisms of epigenetic inheritance.
Results
HFD-induced paternal obesity resulted in cognitive impairments in F1 offspring, potentially due, at least in part, to increased methylation of the BDNF gene promoter, which was inherited from F0 spermatozoa. BDNF/tyrosine receptor kinase B signaling was associated with cognitive impairments in HFD-fed F1 offspring. However, there were no significant changes in F2 offspring.
Conclusions
The findings provide evidence of intergenerational effects of paternal obesity on cognitive function in offspring occurring via epigenetic spermatozoan modifications.
Introduction
Obesity is a growing worldwide public health concern because of its association with many human diseases, including type 2 diabetes, cardiovascular diseases, respiratory diseases, arthritis, and cancers (1). More critically, there has been an increasing awareness that parental obesity may have negative long-term health consequences on subsequent generations. Both epidemiologic and animal studies have provided evidence that either maternal or paternal obesity is an independent risk factor for obesity and metabolic disorders in offspring (2-5). However, the underlying biological mechanisms mediating these effects are still unclear.
Although DNA damage or mutations may contribute to phenotypic consequences, emerging evidence indicates that the inter- and/or transgenerational effect is driven by epigenetic components, such as DNA methylation, histone modifications, and noncoding RNA expression (5-8). DNA methylation is an important form of epigenetic modification that has a crucial role in the epigenetic regulation of mammalian embryonic development (9). In mammals, DNA methylation patterns undergo genome-wide reprogramming, which occurs in both primordial germ cells once they have reached the embryonic gonads and in the zygote immediately after fertilization (10). Although most DNA methylation patterns are erased during genome-wide reprogramming, some specific regions can escape from reprogramming post fertilization, supporting the hypothesis that epigenetic information can be inherited by gametes, thereby affecting the development and health of offspring (11).
Obesity is increasingly considered a risk factor for cognitive dysfunction. Human epidemiologic and animal studies have demonstrated that diet-induced obesity negatively affects cognitive functions (12-15). Moreover, it has been demonstrated that diet-induced maternal obesity impairs hippocampal neurogenesis and spatial learning performance in mice offspring because of decreased hippocampal brain-derived neurotrophic factor (BDNF) production (16, 17). Although there is evidence that obesity remodels the DNA methylation of genes that regulate the development and function of the central nervous system in human spermatozoa (18), the effects of paternal obesity on cognitive function in the next generation remain poorly understood. In this study, using a mouse model of diet-induced paternal obesity, we hypothesized that paternal obesity affects cognitive function in offspring via epigenetic modifications in spermatozoa.
Our objectives were to determine the effects of diet-induced paternal obesity on cognitive function in mice offspring. We also explored the mechanism of epigenetic inheritance involved in conveying cognitive impairments to offspring.
Methods
Animal care
All animal procedures were reviewed and approved by the Zhejiang University Animal Care and Use Committee. C57BL/6J mice were purchased from Shanghai SLAC Laboratory Animal Co. (Shanghai, China) and were housed under specific-pathogen-free conditions in a 12-hour light/dark cycle with food and water available ad libitum. Male F0 founders were randomly assigned to receive either a control diet (CD; 10 kcal% fat; Research Diets, D12450B) or a high-fat diet (HFD; 60 kcal% fat; Research Diets, D12492) beginning at 4 weeks of age and continuing for 10 weeks. At 14 weeks of age, CD-fed and HFD-fed F0 males were individually mated with 8- to 10-week-old normal females to generate F1 offspring. Male F1 offspring from CD-fed or HFD-fed fathers were mated with 8- to 10-week-old normal females to generate F2 offspring. To avoid postnatal nutrition imbalances, only litter sizes between 8 and 12 pups were included, and litters were standardized to 10 pups on day 1 in each group. The pups were weaned by their mothers until postnatal day 21. Male offspring were used for all analyses, and a maximum of two male offspring were taken from each litter to mitigate interlitter effects. Behavioral tests of offspring commenced at 8 weeks of age. All animals except F0 males were maintained on the CD throughout the experiment.
Blood analysis
Blood glucose levels were determined using a glucometer (Accu-Check, Roche, Basel, Switzerland). Total plasma cholesterol and triglycerides were assayed using a biochemical analyzer (TBA120FR; Toshiba), and radioimmunoassays were used to measure insulin and leptin levels (iodine [125I] radioimmunoassay kit; BNIBT).
Behavioral testing
Morris water maze
Spatial learning and memory were evaluated using the hippocampus-dependent Morris water maze task, as previously described (19). In brief, animals underwent six consecutive days of acquisition training with four trials per day. During each trial, the animal was placed in the water facing the pool wall, and the start locations varied pseudorandomly. Each trial was terminated when the animal reached the escape platform or at 60 seconds, whichever came first. During training days, escape latency (the time spent reaching the platform) was recorded, and data from the four daily trials were averaged. After completion of training, animals were tested in a single 60-second probe trial, during which the platform was removed from the pool and the search pattern of the animal was recorded. During probe trials, we evaluated the quadrant occupancy (percentage of time spent in the platform quadrant), target crossings, swimming speed, and distance traveled. The following day, the animals were subjected to four visible platform trials to exclude visual differences between groups.
Contextual fear conditioning
In this task, mice learned to associate an environmental context (fear conditioning chamber) with an aversive stimulus (mild foot shock), enabling testing of hippocampal-dependent contextual fear conditioning. In brief, mice were placed in a fear conditioning chamber and allowed to explore for 2 minutes. The mice were then subjected to three 0.75-mA 2-second foot shocks through the metal grid floor of the chamber. Consecutive foot shocks were separated by a 1-minute interval. The next day, to assess the strength of associative learning, each mouse was placed in the same fear conditioning chamber, but no foot shock was delivered for 3 minutes, and freezing was recorded using a FreezeScan video tracking system (Coulbourn Instruments, Holliston, Massachusetts).
Open field
The test apparatus consisted of a 45×45×45-cm transparent box with a camera at the top. Animals were placed in the center of the field and were allowed to freely explore the field area for 10 minutes. Open fields were subdivided into a peripheral and center zone, each making up 50% of the field area. The time spent in the center zone and total distance traveled were recorded by a video tracking system (ViewPoint, Lyon, France).
Immunohistochemistry and quantification of BrdU-labeled cells
To assess hippocampal cell proliferation, mice were given a single injection of the DNA synthesis marker bromodeoxyuridine (BrdU; 300 mg/kg; intraperitoneally) and euthanized 2 hours post injection. Mice were transcardially perfused with 4% paraformaldehyde, and their brains were postfixed overnight and then sectioned coronally at 20 μm. For BrdU labeling, brain sections were pretreated with 2N HCl at 37°C for 30 minutes before incubation with mouse anti-BrdU (1:200; Cell Signaling Technology, 5292). After incubation overnight, the sections were incubated with a horseradish peroxidase-conjugated secondary anti-mouse antibody and reacted with 0.01% diaminobenzidine (Sigma, D8001). To estimate the total number of BrdU-labeled cells in the entire dentate gyrus, every sixth section was subjected to immunohistochemistry analysis. The number of BrdU-labeled cells in granular and subgranular cell layers of the dentate gyrus was counted in each section, and the total number of BrdU-labeled cells per dentate gyrus was estimated after multiplying by 6. All analyses were performed in a masked fashion regarding the experimental groups.
Sperm isolation
Motile spermatozoa samples for reduced representation bisulfite sequencing (RRBS) and pyrosequencing were isolated using the swim-up method. In brief, the cauda epididymis was dissected from anesthetized mice and punctured in prewarmed (37°C) phosphate-buffered saline (PBS). The epididymis was then incubated at 37°C at a 45-degree angle for 30 minutes. The supernatant was harvested, centrifuged (3,000g; 10 minutes), and washed twice in PBS. Sperm preparations were assayed for purity by microscopy.
RRBS library construction and sequencing
Genomic DNA was extracted from sperm samples, and bisulfite conversion was performed using the EZ DNA Methylation-Gold Kit (Zymo Research; D5006). RRBS library construction and sequencing were performed at Genergy Bio (Shanghai, China) as described previously (20).
Pyrosequencing
Genomic DNA bisulfite conversion of unmethylated cytosines to uracil was performed using the EpiTect Fast DNA Bisulfite Kit (QIAGEN; 59824) according to the manufacturer’s instructions. Polymerase chain reactions (PCRs) were performed in a 25-μL final volume using EpiTaq Hot Start DNA polymerase (TaKaRa; R110Q). For pyrosequencing analysis, bisulfite-PCR products were processed according to the manufacturer’s (QIAGEN, Dusseldorf, Germany) standard protocol. In brief, 20 μL of PCR product was immobilized on 5 μL of Streptavidin Sepharose High Performance beads (GE Healthcare; GE17-5113-01) and then annealed to 1 μL of a 10 μM sequencing primer for 2 minutes at 80°C. Pyrosequencing was then performed on a PSQ 96MA instrument (QIAGEN) using appropriate enzymes, substrates, and nucleotides (PyroMark Gold Q96 Reagents, QIAGEN, 972804). Analyses of CpGs were performed using PSQ 96MA software. Information on the primer sequences and positions of validated CpG sites is shown in Supporting Information Table S1.
Quantitative real-time PCR
Total RNA was extracted from the hippocampus using TRIzol Reagent (Thermo Scientific, 15596026) and was reverse transcribed into complementary DNA using the PrimeScript RT (real time) Reagent Kit (TaKaRa, RR037A). Quantitative real-time PCR was performed on a 7900HT Fast Real-Time PCR System (Applied Biosystems) using SYBR Premix Ex Taq (TaKaRa, RR420L). Data were normalized to β-actin mRNA levels. Primer sequences are listed in Supporting Information Table S2.
Western blot analysis
Mice were euthanized, and their hippocampus tissues were carefully dissected for Western blotting. Briefly, samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride membranes for incubation with the following primary antibodies: BDNF (1:200, Merck, mabn79), tyrosine receptor kinase B (TrkB; 1:250, Santa Cruz, sc-377218), p-Akt (1:2000, Cell Signaling Technology, 4060), p-Erk (1:2000, Cell Signaling Technology, 4370), Akt (1:2000, Cell Signaling Technology, 4691), Erk (1:2000, Cell Signaling Technology, 4695), actin (1:2000, Santa Cruz, sc-58673), and GAPDH (1:5000, Abcam, ab9485). Following incubation with a horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse antibody (1:10,000, Abcam, ab6728), immunoblots were visualized using an enhanced chemiluminescent substrate (SuperSignal West Pico PLUS Chemiluminescent Substrate kit, Thermo Scientific, 34577). Quantification of band intensities was performed using ImageJ software, and both Erk and Akt activation (phosphorylation) was calculated relative to the total protein band intensity under the same conditions.
Statistics
Statistical analyses were performed using SPSS Statistics version 22.0 (IBM, Armonk, New York) and GraphPad Prism version 7 (La Jolla, California). Details of the statistical tests and sample sizes used are provided in the main text, figure legends, and within tables. Significance was set at P < 0.05. All data are presented as the mean ± SEM.
Results
HFD induces obesity and metabolic changes in F0 fathers
We first established an HFD mouse model. After 10 weeks of HFD exposure, F0 males displayed increased body weight, fat mass, total cholesterol, insulin, and leptin compared with CD-fed F0 males (Table 1). Next, we assessed hippocampus-dependent learning and memory in F0 males using the Morris water maze and contextual fear conditioning paradigm. There were no significant differences in these two tasks between CD-fed and HFD-fed F0 mice (for details, see Supporting Information Figure S1).
| Groups and parameters | CD | HFD | P value |
|---|---|---|---|
| Fathers | n = 10 | n = 10 | |
| Body weight, g | 25.1 ± 0.3 | 34.3 ± 0.9 | <0.001 |
| Mesenteric WAT, g | 0.08 ± 0.01 | 0.34 ± 0.05 | <0.001 |
| Retroperitoneal WAT, g | 0.08 ± 0.01 | 0.61 ± 0.09 | <0.001 |
| Gonadal WAT, g | 0.35 ± 0.04 | 1.57 ± 0.14 | <0.001 |
| Total cholesterol, mmol/L | 2.42 ± 0.14 | 4.41 ± 0.14 | <0.001 |
| Triglyceride, mmol/L | 1.31 ± 0.13 | 1.53 ± 0.09 | 0.20 |
| Glucose, mmol/L | 3.89 ± 0.17 | 4.18 ± 0.43 | 0.57 |
| Insulin, ng/mL | 0.62 ± 0.06 | 1.52 ± 0.18 | 0.001 |
| Leptin, ng/mL | 1.84 ± 0.22 | 2.51 ± 0.27 | 0.03 |
| F1 offspring | n = 15 | n = 15 | |
| Body weight, g | 24.2 ± 0.3 | 24.1 ± 0.5 | 0.84 |
| Mesenteric WAT, g | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.86 |
| Retroperitoneal WAT, g | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.59 |
| Gonadal WAT, g | 0.38 ± 0.04 | 0.39 ± 0.03 | 0.91 |
| Total cholesterol, mmol/L | 2.16 ± 0.11 | 2.30 ± 0.07 | 0.33 |
| Triglyceride, mmol/L | 1.10 ± 0.11 | 0.97 ± 0.09 | 0.40 |
| Glucose, mmol/L | 4.12 ± 0.29 | 3.96 ± 0.29 | 0.72 |
| Insulin, ng/mL | 0.55 ± 0.05 | 0.54 ± 0.04 | 0.85 |
| Leptin, ng/mL | 3.17 ± 0.23 | 2.73 ± 0.27 | 0.26 |
- All results are expressed as mean ± SEM.
- CD, control diet; HFD, high-fat diet; WAT, white adipose tissue.
Paternal HFD leads to cognitive impairments in F1 offspring
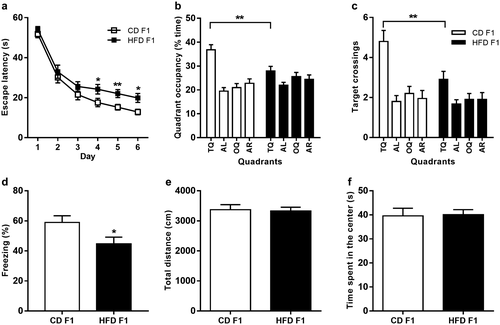
Paternal HFD did not alter the body weight, adiposity, or serum metabolic parameters in F1 male offspring, as assessed at 8 weeks of age (Table 1). Next, we tested F1 offspring using the Morris water maze to examine hippocampus-dependent spatial learning and memory. During 6 days of acquisition, data analysis showed a significant effect of paternal diet on escape latencies (Figure 1a; two-way analysis of variance [ANOVA], P = 0.0002). A further individual t test revealed that HFD-fed F1 mice performed significantly worse than CD-fed F1 mice on days 4, 5, and 6 (Figure 1a; P = 0.0421, 0.0088, and 0.0120 for days 4, 5, and 6, respectively). After completion of training, we administered a single probe trial to assess how accurately the animals had learned the spatial position of the escape platform. During probe trials, HFD-fed F1 mice displayed altered search patterns, and there was a significant interaction between paternal diet and quadrant regarding quadrant occupancy (Figure 1b; two-way ANOVA, P = 0.0007) and target crossings (Figure 1c; two-way ANOVA, P = 0.0391). Further analyses revealed that HFD-fed F1 offspring exhibited a decreased target quadrant occupancy (Figure 1b; unpaired t test, P = 0.0035) and target crossings (Figure 1c; unpaired t test, P = 0.0087) compared with CD-fed F1 offspring. There were no significant differences in swimming speed or distance traveled during probe tests between CD-fed and HFD-fed F1 offspring (Supporting Information Figure S2a-S2b). To exclude visual differences between groups, we performed a hippocampus-independent visible version of the Morris water maze. There were no significant differences in escape latency during the four visible platform trials between CD-fed and HFD-fed F1 mice (Supporting Information Figure S2c). Next, we evaluated hippocampus-dependent learning and memory in F1 mice using an independent contextual fear conditioning task. On the training day, mice were allowed to explore the fear conditioning chamber for 2 minutes before the onset of the shock, and freezing levels were recorded as the baseline activity during this 2-minute period. No significant difference in baseline activity was observed on the training day (Supporting Information Figure S2d). During the contextual memory test, mice were placed back into the same fear conditioning chamber, and time spent freezing during a 3-minute test was measured. Notably, HFD-fed F1 mice exhibited reduced freezing levels compared with CD-fed F1 mice (Figure 1d; t test, P = 0.031), demonstrating impaired contextual fear conditioning in HFD-fed F1 offspring. In addition, we subjected F1 mice to an open field test. There were no significant differences in the total distance (Figure 1e; t test, P = 0.8371) or time spent in the center of the open field (Figure 1f; t test, P = 0.9075) between CD-fed and HFD-fed F1 mice, indicating no differences in processes related to movement or anxiety regulation. Taken together, these data demonstrate that paternal HFD impairs hippocampus-dependent learning and memory in F1 offspring.
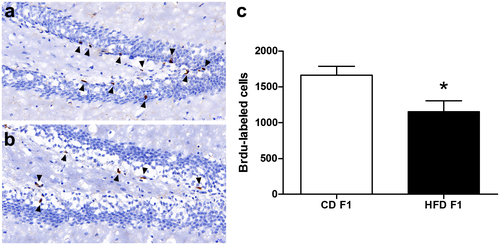
Paternal HFD impairs adult hippocampal neurogenesis in F1 offspring
Next, we investigated adult neurogenesis in the dentate gyrus of the hippocampus, a cellular process involved in hippocampus-dependent learning and memory (21). To assess hippocampal cell proliferation, we administered a single injection of 300 mg/kg BrdU to CD-fed and HFD-fed F1 offspring mice and euthanized them 2 hours later. Next, we counted the total number of BrdU-labeled cells in the dentate gyrus. There was a significant reduction in the number of BrdU-labeled cells in the dentate gyrus of HFD-fed F1 offspring compared with controls (Figure 2; t test, P = 0.0291), indicating impaired adult hippocampal neurogenesis in HFD-fed F1 offspring.
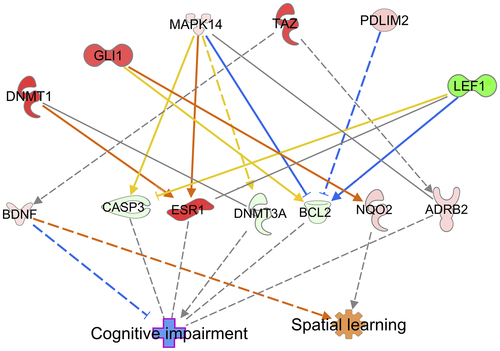
Paternal HFD reprograms DNA methylation patterns in F0 spermatozoa
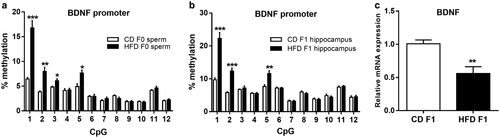
We subsequently explored the possible molecular mechanisms by which paternal HFD exerts these intergenerational effects on cognitive function. We collected mature, motile spermatozoa from CD-fed and HFD-fed fathers and investigated DNA methylation patterns using RRBS (three arrays per group; for each array, we used pools of six mice). Strikingly, we found several differentially methylated genes in promoter regions (Supporting Information Table S3). To identify potential genes associated with the cognitive phenotype in the offspring, we further performed Ingenuity Pathway Analysis (QIAGEN) using the terms “spatial learning” and “cognitive impairment.” Notably, we found that BDNF exhibits a strong relationship with both spatial learning and cognitive impairment (Figure 3). Using pyrosequencing analysis, we confirmed elevated CpG methylation in the BDNF promoter region in HFD-fed F0 spermatozoa (Figure 4a). Moreover, we assessed BDNF promoter methylation in the F1 hippocampus. Similarly, HFD-fed F1 hippocampal samples exhibited an elevated CpG methylation status in the BDNF promoter region (Figure 4b). Furthermore, we examined BDNF mRNA expression in the F1 hippocampus by quantitative PCR. The HFD-fed F1 hippocampus showed reduced BDNF mRNA expression (Figure 4c). Collectively, these data indicate that an altered BDNF methylation status in HFD-fed F0 spermatozoa can transmit to offspring hippocampus, thereby affecting BDNF expression in the HFD-fed F1 hippocampus.
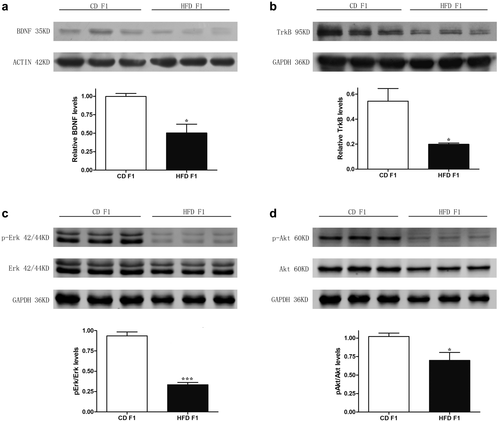
BDNF/TrkB signaling is associated with cognitive impairments in HFD-fed F1 offspring
It is well established that BDNF binds to its receptor TrkB and activates two major effector pathways, the RAS/ERK pathway and the PI3K/AKT pathway, which mediate cell proliferation and survival effects, respectively (22, 23). Therefore, we examined the protein levels of BDNF/TrkB signaling in the F1 hippocampus. Our results revealed decreased BDNF, TrkB, pErk/Erk, and pAkt/Akt protein levels in HFD-fed F1 mice (Figure 5a-5d), indicating that BDNF/TrkB signaling is associated with cognitive impairments in HFD-fed F1 offspring.
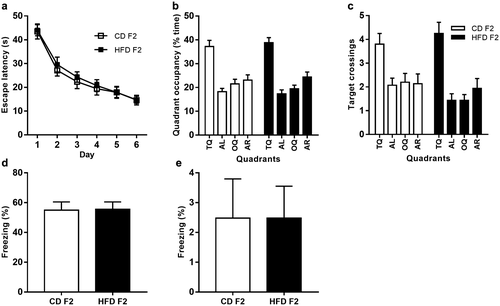
No evidence of transgenerational effects of paternal HFD on cognitive function in the F2 generation
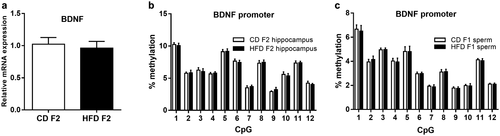
Next, we examined whether paternal HFD could exert transgenerational effects on cognitive functions in F2 offspring. However, behavioral assessment revealed no group differences in the Morris water maze and contextual fear conditioning tasks (Figure 6). Moreover, there were also no differences in BDNF mRNA expression or BDNF promoter methylation in the F2 hippocampus (Figure 7a-7b). Furthermore, we examined BDNF promoter methylation in F1 spermatozoa, and there were also no significant differences between these two groups (Figure 7c).
Discussion
Evidence of paternal obesity affecting the development and health of offspring is growing. Previous studies have reported that diet-induced paternal obesity leads to many types of obesity-related disorders in offspring, such as impaired glucose tolerance and insulin sensitivity (5), altered hepatic lipogenesis (24), and kidney damage (25). However, the effects of paternal obesity on cognitive function in the next generation remain poorly understood. In this study, we describe the first demonstration of paternal obesity influencing the cognitive function of offspring. Moreover, we provide a potential molecular mechanism of epigenetic inheritance via the male germ line. The mechanisms explaining how the father affects the development and health of the offspring are still unclear. The spermatozoa “epigenome’’ was traditionally considered insignificant because its DNA methylation profile was presumably erased immediately after fertilization. However, studies have increasingly demonstrated that epigenetic information carried by spermatozoa can indeed be transmitted between generations (11). In the present study, we show that paternal HFD reprograms the DNA methylation patterns in F0 spermatozoa. Specifically, we identify a key differentially methylated gene, BDNF, associated with the cognitive phenotype of the offspring. BDNF is a member of the neurotrophin family and plays a critical role in hippocampal neurogenesis and cognitive function (21). Knockdown of BDNF in the adult dentate gyrus of the hippocampus has been shown to result in decreased hippocampal neurogenesis (26), whereas administration of BDNF into the hippocampus of adult mice increased neurogenesis in vivo (27). Neurogenesis, the process by which new neurons are produced, is most abundant during embryonic development but continues throughout adult life. In mammals, adult neurogenesis has been shown to occur at two primary locations in the brain: the subventricular zone of the lateral ventricles and the subgranular zone of the dentate gyrus in the hippocampus. Newborn neurons undergo typical morphological differentiation and maturation and then functionally integrate into the existing hippocampal circuitry (21). Previous studies have demonstrated correlations between hippocampal neurogenesis and cognitive function (28-30). Furthermore, studies have revealed that BDNF mediates cell proliferation and survival via its receptor TrkB by activating two main intracellular pathways: the RAS/ERK pathway and the PI3K/AKT pathway (22, 23). Consistently, we demonstrate that BDNF/TrkB signaling is associated with cognitive impairments in HFD-fed F1 offspring. Mechanistically, downregulation of BDNF expression is partially attributed to increased BDNF promoter methylation in the F1 hippocampus, which may be inherited from the spermatozoa of fathers.
Several epidemiologic studies have reported that obesity can be paternally transmitted (31, 32). However, in human studies, it is often difficult to rule out other potential confounding factors, such as the influence of postnatal nutritional environment and a shared unhealthy lifestyle between parents and offspring. Animal models of diet-induced paternal obesity have been developed to address these limitations. However, there are differential effects of paternal obesity on offspring phenotype across different mouse models. For example, Fullston et al. showed that paternal exposure to a 40% fat diet increased body weight and impaired glucose tolerance in male offspring from 8 weeks of age onward (33). However, Masuyama et al. reported no difference in body weight from 4 to 16 weeks of age, followed by increased body weight and homeostatic model assessment of insulin resistance at 24 weeks of age in male offspring from fathers fed a 62% fat diet (34). Moreover, using in vitro fertilization, Huypens et al. found that sperm isolated from F0 mice fed a 60% fat diet did not induce changes in body weight or glucose tolerance in male offspring at 14 weeks of age (35). In our study, using a mouse model of paternal obesity induced by a 60% fat diet, we also found no differences in body weight or blood metabolic parameters in male offspring at 8 weeks of age. However, metabolic disorders may subsequently emerge or not, which remains to be further determined. The discrepancies observed in these studies may result from differences in the dietary compositions and ages of the offspring.
In the present study, we also examined cognitive functions in F2 offspring; however, no significant changes were observed in the F2 generation. Moreover, there were also no group differences in BDNF mRNA expression or BDNF promoter methylation in the F2 hippocampus. Previous animal studies have reported transgenerational effects of paternal diet in the F2 generation. For example, Fullston et al. reported that F0 HFD-fed grandfathers induced obesity and insulin resistance in F2 females but not in males through the male line, indicating that transgenerational effects may be transmitted in a sex-specific way (33). Wei et al. showed that paternal prediabetes led to glucose intolerance and insulin resistance in both F1 and F2 male offspring (36). However, the authors also pointed out that the observed transgenerational effects were most likely due to the prediabetes-associated physiological and metabolic conditions in the F1 generation, which were derived from the prediabetic F0 generation (36). Therefore, in these studies, F1 spermatozoa were still exposed to an adverse metabolic environment at the time of mating. However, a true paternal transgenerational effect can be verified only if the phenotypic consequences are still present in the offspring when the male germ line is not directly exposed. In our mouse model, HFD-fed F1 male offspring exhibited cognitive deficits, with no changes in body weight or blood metabolic parameters at 8 weeks of age, at which point they were mated with normal females to generate F2 offspring. During the development of F1 spermatozoa, DNA methylation patterns undergo complete reprogramming. Therefore, we further examined BDNF promoter methylation in F1 spermatozoa and found no significant differences between the two groups. Taken together, these results suggest that the F0 fathers exerted no transgenerational effects on the F2 offspring in our mouse model.
It should be noted that our results showed that 10 weeks of HFD exposure was insufficient to impair hippocampus-dependent learning and memory in F0 fathers. Previous animal studies have reported that diet-induced obesity exerts negative effects on cognitive function (14, 15). However, in these studies, animals were maintained on an HFD for a longer period and displayed more severe metabolic changes, as measured by elevated fasting blood glucose levels (14). Otherwise, they were subjected to behavioral testing in their old age (15). In contrast, male mice in our model were fed an HFD for only 10 weeks, and they exhibited an increased body weight while maintaining normal fasting glucose levels. Furthermore, they were subjected to behavioral testing at 14 weeks of age, which is a comparatively young age in mice. It is well known that both metabolic syndrome and aging are important risk factors for cognitive impairment (37, 38). Therefore, depending on age and the severity of the metabolic disease, diet-induced obesity could have differential consequences on cognitive performance.
Conclusion
In summary, we demonstrate that paternal obesity induced by a 60% fat diet exerts intergenerational effects on cognition in F1 offspring, potentially via epigenetic modifications in spermatozoa. How the diet induces epigenetic changes in spermatozoa, how these changes escape reprogramming after fertilization, and how the change penetrates in a tissue-specific manner remain to be elucidated.
Funding agencies
This work was supported by the Major Program of National Natural Science Foundation of China (NSFC) (#81490742); the National Key Research and Development Program of China (#2017YFC1001303); the National Natural Science Foundation of China (#31471405 and 81671456); the International Cooperation Project of China and Canada NSFC (#81661128010); the Interdisciplinary Key Program of Shanghai Jiao Tong University (#YG2014ZD08); and the Shen Kang Three Year Action plan (#16CR3003A).
Disclosure
The authors declared no conflict of interest.





