Health Outcomes and Explant Rates After Laparoscopic Adjustable Gastric Banding: A Phase 4, Multicenter Study over 5 Years
Funding agencies: Allergan Medical (Goleta, California) designed and funded the study before it was transferred to Apollo Endosurgery (Austin, Texas). The funding organizations played no role in the design, analysis, or interpretation of data described here nor in preparation, review, or approval of the manuscript.
Disclosure: John Dixon has provided consultancy services to Allergan Medical, Apollo Endosurgery, Bariatric Advantage, Covidien, Nestle Health Science, iNova Pharmaceuticals, and Novo Nordisk. Laura Eaton has provided consultancy services to Allergan Medical and Apollo Endosurgery. Trace Curry has provided consultancy services to Apollo Endosurgery and ReShape Medical. Phong Ching Lee declared no conflict of interest.
Clinical trial registration: ClinicalTrials.gov identifier NCT00953173.
Abstract
Objective
This study aimed to evaluate the real-world safety and effectiveness of the LAP-BAND (Apollo Endosurgery Inc., Austin, Texas) adjustable gastric banding system (LBS) for 5 years following implantation.
Methods
This prospective, longitudinal, phase 4, multicenter study involved 652 patients who had implantation of the LBS system. The primary outcome was the percentage of subjects who had LBS explant over 5 years. The secondary outcomes included the rate of reoperations, clinical and biochemical measures, and patient-reported outcome measures over 5 years.
Results
The study cohort consisted of 79.3% females with a mean age of 44 years and a mean BMI of 45.4 kg/m2. The primary end point was met with an explant rate of 8.74% (95% CI: 6.6%-10.9%) at 5 years. The rates for completer-only analysis and imputed missing data analysis were 12.81% (95% CI: 9.7%-15.9%) and 12.85% (95% CI: 10.2%-15.5%), respectively. All were significantly lower than the historic rate of 39.4% (P < 0.001). There were 43 patients who required reoperations or revisions excluding explants (6.6%). A mean weight loss of 18.7% was maximally achieved by 2 years, and weight loss was maintained through to 5 years. All patient-reported outcomes showed improvement following LBS treatment throughout 5 years.
Conclusions
This study validates the long-term safety and effectiveness of LBS for the treatment of patients with obesity and its related conditions.
Introduction
The ongoing global obesity epidemic is having catastrophic effects on human health, function, and productivity (1, 2). The determinants of obesity are complex and multifactorial, and they involve the interaction between genes and the environment, especially in the first 1,000 days following conception (3, 4). It is now clear that body weight and adiposity are tightly regulated. Behavioral and lifestyle interventions improve health in many ways but have limited efficacy in generating substantial and sustained weight loss in the majority of individuals. A broad range of effective tools are needed to manage weight just as for other chronic diseases, with the ability to combine effective strategies, scale up treatment, and adapt a treatment regimen to the individual.
The past two decades have seen major changes in the safety, morbidity, and mortality associated with bariatric-metabolic surgery (5, 6). There is a strong evidence base for efficacy, safety, and health outcomes following surgery, including mortality advantage, improvement or remission of obesity-related comorbidities, improved function and quality of life, and a favorable health economic profile (7). Laparoscopic adjustable gastric banding (LAGB) led the era of laparoscopic bariatric surgery in 1993 (8) and was soon followed by laparoscopic Roux-en-Y gastric bypass (RYGB) in 1994 (9). The concept of reversibility often has limited appeal to surgical traditional paradigms of bariatric surgical care, but this attribute of LAGB may be attractive for younger groups with lower body mass index (BMI) in whom the impact of advances in the nonsurgical therapies over the coming decades may most readily translate (10, 11). On the other hand, reversibility could also lead to the inappropriate or unnecessary removal of LAGB in patients who may be deriving benefit from it if managed correctly. LAGB is designed for active management with adjustability, and this requires time and ongoing skilled management, as is needed for many other chronic diseases.
In 2001, the LAP-BAND adjustable gastric banding system (LBS) (Inamed Corp., Irvine, California) was approved by the US Food and Drug Administration (FDA) for patients with a BMI of 40 kg/m2 or greater or at least 35 kg/m2 with a severe comorbidity (12). On February 16, 2011, the FDA granted approval for an extension of the indication to lower the BMI cutoff to 30 kg/m2 in individuals with an obesity-associated comorbid condition. The full 5-year follow-up of this lower-BMI study cohort showed durable weight loss in association with improvement in patient-reported outcomes, comorbidity change, and a much lower band explant rate of 12.1% (13) compared with the historic 39.4%.
Simultaneously, a larger health outcome study, Helping Evaluate Reduction in Obesity (HERO), was also under way, with a focus on health economics as well as efficacy and safety. However, given the extreme variation in reported rates of band explanation and reoperations following LBS surgery (14), the focus was changed to examine band explant and reoperation rates. We now report the full 5-year data of the HERO study.
Methods
Study objectives and design
The objective of this sponsor-initiated, phase 4, multicenter, prospective, longitudinal, nonrandomized study was to evaluate the real-world safety and effectiveness of the LBS implanted in patients with a BMI ≥ 40 kg/m2 or a BMI ≥ 35 kg/m2 with one or more comorbid conditions for 5 years following implantation.
While the HERO-001 study was initially designed to examine the effectiveness and safety of implantation with LBS, a sponsor- and FDA-agreed amendment, HERO-002, defined specific outcomes of interest. The primary objective of HERO-002 was to demonstrate in a real-world, postapproval setting that the LBS explant rate over the first 5 years was significantly less than a historic rate criterion of 39.4%. This rate criterion of 39.4% was mutually agreed upon by the sponsor and the FDA and was derived from the explant rates observed in the earlier FDA A – Trial after adjustment for those lost to follow-up. The details of this US pivotal study are available in the LBS Directions for Use manual (15). A secondary aim of HERO-002 was to describe the rate of reoperations over the first 5 years of implantation. Other outcomes of interest in HERO-002 included the following secondary effectiveness measures:
Clinical measures: weight, BMI, anthropometric measures (waist and hip), systolic and diastolic blood pressure, and heart rate.
Biochemical measures: fasting glucose, glycated hemoglobin (HbA1c), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
Patient-reported outcomes: Short Form-12 (SF-12) health survey (16), a generic health-related quality of life instrument; Impact of Weight on Quality of Life-Lite (IWQOL-Lite), an obesity-specific health-related quality of life instrument (17); Euroqol Five Dimensions Questionnaire (EQ-5D), a generic evaluation of health status useful in cost-effectiveness analysis (18); and the Work Productivity and Activity Impairment instrument (WPAI) (19).
The clinical trial sites for the 5-year study were 17 US and Canadian private practices experienced in bariatric surgery, and 671 eligible subject were enrolled. The study was approved by the institutional review boards for each participating practice, and all subjects provided written informed consent. The clinical trial was registered on the National Institutes of Health website (www.clinicaltrials.gov number NCT00953173). The study was initially designed, funded, and conducted by Allergan Medical (Santa Barbara, California). The study was initiated on June 4, 2009. The last subject was enrolled on January 26, 2011, with surgical implantation completed on March 10, 2011. On December 2, 2013, the LBS system, ownership of the postmarketing approval, and responsibility for all ongoing FDA-regulated clinical trials, including HERO-002, were transferred from Allergan Medical to Apollo Endosurgery (Austin, Texas). The last study visit was completed on April 6, 2016, and the database was locked on June 10, 2016. The detailed report on the analysis and results of this study that was submitted to the FDA was used in the preparation of this manuscript.
Inclusion and exclusion criteria
Eligible subjects were adults who, independently of the HERO study, decided to undergo primary implantation of the LBS at one of the participating practices and were accepted into the practice program. In addition, participation required a BMI ≥ 40 kg/m2 or a BMI ≥ 35 kg/m2 with one or more comorbid conditions.
Treatment administration
All practices included in the study have established LBS programs and were experienced in placement, band adjustment, and long-term aftercare. Placement of the LBS and aftercare were performed as per usual practice for the surgeons and their aftercare programs. However, placing the LBS is quite standardized by using the pars flaccida approach to create a small or virtual gastric pouch above the band and gastro-gastric band fixation (20). Band adjustments (via saline injected into or removed from the access port) were performed at the investigator's discretion based on the subject's dietary, appetite, and symptom report, clinical progress, and physical examination. Adjustments were made to target the optimal zone of band volume in which the subject was comfortable, satisfied with small meals, and losing weight (21).
Study data were collected at baseline, at the implantation surgery, and during regular scheduled postimplantation visits at months 3 and 6 and years 1, 2, 3, 4, and 5 following band placement.
Statistical analysis
All analyses were conducted by using the safety analysis population, which consisted of all subjects enrolled in the HERO study at investigative sites within the United States and Canada who were implanted with the LBS, and by using SAS version 9.4 or higher (SAS Institute, Inc., Cary, North Carolina). Statistical tests were performed using a one-sided significance level of 5%, and confidence intervals (CI) were reported by using a 95% level of confidence.
The primary end point was the percentage of subjects who were known to experience removal of the LBS over 5 years. The historic explant rate of 39.4% at 5 years was the comparator, and a superiority hypothesis was tested. Three sensitivity analyses were also performed. The first was a completer-only analysis; the second was performed by using multiple imputation to impute the status of explant in those lost to follow-up by using patient potential risk characteristics of age, sex, comorbidities, and baseline weight; and the third was a tipping point analysis to assess the effect of the missing data on the result of the primary analysis. The pattern of missing data was assumed to be missing at random.
Effect sizes when quoted were based on Cohen's d (mean difference/pooled standard deviation [SD]), indicated by small (0.2 to 0.49), moderate (0.5 to 0.79), or large (≥ 0.8) effect sizes.
Results
Baseline demographics and characteristics of the 652 participants implanted are shown in Table 1. Of the 652 participants, 636 (97.5% of the 652 expected subjects) completed their 3-month visit, 614 (94.5% of the 650 expected subjects) completed their 6-month visit, 592 (91.9% of the 644 expected subjects) completed their 1-year visit, 470 (74% of the 635 expected subjects) completed their 2-year visit, 413 (68.5% of the 603 expected subjects) completed their 3-year visit, 358 (61.5% of the 582 expected subjects) completed their 4-year visit, and 389 (73.1% of the 532 expected subjects) completed their 5-year visit.
| n | % | |
|---|---|---|
| Gender | ||
| Female | 517 | 79.3% |
| Male | 135 | 20.7% |
| Age, mean (SD) | 44.4 (11.3) y | |
| 18-29 | 72 | 12.1% |
| 30-39 | 164 | 25.2% |
| 40-49 | 166 | 30.5% |
| 50-59 | 22 | 25.5% |
| ≥ 60 | 51 | 7.8% |
| BMI, mean (SD) | 45.4 (6.9) kg/m2 | |
| Weight, mean (SD) | 127.3 (24.7) kg | |
| Waist circumference, mean (SD) | 127.2 (14.2) cm | |
| Hip circumference, mean (SD) | 140.2 (15.6) cm | |
| Type 2 diabetes | 168 | 25.8% |
| Hypertension | 315 | 48.3% |
| Dyslipidemia (n = 363)a | 129 | 35.5% |
| Chronic joint pain (n = 366)a | 179 | 48.9% |
| Chronic back pain (n = 366)a | 117 | 32.0% |
| Depression (n = 361)a | 110 | 30.5% |
| Gastroesophageal reflux (n = 366)a | 164 | 44.8% |
- a Questions added after enrollment regarding baseline comorbidity.
Surgery
The LBS system was implanted by using a laparoscopic procedure, and in 99.8% (all but one placement), the pars flaccida approach was used. The standard size band was used for 73.2% (n = 477) and the large band for the remainder. Access ports were fixed to the rectus sheath by using the following methods: suturing (74%), mesh (25%), and dedicated fixation device (RapidPort; Apollo Endosurgery Inc., Austin, Texas) (1%).
Primary safety outcome: LBS explants
The primary safety end point and sensitivity analyses are shown in Table 2. The primary end point of the study was met with the explant rate of 8.74% (95% CI: 6.6%-10.9%) at 5 years. The rates for the completer analysis and imputed missing data analysis were 12.81% (95% CI: 9.7%-15.9%) and 12.85% (95% CI: 10.2%-15.5%), respectively. All were significantly lower than the historic rate of 39.4% (P < 0.001). The tipping point analysis is also conclusive, and it would require a total explant rate of 237 of 652 (36.4%) before superiority is lost. This would mean that 180 of the 207 patients lost to follow-up (87%) had to have their LBS explanted before superiority was lost, a likelihood that would be extremely low.
| n/N | Rate | P valuea | 95% CI | |
|---|---|---|---|---|
| Primary end point | 57/652 | 0.0874 | < 0.0001 | 6.60%-10.90% |
| Completers | 57/445 | 0.1281 | < 0.0001 | 9.70%-15.90% |
| Imputedb | N/A | 0.1285 | < 0.0001 | 10.20%-15.50% |
| Tipping point | 237/652 | 0.3635 | 0.0527 | 32.70%-40.00% |
- a P value based on exact binomial one-sided tests for hypothesis that explant rates would be < 39.4%.
- b Imputed values dependent on examination of potential risk factors, such as age, gender, race, diabetes/hypertension/hyperlipidemia status, and baseline weight.
The reasons for explants are shown in Table 3. Band explants were performed for surgical adverse events in 41 out of 57 patients (71.9%), whereas 16 out of 57 (28.1%) were for dissatisfaction with weight loss and/or conversion to another bariatric procedure. Proximal gastric pouch dilatation, gastric prolapse, band erosion, stoma obstruction, and gastrointestinal symptom-related events (e.g., abdominal pain, dysphagia, gastritis, gastroesophageal reflux disease, vomiting) were the main reasons for explants because of adverse events.
| Total explants | N = 57 | |
|---|---|---|
| 1. Adverse events | 41 (71.9%) | |
| A. Band-related adverse events | 17 | |
| Gastric prolapse/band slippage | 8 | |
| Pouch dilatation | 2 | |
| Stoma obstruction | 1 | |
| Band erosion into the stomach | 6 | |
| B. Gastrointestinal symptom-related events (e.g., abdominal pain, dysphagia, gastritis, gastroesophageal reflux disease, hernia [unspecified], vomiting) | 17 | |
| C. Unknown adverse events (adverse event listed as reason for removal but reason not reported) | 7 | |
| 2. Conversion to another bariatric procedure | 11 (19.3%) | |
| 3. Dissatisfaction with weight loss | 5 (8.8%) |
Secondary safety outcome: reoperations
During the 5-year follow-up period, there were 62 reoperations or surgical revisions performed on 50 subjects (7.7%) (Table 4). Band-related procedures accounted for 38 (58.5%) of the procedures, while adjustment port-related procedures accounted for 24 of the procedures (36%). The reoperations of the LBS excluded procedures to primarily explant bands, with the exception of the seven bands that were removed and replaced with a new LBS simultaneously. However, these reoperations may have preceded a subsequent LBS explant. The total number of reoperations inclusive of explants was 100 (15.3%) over the 5-year period.
| Subjects | Events | |
|---|---|---|
| 652 | 62 | |
| LAP-BAND surgical reoperationa | 50 (7.7%) | 65 |
| Revision to reposition LBS | 20 (3.1%) | 21 (32.3%) |
| Revision with replacement of LBSb | 7 (1.1%) | 7 (10.8%) |
| Band-related reoperation (unspecified) | 5 (0.8%) | 5 (7.7%) |
| Unbuckle LAP-BAND | 4 (0.6%) | 4 (6.2%) |
| Rebuckle LAP-BAND | 1 (0.2%) | 1 (1.5%) |
| Access port revision with preservation | 13 (2.0%) | 13 (20.0%) |
| Access port revision with replacement | 6 (0.9%) | 6 (9.2%) |
| Port removal | 5 (0.8%) | 5 (7.7%) |
| Hernia repair | 2 (0.3%) | 2 (1.5%) |
| Aspiration pneumonia | 1 (0.2%) | 1 (1.5%) |
- Total of 62 reoperations, involving 65 procedures, performed in 50 individual subjects.
- a Multiple selections allowed for this question.
- b The seven LBS removed at surgical revision and replaced simultaneously were included in explant numbers and Table 3. None of the other procedures in this list involved removal of LBS.
Effectiveness
Weight loss
Changes in weight and other markers of cardiometabolic risk after LBS implantation are shown in Table 5. The mean baseline weight was 127.3 kg (± 24.7 kg) with a mean BMI of 45.4 ± 6.9 kg/m2. At the 1-year follow-up visit, a weight loss of 21.7 kg (total body weight loss [TBWL] 17.1%) was observed. The maximal amount of weight loss was achieved by 2 years (TBWL 18.7%), and weight loss was maintained through to the 5-year follow-up visit. The effect sizes of all anthropometric changes were large at all annual follow-up visits.
| Baseline | 1 year | 2 years | 3 years | 4 years | 5 years | |
|---|---|---|---|---|---|---|
| N of subjects | 652 | 592 | 464 | 404 | 339 | 383 |
| % TBWLa | — | 17.1 ± 8.6 | 18.7 ± 12.9 | 17.9 ± 16.9 | 18.6 ± 12.9 | 18.0 ± 12.7 |
| Weight (kg)a | 127.3 ± 24.7 | 105.6 ± 23.2 | 103.1 ± 24.8 | 103.9 ± 27.6 | 103.2 ± 24.5 | 104.6 ± 24.8 |
| BMI (kg/m2)a | 45.4 ± 6.9 | 37.6 ± 6.7 | 36.9 ± 7.7 | 37.1 ± 8.8 | 36.8 ± 7.5 | 37.2 ± 7.5 |
| Waist circumference (cm)a | 127.3 ± 16.7 | 110.7 ± 16.3 | 108.5 ± 16.1 | 109.0 ± 17.7 | 110.7 ± 17.3 | 111.3 ± 17.1 |
| Hip circumference (cm)a | 140.2 ± 15.2 | 125.0 ± 15.2 | 123.2 ± 15.4 | 122.9 ± 16.0 | 125.0 ± 16.4 | 124.7 ± 16.0 |
| Blood pressure | ||||||
| Systolic (mmHg)b | 133.3 ± 16.81 | 126.7 ± 15.2 | 126.6 ± 15.6 | 127.3 ± 14.9 | 126.5 ± 15.4 | 128.7 ± 16.9 |
| Diastolic (mmHg)b | 82.0 ± 10.2 | 78.4 ± 11.3 | 79.6 ± 11.2 | 80.4 ± 9.5 | 79.0 ± 10.2 | 79.8 ± 10.1 |
| Heart rate (beats/min)b | 79.6 ± 12.0 | 74.8 ± 10.7 | 74.6 ± 11.6 | 75.4 ± 11.1 | 75.5 ± 10.7 | 75.1 ± 11.5 |
| Glycemic parameters | ||||||
| HbA1c (%)b | 6.1 ± 1.1 | 5.8 ± 0.9 | 5.9 ± 3.0 | 5.7 ± 0.8 | 5.8 ± 1.0 | 5.8 ± 1.0 |
| Glucose (mmol/L)c | 5.9 ± 2.0 | 5.5 ± 2.8 | 5.4 ± 1.5 | 5.3 ± 1.4 | 5.4 ± 1.5 | 5.7 ± 2.1 |
| Lipid panel | ||||||
| Total cholesterol (mmol/L)c | 4.70 ± 1.03 | 4.77 ± 0.95 | 4.78 ± 0.94 | 4.77 ± 1.00 | 4.78 ± 1.03 | 4.88 ± 1.02 |
| HDL (mmol/L)a | 1.13 ± 0.31 | 1.33 ± 0.34 | 1.43 ± 0.44 | 1.42 ± 0.44 | 1.46 ± 0.53 | 1.45 ± 0.44 |
| LDL (mmol/L)c | 2.88 ± 0.89 | 2.87 ± 0.90 | 2.82 ± 0.84 | 2.81 ± 0.90 | 2.80 ± 0.90 | 2.85 ± 0.90 |
| Triglycerides (mmol/L)b | 1.54 ± 0.83 | 1.30 ± 0.72 | 1.23 ± 0.68 | 1.21 ± 0.61 | 1.21 ± 0.75 | 1.31 ± 0.91 |
| Generic QOL Short Form-12 | ||||||
| SF-12 PCSa | 37.5 ± 10.6 | 50.1 ± 8.6 | 49.8 ± 8.9 | 49.4 ± 9.9 | 48.9 ± 9.6 | 48.8 ± 9.7 |
| SF-12 MCSd | 44.7 ± 11.2 | 50.3 ± 9.9 | 49.6 ± 10.2 | 49.2 ± 10.3 | 49.2 ± 10.5 | 50.3 ± 10.0 |
| Obesity-specific IWQOL-Lite | ||||||
| IWQOL-Lite physical functiona | 41.0 ± 22.8 | 80.5 ± 19.0 | 81.1 ± 20.0 | 80.1 ± 19.4 | 78.5 ± 20. | 78.6 ± 21.2 |
| IWQOL-Lite self-esteema | 35.0 ± 25.1 | 69.9 ± 24.1 | 69.7 ± 25.8 | 68.2 ± 24.7 | 68.2 ± 25.2 | 69.6 ± 25.8 |
| IWQOL-Lite sexual lifea | 51.1 ± 30.4 | 78.4 ± 28.3 | 78.8 ± 26.4 | 79.9 ± 23.5 | 78.3 ± 25.7 | 80.5 ± 25.0 |
| IWQOL-Lite public distressa | 52.6 ± 25.4 | 82.0 ± 21.0 | 82.9 ± 21.8 | 83.8 ± 20.0 | 83.2 ± 20.2 | 84.0 ± 20.9 |
| IWQOL-Lite work scalesa | 63.0 ± 25.4 | 86.2 ± 18.2 | 88.0 ± 17.4 | 88.1 ± 17.4 | 86.8 ± 17.8 | 87.6 ± 17.9 |
| IWQOL-Lite (total score)a | 45.6 ± 19.2 | 78.8 ± 17.7 | 79.4 ± 19.0 | 79.4 ± 19.0 | 78.1 ± 18.4 | 78.8 ± 19.6 |
| EQ-5D | ||||||
| EQ-5D index scored | 0.7 ± 0.18 | 0.9 ± 0.15 | 0.9 ± 0.16 | 0.8 ± 0.17 | 0.8 ± 0.17 | 0.8 ± 0.18 |
| EQ-5D VAS scorea | 54.2 ± 18.4 | 75.9 ± 15.5 | 76.6 ± 16.3 | 76.2 ± 17.4 | 76.2 ± 17.2 | 77.3 ± 17.1 |
| WPAI | ||||||
| Impairment at work due to health (%)d | 24.8 ± 23.6 | 8.3 ± 15.1 | 9.5 ± 16.9 | 8.6 ± 17.7 | 9.1 ± 16.6 | 11.3 ± 20.0 |
| Activity impairment due to health (%)a | 41.5 ± 27.1 | 15.8 ± 22.3 | 16.0 ± 22.9 | 17.5 ± 22.6 | 16.7 ± 23.8 | 17.2 ± 24.6 |
- Effect size reflects change from baseline to 5 years.
- a Large Cohen's d effect size.
- b Small Cohen's d effect size.
- c No relevant Cohen's d effect size.
- d Moderate Cohen's d effect size.
- Abbreviations: TBWL, total body weight loss; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SF-12, Short Form-12; IWQOL, Impact of Weight on Quality of Life; EQ-5D, Euroqol Five Dimensions Questionnaire; WPAI, Work Productivity and Activity Impairment questionnaire; PCS, physical health composite score; MCS, mental health composite score; QOL, quality of life; VAS, visual analogue scale.
Objective changes in cardiometabolic risk
Waist circumference, a marker of cardiometabolic risk, decreased from 127.3 ± 16.7 cm at baseline to 110.7 ± 16.3 cm at 1 year, a reduction of 16.6 cm. This decrease was largely maintained, with a mean waist circumference of 111.3 ± 17.1 cm at 5 years. There were small but significant declines in systolic blood pressure of 5.1 mmHg, diastolic blood pressure of 1.8 mmHg, and heart rate of 4.7 beats per minute at 5 years after LBS implantation compared with baseline.
Compared with baseline, there was a small decrease in triglycerides and a large increase in HDL cholesterol levels at 5 years (Table 5). No significant differences in total and LDL cholesterol levels were observed throughout the 5 years (Table 5). The mean baseline HbA1c was 6.1% and fasting glucose was 5.9 mmol/L. At the 5-year visit, there was a small but significant decrease in HbA1c of 0.3% to 5.8% (Table 5). Of the 25% of the participants with type 2 diabetes mellitus (T2DM), excellent glycemic control (HbA1c < 6.5%) was achieved in 57%, 46%, 29%, 41%, and 29% of those followed at years 1 through 5, respectively.
Patient-reported outcomes
All patient-reported outcomes and measures of generic and obesity-specific quality of life showed improvement following LBS treatment, and the beneficial effect was maintained at all visits through to 5 years (Table 5). The SF-12 quality of life instrument showed greater impairment in physical when compared with mental summary scores at baseline and sustained improvements to community expected normative values for both summary scores (approximately 50) throughout the 5 years. The obesity-specific IWQOL-Lite scores demonstrated a large effect size improvement in all of the five category scores and the total score, as shown in Table 5 and Figure 1. The EQ-5D Index score was 0.7 (SD = 0.18) at baseline and improved to 0.8 (SD = 0.18) at the 5-year visit. Similarly, the EQ-5D visual analogue scale score increased from 54.2 (SD = 18.4) at baseline to 77.3 (SD = 17.1) by 5 years (Table 5). WPAI scoring indicated a sustained reduction in impairment at work because of health and a major sustained reduction in activity impairment because of health (Table 5; Figure 1).
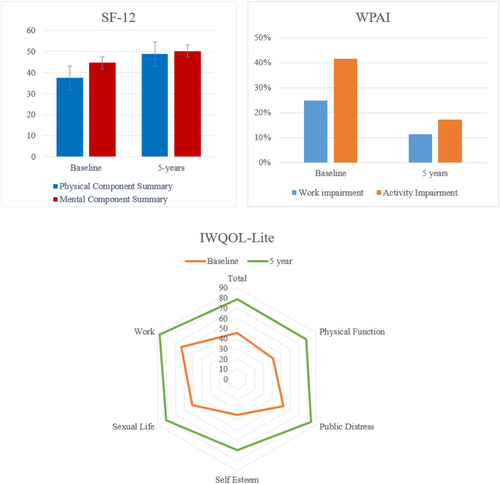
Patient-reported outcomes and health-related quality of life scores at baseline and 5 years after LBS placement. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
This phase 4 study conducted in 17 North American practices showed that the LBS system met its primary end point, which was to demonstrate that the explant rate at 5 years was significantly less than the historic rate of 39.4%. The follow-up rate of 73.1% was achieved at 5 years and the explant rate was 8.7% of the total number implanted. After accounting for missing data by using completer-only analysis or imputed data analysis, the estimated explant rate at 5 years was 12.8%. These findings are consistent with other recent 5-year data from the pivotal study that enabled the LBS to be indicated in patients with class I obesity and obesity-related comorbidities (13).
Several factors could potentially explain the explant rate that was more than three times lower than the historic rate reported to the FDA following the original US pivotal trial. Since the US approval of the LBS by the FDA in 2001, advances in band design, surgical technique, and appropriate band management and aftercare programs have all contributed to improving safety and reducing complications of the LBS system (22, 23). Refinements in surgical techniques include the use of a pars flaccida approach and proximal placement of the LBS just below the gastroesophageal junction, producing a virtual pouch. Experience in band management has also increased over the years, with a focus on early satiation and prolonged satiety rather than restriction or obstruction as the mode of action, targeting the “green zone” for optimal band fill (21).
The reoperation rates of 7.7% were comparable to a previous study in 2013, which showed a revision rate for proximal gastric enlargements of 6.4% over 5 years (24). In that study, the rates of revision also decreased with time. Another systematic review showed that, compared with other forms of bariatric surgery, LAGB has the highest reoperation rate of 12%, followed by sleeve gastrectomy (9%) and RYGB (3%) (25). However, the complexity of reoperations is lower with LAGB compared with the more complex procedures. Notably, similar phase 4 studies are not required following surgical therapy, and reporting bias in retrospective series is problematic. Rates of invasive procedures for small bowel obstruction alone following RYGB are reported to be generally greater than 3%, with 14% at 5 years in adolescents and 16% at 10 years in a quality US practice (26, 27). Recently, high reoperation rates following sleeve gastrectomy for poor weight loss and gastroesophageal reflux disease have also been reported (28, 29).
The HERO study also confirmed, in a real-world setting, the effectiveness of the LBS system in providing durable weight loss. Consistent with other studies, maximal weight loss was achieved at 2 years, and this mean weight loss of around 18% was maintained up until the end of the study at 5 years (13). As expected for weight loss, there was corresponding improvement in cardiometabolic risk parameters, including blood pressure, triglycerides, HDL, HbA1c, and fasting blood glucose. Changes in total and LDL cholesterol are not expected following weight loss, and results are consistent with all other nonmalabsorptive bariatric procedures (30, 31). The magnitude of changes was modest, though only a quarter of the study participants had T2DM, and we would expect to see greater improvement in glycemic parameters in a cohort consisting entirely of T2DM patients.
Perhaps the most important outcomes from the patients’ perspectives were the results of the patient-reported outcomes. This study confirmed the extensive literature indicating clinically important improvement in quality of life following LBS surgery. The most significant and reproducible effect was the sustained community normalization of generic quality of life scores of the SF-12 and the SF-36 in cohorts having clearly impaired physical and mental summary scores at baseline (13, 32). The Swedish Obese Subjects study demonstrated sustained benefit at 10 years (33). The current study was consistent with these observations, with better patient-reported outcomes and measures of quality of life following LBS treatment, which was maintained through to 5 years.
The current study, through additional patient-reported outcomes, IWQOL-Lite, EQ-5D, and WPAI, indicated not only individual health and functional improvement, but also positive change in important health economic and productivity metrics. These support the many studies indicating that LBS has consistently been found to be cost-effective and, in some studies, dominant (34-36).
There were significant limitations to this study. It was not a controlled trial and patients served as their own controls. However, the safety hypotheses did not require contemporaneous controls but rather a comparison with historic controls. The efficacy on a broad range of health outcomes following placement of the LBS system has been well established, and this carefully performed longitudinal study supported the finding of other studies, including randomized controlled trials in which similar or identical metrics have been measured. In addition, we caution interpretation of the SF-12 community normative data, as these were not matched controls and were simply whole population normative data.
Conclusion
The HERO study has met its primary end point with an explant rate that was much lower than the historic rate over a period of 5 years. The study also has validated the long-term mean TBWL of approximately 18% with its resultant improvements in cardiometabolic health and improvements in health-related quality of life. These results are consistent with the more recently published literature and add to the already robust evidence for the use of the LBS for the treatment of patients with obesity and its related conditions.
Acknowledgments
The LAP-BAND HERO study group investigators included Zubin Bhesania, Kevin Montgomery, Joseph Moran, Derek Weiss, Vincent Lusco, Trace Curry, John Olsofka, Jeff Holloway, Hans Schmidt, Dominick Gadaleta, George Fortier, Mark Fusco, Sunil Bhoyrul, Adam Smith, Lee Grossbard, David Voellinger, and Christopher Cobourn.





