Sedentary Time and MRI-Derived Measures of Adiposity in Active Versus Inactive Individuals
Funding agencies: This report is independent research funded by the National Institute for Health Research (NIHR). The research was supported by the NIHR Leicester Biomedical Research Centre, which is a partnership between University Hospitals of Leicester NHS Trust, Loughborough University, and the University of Leicester; the NIHR Collaboration for Leadership in Applied Health Research and Care-Leicestershire, Northamptonshire, and Rutland (NIHR CLAHRC LNR) and East Midlands (NIHR CLAHRC EM); and the Leicester Clinical Trials Unit. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. MRI scans were funded by Unilever Discover, UK.
Disclosure: The authors declared no conflict of interest.
Clinical trial registration: ClinicalTrials.gov identifier NCT00941954.
Abstract
Objective
The aim of this study was to examine cross-sectional associations between objectively measured sedentary time and magnetic resonance imaging (MRI)-assessed adiposity in a population at high risk for type 2 diabetes (T2DM) and to determine whether associations are modified by the recommended levels of moderate-to-vigorous physical activity (MVPA).
Methods
Sedentary time and MVPA were measured objectively by using accelerometers. Linear regression models examined the association of sedentary time with liver, visceral, subcutaneous, and total abdominal fat (quantified by using MRI). Interaction terms determined whether results were consistent across activity categories (active [> 150 min/wk of MVPA] vs. inactive [< 150 min/wk of MVPA]).
Results
One hundred and twenty-four participants (age = 64.0 ± 7.1 years; male = 65.3%; BMI = 31.8 ± 5.6 kg/m2) were included. Following adjustment, each 60 minutes of sedentary time was associated with 1.74 L higher total abdominal fat, 0.62 L higher visceral fat, 1.14 L higher subcutaneous fat, and 1.86% higher liver fat. When results were stratified by MVPA (active vs. inactive), sedentary time was associated with greater liver, visceral, and total abdominal fat in the inactive group only.
Conclusions
These findings suggest that sedentary time is associated with higher levels of inter- and intraorgan fat, but associations with liver, visceral, and total abdominal fat were stronger in those who do not reach the current exercise recommendations for health.
Introduction
It has been well documented that obesity and physical inactivity predispose individuals to insulin resistance and type 2 diabetes (T2DM) (1, 2). More specifically, evidence suggests that the distribution of excess fat is an important determinant of metabolic, cardiovascular, and mortality risk with a predominance of android rather than gynoid being a key contributor (3-5). In particular, ectopic fat, defined as the deposition of triglycerides within locations not classically associated with adipose storage, particularly within the intra- and interabdominal organs, appears to confer a higher metabolic risk than total abdominal fat per se (6, 7).
Physical activity is known to be one of the cornerstone interventions for the prevention of T2DM, with individuals who regularly engage in the recommended levels of moderate-to-vigorous physical activity (MVPA), typically 150 min/wk, manifesting a myriad of physiological benefits (including reduced visceral fat) and a reduced risk of chronic disease and premature mortality (8, 9). Conversely, sedentary time (defined as any sitting/reclining activity with a low energy expenditure (10)) has been shown to be detrimentally associated with homeostatic model assessment of insulin resistance, insulin, insulin sensitivity, T2DM, and mortality (2, 11-13). The evidence appears particularly compelling for those at high risk of, or diagnosed with, T2DM. Objectively measured sedentary behavior quantified by using an accelerometer is strongly associated with markers of insulin resistance (14-16), interleukin-6 (17), and markers of regional adiposity, assessed by magnetic resonance imaging (MRI) (18). Importantly, the majority of these observations persist after further adjustment for body mass index (BMI) or total fat mass and MVPA (15, 17, 18).
Despite these findings, there is emerging evidence that levels of fitness or physical activity may modify the association between sedentary time and health, with stronger associations seen in those who are inactive or unfit (15, 17, 19, 20). Others have also demonstrated that in comparison to adults who are physically inactive with high sedentary time, those who are physically active have a more desirable health profile across multiple cardiometabolic markers (BMI, A1c, and high-density lipoprotein cholesterol) (19). In addition, cross-sectional analyses in individuals at high risk of T2DM have demonstrated that after stratifying by MVPA, the detrimental effects of sedentary time on interleukin-6 are stronger in those who were classed as inactive (17). More recently, a harmonized meta-analysis also found that high levels of moderate physical activity (60-75 min/d) largely negated the increased risk of mortality associated with high sitting time (12).
Taken together, these studies have begun to suggest that the effects of sedentary time may be more relevant in those individuals who do not engage in sufficient levels of MVPA. However, these findings have yet to be explored beyond traditional markers of adiposity (BMI and waist circumference). The way in which sedentary time is accumulated may also influence health outcomes. For example, sedentary time that occurs in long periods without interruption may be more detrimental to health than shorter bouts (21). Moreover, previous research has shown that breaks in sedentary time are strongly associated with traditional measures of adiposity (14). Therefore, the aim of this study was to examine the associations between objectively measured sedentary time (total and prolonged bouts), breaks in sedentary time, and MRI-assessed body composition in a population at high risk of T2DM and to determine whether associations are mediated by the recommended levels of MVPA.
Methods
Subjects
This was a nested study within a randomized controlled trial for a subset of participants. Individuals were recruited from the Walking Away from Type 2 Diabetes trial, reported in detail previously (22, 23). Participants at an increased risk of T2DM were recruited through 10 primary care practices in Leicestershire, United Kingdom, from 2010 to 2011. Individuals with an increased risk of impaired glucose regulation (any combination of impaired glucose tolerance and/or impaired fasting glycemia or undiagnosed T2DM) were identified by using a modified version of the Leicester Risk Score (24). Individuals were unaware of their diabetes risk status before entering the study. At baseline, individuals were randomized to usual care or the Walking Away structured education program (23). There was no difference between groups in levels of MVPA, sedentary behavior, or markers of metabolic health at 12 months (22). Therefore, this paper reports cross-sectional data at 12 months from a sample of 124 participants collected during 2011. Ethical approval was obtained from the Nottingham Research Ethics Committee in the United Kingdom. Informed consent was obtained from all individual participants included in the study.
Quantification of sedentary time (total and bouts), breaks in sedentary time, and MVPA
All eligible participants were asked to wear an accelerometer at their 12-month visit (ActiGraph GT3X; ActiGraph, LLC, Pensacola, Florida) for seven consecutive days during waking hours. Data were collected in 15-second epochs and reintegrated into 60-second epochs for the purposes of this study. Freedson cut points were used to categorize an epoch as sedentary (< 100 counts per 60 seconds) or MVPA (≥ 1,952 counts per 60 seconds) (25). Breaks in sedentary time were defined as a transition from a sedentary (< 100 counts per 60 seconds) to an active state (≥ 100 counts per 60 seconds). Bouts of sedentary behavior were categorized into time periods of either 0 to 30 minutes, 30 to 60 minutes, or 60 + minutes. Nonwear time was defined as a minimum of 60 minutes of continuous zero counts, and days with at least 600 minutes of wear time were considered valid (26). In order to be included in the analysis, participants were required to have a minimum of four valid days (27). In order for individuals to be classed as active, they needed to have undertaken an average of at least 150 minutes of MVPA (28).
A data analysis tool (KineSoft version 3.3.76; KineSoft, Loughborough, UK) was used to process the accelerometer data.
MRI-derived measures of adiposity
At the 12-month follow-up measurement, participants were invited to undergo an MRI scan in addition to their other study assessments. MRI scans were performed at Glenfield Hospital in Leicester, United Kingdom, where liver, visceral, subcutaneous, and total abdominal fat (includes liver, subcutaneous and visceral fat) was quantified. MRI is a reliable modality for the assessment of adipose tissue and is capable of measuring fat distribution with a high spatial resolution (29, 30).
Scanning was performed using a 1.5 Tesla Avanto system (Siemens Medical, Erlangen, Germany). Flexible body array coils were applied to the thorax and abdomen for signal reception. For lipid volume quantification, a 2-point Dixon gradient-echo pulse sequence was used to separate tissue water signal from lipid signal. With this technique, two images are acquired: in the first, the fat and water signals are aligned in-phase; in the second, the fat and water signals have opposed phase directions. After the correction of phase distortions because of magnetic field inhomogeneity, the algebraic addition of the two images produces an image containing the signal largely from water, while subtraction produces an image-containing the signal largely from fat (31). Three-dimensional images were acquired axially with 5-mm slice thickness and in-plane resolution of 1.56 mm, interpolated to 0.78 mm. The field of view was 500 mm (left to right) by 375 mm (anterior to posterior). Images were acquired in three contiguous blocks, covering the thoracic, abdominal, and pelvic regions, with each block acquired in a breath-hold at full inspiration to minimize motion-related artifacts and to negate changes in slice position. The acquisition time for each block was 18 seconds. All scans were performed by the same team of trained staff according to standardized procedures.
The analysis of the MRI images was performed by a trained individual using Java Image Manipulation analysis software (version 7; Xinapse Systems, West Bergholt, UK). All analysis was undertaken by the same researcher who was blinded to the clinical, anthropometric, and accelerometer data.
For analysis, the “fat” and “water” images were algebraically combined to create a “fat percentage” image. Fat-containing voxels were then defined as those with an intensity between 51% and 99% (100% being due to image artifact). In order to expedite the analysis, the images were then down sampled in the slice direction into 15-mm-thick contiguous slices from the top of the pulmonary trunk extending to the bottom of the symphysis pubis. Volumes of interest for total abdominal fat were created by outlining the perimeter of the body on each relevant slice by using a mouse-controlled pointer and excluding those voxels outside the structures.
The visceral (and retroperitoneal) fat was further separated by outlining the abdominal and chest wall muscles and excluding the voxels for subcutaneous fat. The fat volume was calculated automatically by multiplying the cross-sectional areas of the fat-containing voxels, summed over all slices on which the tissue was outlined by the slice thickness. This created two fat volumes: total abdominal fat and visceral fat (from the top of the pulmonary trunk to the bottom of the symphysis pubis). Subcutaneous fat volume was calculated by subtracting visceral fat from total abdominal fat. The liver fat percentage was also measured by using a representative region of interest (1,000 mm2) placed in the right lobe of the liver, avoiding the main portal veins.
Covariates
Information on ethnicity (coded according to census criteria), current smoking status, and lipid-lowering medication use was obtained following an interview-administered questionnaire with a health care professional. Height and weight (Tanita TBF-611 body fat monitor/scale; Tanita, West Drayton, UK) were obtained by trained staff according to standard operating procedures, and the subsequent values were used to compute BMI (kilograms/meters2). Social deprivation was determined by assigning an index of multiple deprivation score to the participant's resident area (based on postcode). Index of multiple deprivation scores are publicly available continuous measures of compound social and material deprivation linked to health outcomes (including income, employment, education, living environment, and health). The dietary habits of participants were assessed by using the Dietary Instrument for Nutrition Education food frequency questionnaire, which is a method of measuring fiber, fat, and unsaturated fat intake in primary care (32). Only self-reported dietary fat intake is reported within this paper.
Statistical analysis
SPSS Statistics version 24.0 (IBM Corp., Armonk, New York) was used to conduct all statistical analyses. Linear regression analysis was used to examine the independent association of total, bouts, and breaks in sedentary time with liver, subcutaneous, visceral, and total abdominal fat. We display the main results per 60 minutes of sedentary time for ease of interpretation.
Model 1 was adjusted for age (continuous), sex, ethnicity (white European/South Asian/other), social deprivation (continuous), smoking status (current/ex/never smoked), lipid-lowering medication (yes/no), study arm (intervention/control), dietary fat intake, and time the accelerometer was worn (average number of minutes per day). Model 2 was additionally adjusted for MVPA or sedentary time and MVPA (breaks). In order to examine the extent to which total adiposity attenuated these relationships, model 3 was further adjusted for BMI. Models were assessed for normality and multicollinearity was assessed through the variance inflation factor.
Significant observations in model 3 were followed up with interaction terms to assess whether associations between total sedentary time and adiposity were modified by levels of MVPA (active vs. inactive) or sex (male vs. female). Analysis was further stratified by MVPA status to show the direction of significant interactions.
Two-tailed P values ≤ 0.05 were considered statistically significant for main effects. P < 0.1 was considered significant for interactions. The results of the generalized linear regression analysis are presented as the unstandardized beta coefficient (β; 95% confidence interval [CI] per 60 minutes of sedentary time). Adjustment was not made for multiple comparisons; therefore, data were viewed with caution and in relation to the overall pattern of results.
Results
In total, 124 participants (age = 64.0 ± 7.1 years; male = 65.3%) of a possible 141 had valid measures of objective activity, MRI, and covariate data. There were no significant differences (P > 0.05) in anthropometric, metabolic, and demographic measures between participants who were included in this analysis versus those not included. Table 1 displays the demographic, anthropometric, MRI-derived, and accelerometer characteristics of all included participants and when stratified by MVPA levels.
| All | Inactive | Active | P for difference | |
|---|---|---|---|---|
| (N = 124) | (n = 66) | (n = 58) | ||
| Age (y) | 64.0 ± 7.1 | 64.4 ± 7.1 | 62.4 ± 6.9 | 0.087 |
| Male | 81 (65.3) | 37 (56.1) | 44 (75.9) | < 0.001 |
| Current smokers | 8 (6.5) | 4 (6.1) | 4 (6.9) | 0.851 |
| IMD score | 21.8 ± 16.1 | 23.5 ± 15.9 | 19.9 ± 16.1 | 0.212 |
| Cardiometabolic variables | ||||
| BMI (kg/m2) | 31.8 ± 5.6 | 32.8 ± 6.0 | 30.8 ± 5.0 | 0.034 |
| Waist circumference (cm) | 105.4 ± 13.2 | 107.7 ± 14.5 | 103.0 ± 11.3 | 0.032 |
| Weight (kg) | 90.1 ± 17.3 | 91.3 ± 19.3 | 88.8 ± 15.1 | 0.495 |
| MRI-derived variables | ||||
| Visceral fat (L) | 6.6 ± 2.4 | 6.9 ± 2.5 | 6.4 ± 2.3 | 0.323 |
| Liver fat (%) | 10.6 ± 5.7 | 11.9 ± 6.1 | 9.0 ± 4.9 | 0.005 |
| Subcutaneous fat (L) | 13.1 ± 0.6 | 14.3 ± 5.9 | 11.9 ± 5.4 | 0.007 |
| Total abdominal fat (L) | 19.7 ± 6.7 | 21.2 ± 7.0 | 18.3 ± 6.0 | 0.007 |
| Ethnicity | ||||
| White European | 113 (91.1) | 58 (87.9) | 55 (94.8) | 0.216 |
| South Asian | 8 (6.5) | 7 (10.6) | 1 (1.7) | 0.045 |
| Other | 3 (2.4) | 1 (1.5) | 2 (3.4) | 0.485 |
| Diagnosis | ||||
| Normal glucose tolerance | 96 (77.4) | 48 (72.7) | 48 (82.8) | 0.183 |
| Isolated impaired fasting glycemia | 5 (4.0) | 2 (3.0) | 3 (5.2) | 0.545 |
| Isolated impaired glucose tolerance | 15 (12.1) | 11 (16.7) | 4 (6.8) | 0.096 |
| Both | 8 (6.5) | 5 (7.6) | 3 (5.2) | 0.587 |
| All (impaired glucose regulation) | 28 (22.6) | 18 (27.3) | 10 (17.2) | 0.183 |
| Dietary fat intake | 26.4 ± 12.7 | 25.1 ± 13.2 | 27.8 ± 12.1 | 0.223 |
| Accelerometer variables | ||||
| Time accelerometer worn (min/d) | 858 ± 96 | 855 ± 98 | 861 ± 96 | 0.977 |
| Sedentary time (min/d) | 546 ± 114 | 576 ± 120 | 516 ± 96 | 0.002 |
| Sporadic, 0- to 30-minute bout (min/d) | 364.7 ± 75.9 | 373.9 ± 78.7 | 354.2 ± 71.9 | 0.149 |
| Continuous, 30- to 60-minute bout (min/d) | 120.3 ± 57.4 | 132.3 ± 64.4 | 106.7 ± 45.0 | 0.013 |
| Continuous, 60 + minute bout (min/d) | 61.4 ± 51.9 | 70.0 ± 58.1 | 51.6 ± 42.4 | 0.045 |
| Light activity (min/d) | 284 ± 80 | 270 ± 84 | 299 ± 72 | 0.042 |
| MVPA (min/d) | 20.2 (12.5-39.8) | 12.5 (6.1-16.7) | 39.5 (28.3-55.1) | < 0.001 |
| Breaks in sedentary time (average per day) | 80 ± 16 | 80 ± 15 | 80 ± 17 | 0.837 |
| Total physical activity counts (× 1,000/d) | 229 (175-333) | 176 (145-212) | 330 (253-382) | < 0.001 |
| Steps per day | 6,802 ± 2,940 | 4,937 ± 1,866 | 8,731 ± 2,856 | < 0.001 |
- Continuous parametric results as mean ± standard deviation (SD), number (column percentage), and continuous nonparametric results as median (interquartile range).
- Sedentary time = < 100 counts per 60 seconds; MVPA = ≥ 1,952 counts per 60 seconds; total physical activity counts = summation of counts within each epoch. Inactive = < 150 min/wk of MVPA; active = ≥ 150 min/wk of MVPA.
- IMD, index of multiple deprivation.
Total sedentary time
Following adjustment for age, sex, ethnicity, social deprivation, smoking status, lipid-lowering medication, randomization arm, dietary fat intake, and time the accelerometer was worn, each 60 minutes of sedentary time was associated with higher liver (1.86%; CI: 1.14-2.52), visceral (0.62 L [0.32-0.92]), subcutaneous (1.14 L [0.54-1.74]), and total abdominal fat (1.74 L [0.96-2.46]). Results are displayed in Table 2. All associations persisted after further adjustment for MVPA. The association of sedentary time with visceral and liver fat remained after additional adjustment for BMI.
| Sedentary time, β (95% CI) | P | |
|---|---|---|
| Model 1 | ||
| Liver fat (%) | 1.86 (1.14 to 2.52) | < 0.001 |
| Visceral fat (L) | 0.62 (0.32 to 0.92) | < 0.001 |
| Subcutaneous fat (L) | 1.14 (0.54 to 1.74) | < 0.001 |
| Total abdominal fat (L) | 1.74 (0.96 to 2.46) | < 0.001 |
| Model 2 | ||
| Liver fat (%) | 1.80 (0.96 to 2.58) | < 0.001 |
| Visceral fat (L) | 0.60 (0.30 to 0.90) | < 0.001 |
| Subcutaneous fat (L) | 0.96 (0.30 to 1.62) | 0.006 |
| Total abdominal fat (L) | 1.56 (0.72 to 2.40) | 0.001 |
| Model 3 | ||
| Liver fat (%) | 1.44 (0.66 to 2.22) | < 0.001 |
| Visceral fat (L) | 0.36 (0.06 to 0.60) | 0.013 |
| Subcutaneous fat (L) | 0.18 (−0.24 to 0.66) | 0.348 |
- Model 1 adjusted for age, sex, ethnicity, social deprivation, smoking status, lipid-lowering medication, arm (control/intervention), dietary fat intake, and time accelerometer worn.
- Model 2 adjusted for the above covariates and MVPA.
- Model 3 adjusted for the above covariates and BMI. Total abdominal fat was not additionally adjusted for BMI because of multicollinearity.
Bouts of prolonged sedentary time
Results for bouts and breaks are displayed in Supporting Information Table S1. In the fully adjusted model, there were no significant associations between 0- to 30-minute bouts of sedentary time and MRI outcomes. Continuous 30- to 60-minute bouts of prolonged sedentary time were significantly associated with liver (1.44% [0.18-2.70]), visceral (0.78 L [0.36-1.26]), subcutaneous (1.08 L [0.12-2.04]), and total abdominal fat (1.86 L [0.66-3.12]). When further adjusted for BMI, the results remained significant for visceral fat (0.48 L [0.18-0.78]).
A similar pattern was observed for continuous 60+ minute bouts. In the fully adjusted model, continuous 60+ minute bouts of sedentary time were significantly associated with liver (1.44% [0.66-2.22]), visceral (0.66 L [0.24-1.08]), and total abdominal fat (2.88 L [1.62-4.08]) (not additionally adjusted for BMI).
Breaks in sedentary time
The number of breaks in sedentary time was significantly inversely associated with visceral (−0.06 L [−0.85 to −0.26]), subcutaneous (−0.68 L [−1.35 to −0.01]), and total abdominal fat (−0.12 L [−0.21 to −0.04]) in model 1. However, all associations were attenuated once adjusted for total sedentary time, MVPA, and BMI.
Interaction analysis
Interaction analyses indicated MVPA status modified some associations, with inactive individuals displaying stronger associations of total sedentary time with liver, visceral, and total abdominal fat (interaction values shown in Supporting Information Table S2). After stratification, no significant associations were found in active individuals. Conversely, each 60 minutes of sedentary time in the inactive cohort was associated with 2.16% higher liver fat (1.08-3.30), 0.54 L (0.12-0.96) higher visceral fat, and 1.80 L (0.54-3.06 L) higher total abdominal fat (Table 3). There were no significant interactions for bout length or breaks in sedentary time (all P > 0.1; data not shown).
| Inactive (n = 66) | Active (n = 58) | |||
|---|---|---|---|---|
| Sedentary time, β (95% CI) | P | Sedentary time, β (95% CI) | P | |
| Liver fat (%) | 2.16 (1.08 to 3.30) | < 0.001 | 0.60 (−0.72 to 1.92) | 0.364 |
| Visceral fat (L) | 0.54 (0.12 to 0.96) | 0.014 | 0.30 (−0.30 to 0.84) | 0.348 |
| Total abdominal fat (L) | 1.80 (0.54 to 3.06) | 0.005 | 0.18 (−1.26 to 1.62) | 0.817 |
- Adjusted for age, sex, ethnicity, social deprivation, smoking status, lipid-lowering medication, arm (control/intervention), dietary fat intake, time accelerometer worn, sedentary time, MVPA, interaction term, and BMI (not for total abdominal fat).
Figure 1 displays fat percentage images (from the bottom of the symphysis pubis to the top of liver) depicting total abdominal fat in two male participants with similar sedentary time but different amounts of MVPA. Figure 2 displays the multiple linear regression coefficients for 0 to 30 minutes, 30 to 60 minutes, and 60+ minute bouts of sedentary time (95% CI) with MRI-derived measures of adiposity.
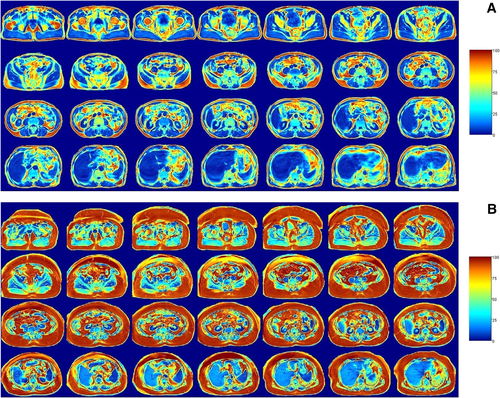
Fat percentage images (from the bottom of the symphysis pubis [top left] to the top of the liver [bottom right]) depicting total abdominal fat in two male participants with similar sedentary time but different amounts of MVPA. Colors represent the magnitude of adipose tissue density from blue (0%) to red (100%). (A) Sedentary time = 65.9%; MVPA = 5.5% (344 min/wk); ACTIVE. (B) Sedentary time = 66.6%; MVPA = 0.2% (11 min/wk); INACTIVE.
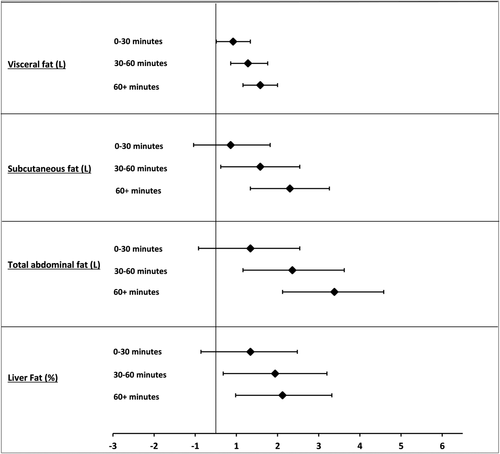
Multiple linear regression coefficients for sporadic (0-30 minutes) and continuous (30-60 minutes and 60+ minutes) bouts of sedentary time (95% CI). Adjusted for age, sex, ethnicity, social deprivation, smoking status, lipid-lowering medication, arm (control/intervention), dietary fat intake, MVPA, and time accelerometer worn. Results for visceral and liver fat remained significant after further adjustment for BMI. Total abdominal fat was significant but not additionally adjusted for BMI because of multicollinearity. CI, confidence interval.
Discussion
This study demonstrates that for individuals at high risk of T2DM, sedentary time is detrimentally associated with liver, visceral, and total abdominal fat, independent of important confounders (including MVPA). These findings extend previous cross-sectional results observed in the general population (33) and in those at high risk of T2DM (18) by showing that associations between sedentary time and liver, visceral, and total abdominal fat are stronger in those who do not reach the current exercise recommendations for health (< 150 min/wk), whereas no significant associations were observed in the active (> 150 min/wk) cohort. Furthermore, the bout length of sedentary time influenced the strength of the association.
To our knowledge, this study is the first to look at the modifying influence of MVPA on associations between sedentary time and MRI-derived fat distribution. The finding that the association between sedentary time and visceral fat is stronger in the inactive cohort is intriguing and lends credence to the proposition that physical activity levels may be an important determinant in the accrual of adipose tissue (9). Broadly, visceral fat has been associated with incident cardiovascular disease, cancer, and mortality after adjustment for clinical risk factors and generalized adiposity. In particular, it has been suggested to be an important link between cardiorespiratory fitness (with its high correlation with MVPA) and markers of the metabolic syndrome (34, 35), with exercise training studies showing inverse associations between visceral fat and insulin sensitivity in subjects with obesity (36). Furthermore, regular MVPA may induce selective reductions in visceral abdominal adipose tissue and other region-specific fat deposits (subcutaneous) that would not necessarily be apparent when using more traditional measures to quantify body mass or adiposity (e.g., BMI, waist circumference).
Similarly, the observation that liver fat is strongly associated with sedentary time in the inactive cohort is a novel finding, which may be partially explained by excess visceral fat, in which nonesterified fatty acids (NEFA), glycerol, and hormones released from adipose tissue within the visceral peritoneum are drained into the hepatic portal vein that feeds directly to the liver (37). Sustained exposure of the liver to an increased flux of NEFA via the portal circulation is the antecedent to many of the disturbances in glucose and lipids, thus predisposing an individual to peripheral insulin resistance, hyperlipidemia, and hypertension (38). This process may be further exacerbated by high levels of sedentary time and low levels of physical activity. Indeed, it has been shown that a prolonged bout of sitting (7.5 hours) elicits a less favorable NEFA postprandial response when compared with breaking sitting with either standing or walking (5 minutes every 30 minutes), which may reflect an increase in the lipolysis of triglycerides stored in adipose tissue in order to supply the working muscle (39).
Our observations for a modifying effect of MVPA status on the association of sedentary time with visceral, liver, and total abdominal fat broadly corroborates other cross-sectional research, which has demonstrated that those who are physically active have a more desirable cardiometabolic and inflammatory profile, even when combined with high sedentary time (17, 19). Similar results have also been shown in individuals recently diagnosed with T2DM, for whom results were suggestive of a stronger association between sedentary time and subcomponents of metabolic risk among individuals below the median for cardiorespiratory fitness (15). This hypothesis appears to extend beyond traditional markers of health, as a recent meta-analysis, which included more than 1 million people and used self-reported data, found that when compared with the referent group (< 4 h/d of sitting and ∼60-75 min/d of moderate-intensity activity; 9.0% of the cohort), there was no increased risk of mortality during follow-up in those who sat for more than 8 h/d but also engaged in ∼60 to 75 minutes of activity (hazard ratio: 1.04; 95% CI: 0.99-1.10; 4.4% of the cohort). However, these high activity levels were shown to attenuate, rather than eliminate, the association with mortality of those watching 5 h/d or more of TV (1.15; 1.05-1.29) (12).
The aforementioned observational findings have also been explored in an experimental context, which showed that individuals with higher fitness levels demonstrated a lower incremental area under the curve response for glucose and insulin when subjected to 7.5 hours of prolonged sitting (38). Similarly, they also accrued less of a metabolic benefit from breaking sitting time with light walking when compared with those with lower fitness levels, although small benefits were still seen for the reduction in insulin levels in individuals with higher fitness. Taken together, the experimental and cross-sectional literature have implied that sedentary time may be not be as pertinent in individuals with a relatively high fitness level or in those who engage in recommended levels of MVPA.
We found no significant associations between overall breaks in sedentary time and MRI outcomes. However, our findings do reinforce previous studies that have demonstrated that prolonged bouts of sedentary time are detrimental to health, particularly when examining markers of cardiometabolic health (21). We found that as the prolonged sedentary bout length increased (from 0-30 minutes, to 30-60 minutes, to 60+ minutes), so did the strength of the association with MRI-derived markers. This further reiterates the importance of targeting prolonged sitting time as well as MVPA in future disease prevention interventions.
Our study has multiple strengths. Most notably, it provides novel evidence of the modifying effect of MVPA on MRI-derived adiposity in a sedentary population with a high risk of T2DM. Secondly, we used gold standard measures in order to quantify regional fat deposition and physical activity levels. All measurements (including MRI scans) were also performed by the same team of trained staff following identical standard operating procedures. Thirdly, participants were recruited from a multiethnic community.
Limitations include the cross-sectional design, which limited inference about the direction of causality between sedentary time and markers of MRI-derived adiposity; as such, reverse causality remains a possibility. It is also plausible that unmeasured lifestyle variables (e.g., alcohol intake) and residual confounding may have influenced the observed relationships. Exposures were only carried out at one time point (up to 7 days), which may not be an accurate reflection of habitual activity. Finally, accelerometers rely on categorizing movement (acceleration) as opposed to distinguishing between specific postures (sitting, lying, and standing behaviors), which may lead to an underestimation of the true association between sedentary time and markers of adiposity.
Conclusion
These novel findings from a cohort with a high risk of T2DM suggest that the deleterious associations of objectively measured sedentary time with visceral, liver, and total abdominal fat may be particularly pertinent for those individuals who do not undertake sufficient amounts of MVPA. Moreover, the manner in which sedentary time is accumulated may influence the strength of the associations with markers of MRI-derived adiposity.





