Usual breakfast eating habits affect response to breakfast skipping in overweight women
Funding agencies: NIH (5 T32 DK007446-30), Endocrine Fellows Foundation grant: Fellows Development Research Grant Program in Diabetes, Obesity and Fat Cell Biology; Nutrition Obesity Research Center (NORC) (5P30DK048520), NIH/NCATS Colorado CTSI (UL1 TR001082).
Disclosure: The authors declared no conflict of interest.
Author contributions: EAT, MAC, and JH designed research; EAT conducted research; BM and EAT performed statistical analyses; EAT, MAC, JH and DHB wrote the manuscript; all authors approved the final manuscript.
Abstract
Objective
This randomized, cross-over trial was designed to investigate the metabolic and appetitive responses to skipping breakfast in overweight women who were habitual breakfast Eaters or Skippers.
Methods
Nine Eaters and nine Skippers were studied on two separate days during which subjects ate breakfast (B) or had no breakfast (NB), followed by a standard lunch meal 4 h later. Blood sampling for hormones and metabolites was performed after lunch, and appetite was rated throughout the day.
Results
Interactions between day and habitual breakfast pattern were seen for area under the curve (AUC) for insulin and free fatty acids (FFA). Both insulin (P = 0.020) and FFA (P = 0.023) AUC were higher on the NB day for Eaters but similar on both days for Skippers. Eaters had higher pre lunch hunger AUC on the NB day (P = 0.015) as well as lower pre lunch satiety AUC under both conditions (P = 0.019).
Conclusions
Overall, this study showed that the adverse effects of skipping breakfast (higher insulin and FFA responses to lunch, increased hunger, and decreased satiety) were found primarily in habitual breakfast eaters. This suggests that meal skipping may have enhanced effects in habitual Eaters due to entrainment of metabolic and appetitive regulatory systems.
Introduction
Obesity is a serious public health problem in the United States, with a majority of Americans now being either overweight or obese (1). Though many factors contribute to the obesity epidemic, it is possible that breakfast skipping is one such factor. Epidemiological studies have shown that breakfast skipping is associated with higher BMI in children, adolescents and adults (2-11), and most longitudinal studies have shown breakfast skipping to be associated with greater weight gain (4, 12). Prospective studies have shown that skipping breakfast is associated with dyslipidemia (13, 14), increased blood pressure (14, 15), higher risk of type 2 diabetes and metabolic syndrome (12, 15), and increased risk of coronary heart disease (16).
However, a causal relationship between breakfast skipping and weight gain has not been established. The fact that breakfast skippers have a higher risk of type 2 diabetes mellitus suggests that skipping breakfast might lead to changes in glucose metabolism. Several small studies have investigated the metabolic response to food after eating or omitting breakfast. These studies have shown breakfast skipping to be associated with impairment of insulin sensitivity (17, 18), decreased fullness and decreased levels of hormones related to satiety (19), and deterioration of lipid profiles (17, 20).
However, none of these studies evaluated obese adults or assessed the effects of habitual breakfast pattern. Thus, in order to investigate the metabolic, hormonal, and appetitive responses to skipping breakfast, we carried out a randomized, cross-over trial of overweight and obese women who were habitual breakfast eaters or breakfast skippers. The primary hypothesis was that skipping breakfast would lead to an increased insulin response following lunch, and that this effect would be more pronounced in habitual breakfast skippers. Secondary outcomes included response of additional hormones and metabolites, appetitive response, energy intake, energy expenditure, substrate oxidation, and differences between breakfast eaters and skippers.
Methods
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Colorado Multiple Institutional Review Board. All patients provided written informed consent for the collection of samples and subsequent analysis.
Subjects
Healthy women of all ethnic groups, ages 25−40, with BMI 27−35 kg/m2, without eating disorders, and who were either habitual breakfast eaters (Eaters) or breakfast skippers (Skippers) were recruited. Subjects were asked to identify themselves as Eaters or Skippers by answering the question: “How many days per week do you eat breakfast?” Eaters reported eating breakfast ≥ 5 days per week and Skippers reported eating breakfast ≤2 days per week. Women without menses were excluded, and all women reported no active dieting or intensive exercise training. Additionally, subjects were excluded if they were identified as having night eating syndrome (at least 25% of food intake is consumed after the evening meal and/or at least two episodes of nocturnal eating per week) (21).
Prestudy procedures
Prestudy procedures included measurement of height, weight, vital signs, and resting metabolic rate (RMR). After 30 min of quiet rest, RMR was measured using standard indirect calorimetry with the ventilated hood technique (Parvo Medics Truemax 2400, Salt Lake City, UT). Subjects remained awake, supine, lightly clothed in a thermoneutral (68−74°F) quiet room. The following equation was used to estimate total energy expenditure (TEE): TEE = measured RMR (kcals) × activity factor. A standard activity factor of 1.4 was used for all subjects. Prior to the first study visit day, all subjects completed a 3-day diet diary in order to obtain usual dietary intake patterns and confirm reported breakfast eating pattern (Eater or Skipper).
Study visits
Each subject underwent 2 study conditions (Breakfast – B and No Breakfast – NB) with the order of study conditions determined using a computer-generated randomization table. Four Skippers and three Eaters underwent the NB condition first, and six Eaters and five Skippers underwent B first. Subjects were not aware of the order of study visits until the first study day. The study visits were timed to occur during the follicular phase of the menstrual cycle with one month between visits (22). Subjects were asked not to do purposeful exercise in the 48 h prior to each study day. Each subject's total daily energy intake (EI) for study visits was estimated based upon TEE as described above. Subjects were provided with a standard dinner meal (35% of daily EI; 15% protein, 30% fat, 55% carbohydrate) to be eaten the night before each test day between 6 and 8 PM and were asked not to eat anything after 8 PM. On the morning of the test day, subjects presented for baseline measurements at 7:00 AM. Their height, weight and vital signs were measured and then they were asked to lie quietly for 30 min. Subsequently, RMR was measured at baseline, as described above, and subjects were then asked to rate hunger and satiety using visual analog scales (VAS). Hunger was rated on a 100-mm line preceded by the question, "How hungry do you feel right now?" and anchored by "not at all hungry" on the left and "extremely hungry" on the right. Satiety was rated by VAS with the question, “How full do you feel right now?” with the anchors "not at all" and "extremely (23). When the appetite ratings were complete, an intravenous catheter was inserted into an antecubital vein and blood was drawn for fasting glucose, insulin, free fatty acids (FFA), ghrelin, PYY (peptide YY), GLP-1 (glucagon-like peptide 1), and triglycerides (TG). When all baseline measurements were complete, subjects were provided with breakfast along with 250mL water on the B test day, or 250mL water alone on the NB test day. The breakfast meal contained 25% of total daily EI (15% protein, 30% fat, 55% carbohydrate). The content of the breakfast was wheat flakes plus milk, scrambled eggs, and orange juice. Time zero was the time that the subject began eating (or drinking) and subjects were asked to consume the breakfast (or water) within 20 min. Blood sampling was performed prior to and every 30 min after the lunch meal for 3 h. Subjects rated hunger and satiety using VAS hourly prior to lunch, every 30 min for 3 h following lunch, and hourly until the end of the study day. Indirect calorimetry was performed hourly throughout each testing day (Figure 1).
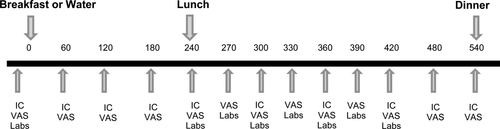
Overview of study day. Study procedures are shown by time (min). IC, indirect calorimetry; VAS, visual analog scales for appetite ratings; Labs, glucose, insulin, free fatty acids, triglycerides, GLP-1, PYY, ghrelin.
At 240 min, subjects were provided with a standard lunch containing 35% of daily EI (15% protein, 30% fat, 55% carbohydrate). The lunch meal was comprised of the same foods in the same amounts on both test days, and was made up of a sandwich, chips, and fruit. Subjects were asked to consume all contents of the lunch meal within 20 min.
At 540 min, subjects were provided with an ad libitum dinner meal and told to eat as much or as little as they needed to feel full. The dinner meal was comprised of pepperoni pizza, fettucine alfredo, vanilla yogurt and chocolate pudding and provided a total of 2,600 kcal (15% protein, 30% fat, 55% carbohydrate). After the meal was finished the subjects were allowed to leave. They were provided with a box of snack foods to take home and were told that if they wanted to eat anything that evening, they should eat only items contained in the box (and no items from their home). The box contained soda (diet and regular), grapes, plain M&M's, cashews, vanilla ice cream, pretzels, and potato chips. They returned the uneaten portion of snacks the following day, and the amount eaten both at dinner and from snack foods was determined by weigh-back methods.
Laboratory analyses
Blood samples were collected in EDTA-containing tubes, centrifuged, placed in aliquot tubes, and stored at −70 to −80°C until analysis. All assays were run after both study days were complete for each subject. For GLP-1, 30ɥl of dipeptidyl peptidase IV inhibitor was added to the 4ml EDTA tube prior to collection. GLP-1 assays were performed with Alpco Diagnostics ELISA (43-GPTHU-E01). Insulin concentrations were measured using competitive radioimmunoassay (Millipore). Radioimmunoassay was used to analyze serum leptin (Millipore), serum PYY concentrations (Millipore Cat. #PYYT-66HK) and total serum ghrelin concentrations (Millipore Cat. #GHRT-89HK). All radioimmunoassays were performed with a Perkin Elmer Wallac Gamma counter using Maciel RIA-AID data reduction software. Assays for glucose, TG and FFA were performed on the Olympus AU400e Chemistry Analyzer (Beckman). Reagents were purchased from Beckman Coulter for glucose and TG and from WACO for FFA.
Statistical analysis
Sample size calculations were performed using SigmaPlot version 12 (San Jose, CA). Sample size was determined with the use of glucose and insulin data from a study of over- and underfeeding done by our group (24). Power was estimated for a range of possible differences for a paired t-test at the 0.05 level. A sample of 18 subjects would give us 80% power to detect a difference in insulin AUC of 3,000 µIU/mL × 180 min. Assuming a 25% dropout rate, a sample size of 24 women enrolled would provide about 18 subjects for analysis. Baseline characteristics of the two groups (Skippers vs. Eaters) and EI were analyzed with the use of Wilcoxon rank sum tests. Area under the curve (AUC) was calculated for all outcomes with the exception of energy intake (laboratory values, appetite ratings, energy expenditure, and respiratory quotient [RQ]) using the trapezoid method (25). For laboratory values, AUC for the 3-h period following lunch was used. For appetite ratings, energy expenditure and RQ, AUC was calculated for the prelunch period (time zero through 240 min) and postlunch period (270 through 540 min). Energy intake (dinner, evening snack, and total EI) and all AUC outcomes were modeled using mixed-model linear regression (SAS 9.3, SAS Institute, Cary, NC). Day, Skipper/Eater, and order were included as predictors in every model. The day by Skipper/Eater interaction was tested in every model. The interaction term was left in the final model in models where it was significant at the 0.05 alpha-level, but was removed from the final model if not significant. All models included random intercepts for subjects to account for the repeated measures on subjects. All data for all 18 subjects were included in analyses, with the exception of EI data for one subject in the Skipper group (due to failure to follow protocol in returning the snack box items) and glucose data for one subject in the Eater group (due to missing data).
Results
Subjects and baseline characteristics
A total of 24 women were enrolled in the study. Two subjects completed one study day and then dropped out of the study due to scheduling conflicts, and 4 other subjects dropped out prior to the first study day, also due to scheduling conflicts. A total of 18 women completed the study, 9 Skippers and 9 Eaters. Subjects were 72% Caucasian, 22% African American, and 6% Asian. They were 88% Non-Hispanic and 22% Hispanic. There was no difference among groups in race or ethnicity, and no difference in age, BMI or RMR (Table 1).
| All subjects, n=18 | Breakfast eaters, n=9 | Breakfast skippers, n=9 | |
|---|---|---|---|
| Age (years) | 29 (27, 32) | 29 (27.5, 31.5) | 28 (26.5, 34) |
| BMI (kg/m2) | 30.2 (28.6, 33.7) | 30.2 (29.2, 32.7) | 30 (27.8, 34.3) |
| Breakfast normally consumed (day/wk) | 3.5 (1, 6) | 6 (5.5, 7) | 1 (1, 2)* |
| RMR (kcal/day) | 1549 (1463, 1688) | 1563 (1465, 1755) | 1536 (1460, 1594) |
- Values are medians (interquartile range). *Different from breakfast eaters, P<0.001.
- BMI, body mass index; RMR, resting metabolic rate.
Laboratory measures
There were significant interactions between day and breakfast pattern (Skipper/Eater) for insulin and FFA AUC, such that values were higher on the NB day for Eaters but similar on both days for Skippers (Figure 2 and Table 2). The AUC's for TG, GLP-1 and PYY were higher on the B day, whereas AUC was lower on the B day for glucose. Order effect was evaluated and was not found to be significant.
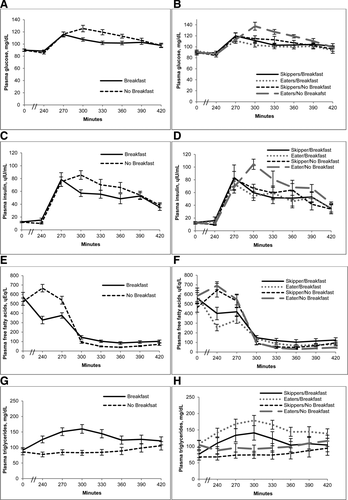
Metabolic response to breakfast skipping in habitual breakfast eaters and skippers. (A) Glucose, (C) insulin, (E) free fatty acids, and (G) triglycerides are shown at 0 min, 240 min (prior to lunch meal), and every 30 min for 180 min following the lunch meal on the breakfast and no breakfast days in all subjects combined. Values are means ± SEM, n = 18. Also shown are differences between the response in breakfast Eaters vs. Skippers for (B) glucose, (D) insulin, (F) free fatty acids, and (H) triglycerides. Values are means ± SEM, n = 18 (9 Skippers; 9 Eaters).
| Outcome | Effect | Category | Estimate | 95% CI | P-value |
|---|---|---|---|---|---|
| FFA AUC, mEq/L × 180 min | Breakfast/No Breakfast | 0.24 | (−5.34, 5.83) | 0.927 | |
| Skipper/Eater | 0.45 | (−8.87, 9.78) | 0.919 | ||
| Day × Skipper/Eater | −9.66 | (−17.8, −1.52) | 0.023 | ||
| Breakfast/Eater | 25.7 | (18.8, 32.5) | |||
| Breakfast/Skipper | 34.9 | (28.5, 41.3) | |||
| No Breakfast/Eater | 35.1 | (28.2, 41.9) | |||
| No Breakfast/Skipper | 34.6 | (28.2, 41.0) | |||
| GLP-1 AUC, pM × 180 min | Breakfast/No Breakfast | 321 | (177, 464) | <0.001 | |
| Breakfast | 1198 | (876, 1520) | |||
| No Breakfast | 877 | (555, 1200) | |||
| Skipper/Eater | −138 | (−757, 480) | 0.642 | ||
| Eater | 969 | (513, 1420) | |||
| Skipper | 1100 | (682, 1530) | |||
| Ghrelin AUC, ng/L × 180 min | Breakfast/No Breakfast | −2.67 | (−13.2, 7.91) | 0.600 | |
| Breakfast | 13.0 | (11.1, 14.9) | |||
| No Breakfast | 13.3 | (11.4, 15.1) | |||
| Skipper/Eater | −5.47 | (−41.2, 30.2) | 0.750 | ||
| Eater | 12.9 | (10.2, 15.5) | |||
| Skipper | 13.4 | (10.9, 15.8) | |||
| Glucose AUC, g/L × 180 min | Breakfast/No Breakfast | −14.6 | (−24.7, −4.59) | 0.007 | |
| Breakfast | 187 | (179, 195) | |||
| No Breakfast | 201 | (193, 209) | |||
| Skipper/Eater | 331 | (−922, 158) | 0.582 | ||
| Eater | 196 | (186, 205) | |||
| Skipper | 192 | (184, 201) | |||
| Insulin AUC, IU/L × 180 min | Breakfast/No Breakfast | −0.31 | (−1.97, 1.35) | 0.698 | |
| Skipper/Eater | 2.49 | (−8.96, 5.87) | 0.138 | ||
| Day × Skipper/Eater | −2.95 | (−5.37, −0.53) | 0.020 | ||
| Breakfast/Eater | 9.26 | (6.77, 11.7) | |||
| Breakfast/Skipper | 9.72 | (7.40, 12.0) | |||
| No Breakfast/Eater | 12.5 | (10.0, 15.0) | |||
| No Breakfast/Skipper | 10.0 | (7.71, 12.4) | |||
| PYY AUC, ng/L × 180 min | Breakfast/No Breakfast | 2.25 | (0.65, 3.86) | 0.008 | |
| Breakfast | 19.6 | (18.1, 21.1) | |||
| No Breakfast | 17.4 | (15.9, 18.8) | |||
| Skipper/Eater | −0.37 | (−2.82, 2.07) | 0.750 | ||
| Eater | 18.3 | (16.5, 20.1) | |||
| Skipper | 18.7 | (17.0, 20.4) | |||
| TG AUC, g/L × 180 min | Breakfast/No Breakfast | 88.6 | (447, 133) | <0.001 | |
| Breakfast | 241 | (196, 285) | |||
| No Breakfast | 152 | (107, 196) | |||
| Skipper/Eater | 40.1 | (−36.2, 116) | 0.281 | ||
| Eater | 216 | (160, 273) | |||
| Skipper | 176 | (124, 229) |
- a Values are mean AUC (95% CI), n=18. For outcomes that do not include interactions, estimates, CIs, and P-values are given for each predictor, and least-squares means and CIs are given for each predictor group. For outcomes that do include interactions, least-squares means are given for each day by Skipper/Eater combination rather than for day groups and Skipper/Eater groups separately.
- b AUC, area under curve; FFA, free fatty acids; GLP-1, glucagon-like peptide 1; PYY, peptide YY; TG, triglycerides.
Appetite ratings
There were significant interactions between condition and breakfast pattern for prelunch hunger AUC and postlunch satiety AUC (Figure 3 and Table 3). Prelunch hunger AUC was higher on the NB day for Eaters as compared with Skippers. Overall prelunch hunger under both the B and NB conditions was higher in Eaters. Prelunch satiety under both conditions was lower in Eaters as compared to Skippers. Postlunch satiety AUC was similar on both days for Eaters, whereas it was higher on the B day for Skippers. There was no significant order effect.
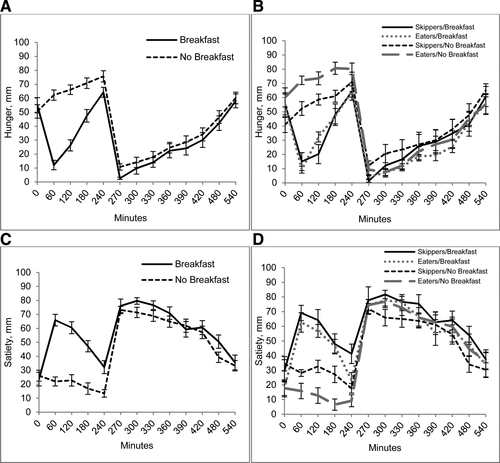
Appetitive response to breakfast skipping in habitual breakfast eaters and skippers. (A) Hunger and (C) satiety at 0 min and every hour prior to the lunch meal, every 30 min for 180 min following lunch, and hourly until end of study day are shown on the breakfast and no breakfast day for all subjects. Values are means ± SEM, n = 18. (B) Hunger and (D) satiety in breakfast Eaters vs. Skippers at 0 min and every hour prior to the lunch meal, every 30 min for 180 min following lunch, and hourly until end of study day are shown on the breakfast and no breakfast day. Values are means ± SEM, n = 18 (9 Skippers, 9 Eaters).
| Outcome | Effect | Category | Estimate | 95% CI | P-value |
|---|---|---|---|---|---|
| Pre lunch hunger AUC, cm × 240 min | Breakfast/No Breakfast | −51.1 | (−72.1, −30.1) | <0.0001 | |
| Skipper/Eater | 42.0 | (15.8, 68.2) | 0.003 | ||
| Day × Skipper/Eater | −38.0 | (−67.7, −8.33) | 0.015 | ||
| Breakfast/Eater | 90.1 | (71.3, 109) | |||
| Breakfast/Skipper | 86.2 | (67.7, 105) | |||
| No Breakfast/Eater | 179 | (160, 198) | |||
| No Breakfast/Skipper | 137 | (119, 156) | |||
| Post lunch hunger AUC, cm × 270 min | Breakfast/No Breakfast | −10.7 | (−24.4, 2.93) | 0.116 | |
| Breakfast | 76.5 | (54.9, 98.1) | |||
| No Breakfast | 87.3 | (65.7, 109) | |||
| Skipper/Eater | −16.3 | (−56.5, 23.9) | 0.405 | ||
| Eater | 73.8 | (44.7, 102.8) | |||
| Skipper | 90.0 | (61.7, 118) | |||
| Pre lunch satiety AUC, cm × 240 min | Breakfast/No Breakfast | 72.1 | (54.1, 90.1) | <.0001 | |
| Breakfast | 121 | (106, 136) | |||
| No Breakfast | 49.1 | (34.2, 64.0) | |||
| Skipper/Eater | −28.4 | (−51.7, −5.21) | 0.019 | ||
| Eater | 70.9 | (54.1, 87.7) | |||
| Skipper | 99.4 | (83.0, 116) | |||
| Post lunch satiety AUC, cm × 270 min | Breakfast/No Breakfast | 31.5 | (11.2, 51.8) | 0.005 | |
| Skipper/Eater | 20.6 | (−16.7, 57.9) | 0.259 | ||
| Day × Skipper/Eater | −32.5 | (−61.2, −3.83) | 0.029 | ||
| Breakfast/Eater | 162 | (136, 189) | |||
| Breakfast/Skipper | 174 | (148, 201) | |||
| No Breakfast/Eater | 163 | (137, 190) | |||
| No Breakfast/Skipper | 143 | (117, 169) |
- a Values are mean AUC (95% CI), n=18. For outcomes that do not include interactions, estimates, CIs, and P-values are given for each predictor, and least-squares means and CIs are given for each predictor group. For outcomes that do include interactions, least-squares means are given for each day by Skipper/Eater combination rather than for day groups and Skipper/Eater groups separately.
- b AUC, area under curve.
Energy intake
EI for breakfast and lunch did not differ by day, per study design (Table 4). Dinner, snack, and total EI, as well as macronutrient composition, were examined with the use of the mixed model, and there were no interactions, differences between Eaters and Skippers, or order effect, so data presented are for the difference between B and NB only. Dinner, snack and total EI did not differ by day. Protein, carbohydrate and fiber intake were higher on the B day.
| Energy (kcal) | Protein (g) | Fat (g) | Carbohydrate (g) | Fiber (g) | ||
|---|---|---|---|---|---|---|
| Breakfast | Breakfast | 497 (463, 529) | 20 (19, 22) | 18 (16, 19) | 75 (70, 80) | 8 (8, 9) |
| No Breakfast | N/A | N/A | N/A | N/A | N/A | |
| Lunch | Breakfast | 713 (691, 782) | 29 (27, 30) | 25 (23, 27) | 103 (98, 110) | 6 (5, 7) |
| No Breakfast | 713 (691, 782) | 29 (27, 30) | 25 (23, 27) | 103 (98, 110) | 6 (5, 7) | |
| Dinner | Breakfast | 815 (550, 1072) | 33 (22, 42) | 27 (18, 36) | 112 (75, 148) | 4 (3, 5) |
| No Breakfast | 837 (594, 972) | 33 (25, 39) | 28 (19, 32) | 116 (82, 134) | 4 (3, 5) | |
| Snack | Breakfast | 701 (177, 1023) | 8 (3, 12) | 24 (10, 48) | 110 (33, 140) | 3 (1, 7) |
| No Breakfast | 716 (360, 1197) | 10 (4, 18) | 37 (12, 58) | 101 (49, 121) | 4 (1, 6) | |
| Total | Breakfast | 2516 (2363, 3324) | 85 (77, 103) | 98 (76, 120) | 380 (328, 459) | 23 (19, 28) |
| No Breakfast | 2344 (1913, 2777) | 75 (64, 86)* | 94 (64, 127) | 318 (262, 368)* | 15 (12, 19)** |
- Values are medians (interquartile range), n=17 (9 Eaters, 8 Skippers).
- g, grams; kcal, kilocalorie.
- *Different from breakfast, P<0.05.
- **Different from breakfast, P<0.001.
Energy expenditure
The prelunch EE AUC was higher on the B as compared with NB day (Table 5). Postlunch EE AUC showed a trend toward being higher on the B day, but did not reach significance (P = 0.051). There were no group differences, interactions, or order effect for EE.
| Outcome | Effect | Category | Estimate | 95% CI | P-value |
|---|---|---|---|---|---|
| Pre lunch EE AUC, Mcal/day × 240 min | Breakfast/No Breakfast | 459 | (372, 545) | <0.0001 | |
| Breakfast | 411 | (388, 434) | |||
| No Breakfast | 365 | (342, 388) | |||
| Skipper/Eater | 177 | (−271, 626) | 0.415 | ||
| Eater | 397 | (364, 429) | |||
| Skipper | 379 | (347, 411) | |||
| Post lunch EE AUC, Mcal/day × 270 min | Breakfast/No Breakfast | 11.7 | (−90.8, 234) | 0.051 | |
| Breakfast | 430 | (404, 456) | |||
| No Breakfast | 418 | (392, 444) | |||
| Skipper/Eater | 28.3 | (−21.5, 78.2) | 0.247 | ||
| Eater | 438 | (402, 474) | |||
| Skipper | 410 | (375, 445) | |||
| Pre lunch RQ AUC, RQ × 240 min | Breakfast/No Breakfast | 14.1 | (8.59, 19.6) | <0.0001 | |
| Skipper/Eater | −3.39 | (−11.36, 4.59) | 0.381 | ||
| Day × Skipper/Eater | 8.71 | (0.92, 16.5) | 0.031 | ||
| Breakfast/Eater | 206 | (200, 212) | |||
| Breakfast/Skipper | 201 | (195, 206) | |||
| No Breakfast/Eater | 184 | (177, 189) | |||
| No Breakfast/Skipper | 186 | (181, 192) | |||
| Order | −7.39 | (−14.5, −0.25) | 0.043 | ||
| Breakfast First | 190 | (186, 195) | |||
| No Breakfast First | 198 | (192, 203) | |||
| Post lunch RQ AUC, RQ × 270 min | Breakfast/No Breakfast | 3.46 | (−0.68, 7.59) | 0.096 | |
| Breakfast | 204 | (201, 207) | |||
| No Breakfast | 201 | (198, 204) | |||
| Skipper/Eater | 8.15 | (4.00, 12.3) | 0.001 | ||
| Eater | 206 | (203, 209) | |||
| Skipper | 198 | (195, 201) | |||
| Order | −7.25 | (−11.5, −2.99) | 0.002 | ||
| Breakfast First | 199 | (196, 201) | |||
| No Breakfast First | 206 | (203, 209) |
- a Values are mean AUC (95% CI), n=18. For outcomes that do not include interactions, estimates, CIs, and P-values are given for each predictor, and least-squares means and CIs are given for each predictor group. For outcomes that do include interactions, least-squares means are given for each day by Skipper/Eater combination rather than for day groups and Skipper/Eater groups separately.
- b AUC, area under curve; EE, energy expenditure; RQ, respiratory quotient.
Carbohydrate and fat oxidation
There was an interaction between prelunch RQ AUC and habitual breakfast pattern, such that the AUC was lower on the B day for Skippers as compared to Eaters (Table 5). Prelunch RQ AUC was higher overall on the B as compared with NB day. Postlunch RQ AUC was lower in Skippers as compared to Eaters under both conditions. There was a significant order effect for both prelunch and postlunch RQ AUC, with higher values for both if the NB day was first.
Discussion
This randomized, cross-over trial was designed to investigate the hormonal, metabolic, and appetitive effects of breakfast skipping in overweight women, and to identify potential differences between habitual breakfast skippers and breakfast eaters. Our primary findings suggest that the effects of breakfast skipping differ depending on habitual breakfast pattern. Eaters demonstrated greater insulin and FFA responses to lunch after skipping breakfast, reported greater hunger before lunch, and had lower rates of fat oxidation after lunch on both days.
While the glucose response was higher after skipping breakfast for both Eaters and Skippers, the insulin and FFA responses were higher only in Eaters. Two other studies done in breakfast eaters have shown that skipping breakfast for 1 day (18) or 14 days (17) resulted in an increased glucose and insulin response to a test meal. One of these studies also measured FFA and found an increased FFA response, similar to our finding of increased FFA in Eaters on the NB day (18). The higher FFA levels prior to lunch on the NB day were expected as subjects were fasting and were therefore undergoing lipolysis. FFA are widely believed to be involved in the causal association between obesity and insulin resistance, though the mechanisms have not been fully elucidated (26, 27). The “second meal phenomenon,” or the observation that the rise in blood glucose is less after the second of two similar meals (in both diabetics and nondiabetics), has been previously studied and found to be associated with enhanced muscle glycogen storage and appears to be determined by suppression of plasma FFA's (28). However, the lack of increased insulin and FFA response after breakfast skipping in habitual breakfast skippers is a novel finding. As the B day represents the typical diet pattern for Eaters, it is possible that the NB condition causes a greater increase in lipolysis and subsequent FFA levels, thus leading to higher insulin levels following lunch. In contrast, the NB day for Skippers represents their typical pattern, so they do not exhibit the same changes in glucose metabolism.
Skipping breakfast was also found in this study to result in changes in appetite and appetite-related hormones. Both groups reported greater hunger on the NB day, but Eaters reported greater hunger and less satiety as compared to Skippers. Both groups were found to have reduced PYY and GLP-1 responses to lunch after skipping breakfast. Since both hormones are thought to induce satiety (29), it could be hypothesized that the lower levels after lunch on the NB day would lead to decreased satiety and increased food consumption later in the day. However, our results showed that the calories consumed at breakfast were compensated for over the course of the day, resulting in similar EI on both days. It is possible that this finding is a product of the study design, as subjects were required to consume the same lunch meal in the same amounts on both study days. Since the greatest difference in appetite occurred at the prelunch time point, it is likely that any potential change in EI would have occurred at the lunch meal if subjects had been allowed ad libitum food intake.
Our study also examined the effects of breakfast skipping on EE and measures of carbohydrate and fat oxidation. Skipping breakfast was found to result in lower EE overall, with the difference being driven mainly by the prelunch values. The higher prelunch EE on the B day is attributable to the thermic effect of food (TEF) after breakfast, because other variables are kept constant on the two testing days, and TEF has been shown to last up to 6 h (30). Postlunch EE was also slightly increased on the B day, suggesting possible continued effects on postlunch TEF related to breakfast consumption (although this difference was small). Skipping breakfast was found to result in a lower RQ, with the difference driven by prelunch values. This suggests greater fat oxidation on the NB day, likely due to ongoing lipolysis. Of interest, we found that Skippers had a lower prelunch RQ on the B day and lower postlunch RQ on both days, suggesting greater fat oxidation, consistent with the slightly higher FFA in Skippers on B days. We did find evidence of an order effect, such that both pre- and postlunch RQ were lower if the B day was first. It is possible that the combination of new experimental procedures (eating breakfast in an experimental setting, along with the first experience of repeated indirect calorimetry measurements) caused greater anxiety and release of catecholamines, leading to lipolysis and lower RQ, as it has been shown that anxiety affects measurements using indirect calorimetry (31)
Perhaps the most novel finding of the present study is that the adverse effects of breakfast skipping were seen only in habitual breakfast eaters. This suggests a “metabolic and behavioral memory” for usual eating patterns. It has been shown that circadian rhythms and eating patterns are related, but it has not been demonstrated whether eating patterns drive circadian rhythms or whether the reverse occurs (32, 33). Similar to our findings, patterns of increased insulin and FFA have been seen in studies of circadian disruption (34, 35). Thus, it is possible that breakfast skipping represents a disruption to usual patterns in the Eaters, thus resulting in changes in glucose metabolism, but that habitual Skippers have adapted to this pattern and therefore do not show such changes. It is interesting to note that Skippers did not react adversely to eating breakfast, even though this would also represent a disruption to their usual eating pattern, and this may represent an area of future study.
There are several limitations to this study that should be acknowledged. First, we have studied only a small group of overweight women, so findings may not be applicable to men or individuals of normal weight. However, since the majority of Americans are now overweight, it could be argued that our findings apply to a clinically more relevant population. We did not include a true “run-in diet” period prior to the study days, although we did provide a dinner meal prior to each study day to ensure that the energy intake at dinner was the same prior to both study days. Therefore, our findings relate to the acute effects of skipping breakfast, and did not address possible longer-term adaptations. Given our findings in Skippers, it is possible that the metabolic effects seen in Eaters would disappear after a period of adaptation to skipping breakfast. Last, qualitative data regarding reasons for diet pattern choice (Eater vs. Skipper) were not collected in this study. It is possible that such factors might affect outcomes, and should be addressed in future studies.
Further studies are needed to confirm these results in a larger population and to assess longer-term effects of breakfast skipping. Additional studies would also be of benefit from a clinical standpoint. Current recommendations from health care providers and US dietary guidelines advocate for breakfast consumption (36). However, several recent studies have questioned the benefit of breakfast consumption (37-40). This study shows that the adverse effects of skipping breakfast occur only in breakfast eaters, suggesting that there is some adaptation to breakfast skipping. As it is not known whether the beneficial effects of eating breakfast would occur after a longer period of time, it is not certain whether a public health campaign to increase breakfast consumption would be of benefit to breakfast skippers.
In conclusion, this study found that in overweight women, the adverse effects of 1 day of breakfast skipping (increased insulin and FFA response, increased hunger and decreased satiety) were found primarily in the breakfast eaters. These results suggest that habitual food intake patterns entrain metabolic systems in overweight breakfast Eaters such that skipping breakfast results in adverse metabolic consequences.
Acknowledgments
The authors would like to acknowledge and thank the University of Colorado Clinical and Translational Science Institute (CCTSI) as well as the CCTSI Metabolic Kitchen for their support of this study. They would also like to thank the study participants for their time.





