A common variant in the MTNR1b gene is associated with increased risk of impaired fasting glucose (IFG) in youth with obesity
Funding agencies: The authors are grateful to the patients and their families as well as to the Yale Center for Genome Analyses (YCGA) and Yale Center for Clinical Investigation (YCCI) and Hospital research Unit (HRU) personnel. CZ is funded by the National Natural Science Foundation of China (NSFC) (No. 81200630), Natural Science Foundation of Zhejiang Province, China (No. LQ12H07001), and Foundation of Zhejiang Public Health Committee (No.2010KYB073). NS is funded by the American Heart Association (AHA) (13SDG14640038) and by the Yale Center for Clinical Investigation (2012 YCCI scholar award and YCCI just in time grant), SC is funded by NIH (grants R01-HD-40787, R01-HD- 28016) and ADA (Distinguished Clinical Scientist Awards from the American Diabetes Association, DK-49230). This work was also made possible by DK045735 to the Yale Diabetes Research Center and Clinical and Translational Science Awards Grant UL1-RR- 024139 from the National Center for Advancing Translational Sciences, a component of the NIH, and NIH Roadmap for Medical Research.
Disclosure: The authors have no conflicts of interest to disclose.
Author contributions: CZ, NS, and HZ analyzed the data. CDM, CC, BP, ED, MS, AEB and LG researched the data. CZ, SC and NS reviewed the data, wrote and edited the MS. SC and NS are the guarantor of this work and as such had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the analyses.
Abstract
Objective
To explore the role of MTNR1B rs10830963 and G6PC2 rs560887 variants in the pathogenesis of impaired fasting glucose (IFG) in obese adolescents.
Methods
A total of 346 Caucasians, 218 African–Americans, and 217 Hispanics obese children and adolescents underwent an oral glucose tolerance test (OGTT) and 518 underwent the evaluation of insulin secretion by the oral minimal model (OMM). Also, 274 subjects underwent a second OGTT after 3.0 ± 2.1years.
Results
The MTNR1B rs10830963 variant was associated with higher fasting glucose levels and lower dynamic beta-cell response in Caucasians and Hispanics (P < 0.05) and conferred an increased risk of showing IFG to Caucasians (P = 0.05), African–Americans (P = 0.0066), and Hispanics (P = 0.024). Despite the association between the G6PC2 rs560887 and higher fasting glucose levels (P < 0.05), there was no association between this variant and IFG at baseline or at follow-up (all P > 0.10).
Conclusions
It has been shown for the first time in obese youth that the MTNR1B variant is associated with an increased risk of IFG.
Introduction
Paralleling the rise of pediatric obesity, the prevalence of type 2 diabetes is increasing among youths. (1-4). Recent population-based data from the Search for Diabetes in Youth (SEARCH) study indicate that type 2 diabetes is diagnosed in about 3,700 obese youths annually in United States. (5) Onset of type 2 diabetes during adolescence heralds many years of disease and an increased risk of the full range of both micro- and macro-vascular complications that will occur when affected individuals are still relatively young. (6-9) The tempo of progression from prediabetes to full blown diabetes is faster in youth than in adults (10-13), and therefore, it is imperative to identify young subjects at increased risk of type 2 diabetes early when they are in the prediabetic state. Noteworthy, the metabolic abnormalities leading to hyperglycemia are established long before overt diabetes (14) and classified to as prediabetes. Impaired fasting glucose (IFG) is a prediabetic condition defined by the fasting glucose levels between 100 and 125 mg/dl. In the last decade, genomewide association studies (GWAS) have discovered a large number of loci associated with fasting glucose and IFG in adults, (15-18) but to date little information is available regarding the genetic defects predisposing obese adolescents to IFG. The genetic regions showing the strongest association with fasting plasma glucose in both adults and children are those including the rs10830963 in the melatonin receptor 1B gene (MTNR1B) and the rs560887 in the glucose-6-phosphatase 2 gene (G6PC2). (11, 19-21) In particular, a meta-analysis of over 6000 children of European descents showed that MTNR1B rs10830963 and G6PC2 rs560887 are associated with fasting glucose levels in healthy children and adolescents, (21) and this observation was replicated by the European Youth Heart Study in 2025 healthy European children and adolescents. (20) Those two studies showed independently that the effect size of the association of G6PC2 rs560887 and MTNR1B rs10830963 with fasting glucose was 2–3 times larger than that observed for any other variants associated with this trait (20, 21). Moreover, there are functional in vitro studies providing evidence that these variants affect the expression of the two genes, likely driving the association between their genetic regions and the glucose phenotype (22, 23). Despite these studies, so far no data showing the relationship between those loci and IFG in a pediatric cohort is available. Therefore, in our study, we sought to determine (1) whether the MTNR1B rs10830963 and the G6PC2 rs560887 variants might predispose obese youth to IFG; (2) and whether the associations might be mediated through alterations in beta-cell function examined by the oral minimal model (OMM).
Methods
Study cohort
Subjects were recruited from a multiethnic cohort participating in the Yale Pathophysiology of Type 2 Diabetes Study, a long-term project aimed at studying early alterations in glucose metabolism in obese children and adolescents. All subjects needed to be obese and not to take medications that affect glucose metabolism when enrolled. The study was approved by the Human Investigations Committee of the Yale University School of Medicine. Parental informed consent and child assent were obtained from all participants. We recruited 781 obese children and adolescents with a BMI greater than the 85th percentile (319 boys, 462 girls; mean age 13.4 ± 3.6 years; mean z-score BMI 2.35 ± 0.37) referred to the Yale Pediatric Obesity Clinic, no lean individuals were included in the study. The study population consisted of the following three ethnicities/races: 346 Caucasians (212 girls), 218 African–Americans, (129 girls), and 217 Hispanics (121 girls). Since puberty is accompanied by a transient state of insulin resistance, (24) the pubertal status was assessed according to Tanner (25) and pubertal stage was included in the analyses. All of them underwent a 3-h oral glucose tolerance test (OGTT), and 533 subjects showed normal glucose tolerance (NGT), 63 IFG, 60 IFG + impaired glucose tolerance (IGT), and 125 IGT.
To evaluate the effect of the gene variants on the risk of developing prediabetes over time, 274 subjects underwent a second OGTT after a follow-up of 3.0 ± 2.2 years. During the follow-up, all participants received standard nutritional guidance and recommendations for physical activity and were not on any medications affecting glucose metabolism. The study population followed up was constituted by 101 Caucasians, 82 African–Americans, and 91 Hispanics; 101 boys and 173 girls (mean age 12.4 ± 2.8 years, mean z-score BMI 2.32 ± 0.50). Of them, at baseline 142 were NGT, 28 showed IFG, 38 had IFG + IGT and 66 IGT. The time interval for the follow-up was based on our previous study suggesting that changes in categories of glucose tolerance in obese adolescents are likely to occur over a relatively short period of time. (13, 26). The procedures are described in the Supporting Information.
Statistical analyses
Distribution of continuous variables was examined for skewness and when appropriate data were statistically transformed to approximate univariate normality before association analyses by inverse normal scores. A Chi square test was used to assess the Hardy–Weinberg equilibrium for each of the studied SNPs and to compare proportions.
The primary outcome of the study was isolated IFG. At baseline, the odds ratio of showing prediabetes according to the genotypes was evaluated by a logistic regression analysis and age, gender, pubertal stage, and z-score BMI were used as covariates. The risk of NGT subjects to progress to prediabetes or T2D at follow-up was calculated by running a Cox regression analysis and including as covariates age, gender, pubertal stage, ethnicity, z-score BMI at baseline, delta z-score BMI, and follow-up time. The association between the genotypes and quantitative traits was evaluated by a regression model after coding the genotype with an additive model of inheritance, that is, the genotype is coded with 0, 1, or 2 corresponding to the number of minor alleles carried by each individual and age, sex, pubertal stage, z-score BMI, and glucose tolerance status were used as covariates when appropriate. Statistical power and sample size was assessed using the “Genetic Power Calculator” available at http://pngu.mgh.harvard.edu/~purcell/gpc/cc2.html. All data were expressed as mean ± standard deviations (SD). Statistical analyses were performed with SPSS (19.0 for Windows, SPSS Inc., Chicago, IL).
Results
Association between MTNR1B rs10830963 and fasting glucose, insulin secretion and IFG
The frequency of the MTNR1B rs10830963 minor allele (G) was 0.262 in Caucasians, 0.091 in African–Americans, and 0.215 in Hispanics (P < 0.0001). The allele frequencies were consistent with those shown in similar ethnic groups in HAPMAP (http://hapmap.ncbi.nlm.nih.gov/). Within each ethnic group, there was no evidence against the null hypothesis that the genotype distribution was in Hardy–Weinberg equilibrium (all P > 0.05). The clinical features of the study population according to the MTNR1B rs10830963 genotype are shown in Table 1. Subjects carrying the minor allele for the rs10830963 in MTNR1B showed higher fasting glucose levels in Caucasians (P = 0.005), and Hispanics (P = 0.0001) independent of age, gender, pubertal stage, and z-score BMI, and the same trend was observed in African–Americans (P = 0.090) (Figure 1A). Of note, the Φd, evaluated with OMM, was significantly decreased in subjects carrying the risk allele (Figure 1B). The MTNR1B rs10830963 was associated with IFG in Caucasians (OR = 2.098; 95% CI: 0.983-4.481, P = 0.055), African–Americans (OR = 3.724; 95% CI: 1.360-10.119, P = 0.0105), and Hispanics (OR = 2.497; 95% CI: 1.009-6.176, P = 0.040).
| Caucasians | African-Americans | Hispanics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CG | GG | Beta (SE) | P | CC | CG | GG | Beta (SE) | P | CC | CG | GG | Beta (SE) | P | |
| N | 184 | 135 | 22 | 178 | 33 | 3 | 129 | 73 | 9 | ||||||
| Age (years) | 14.3 ± 3.9 | 13.3 ± 3.4 | 14.1 ± 2.7 | - | 0.03 | 13.3 ± 3.8 | 13.5 ± 2.7 | 13.6 ± 0.8 | - | 0.97 | 12.4 ± 3.5 | 12.7 ± 3.2 | 13.8 ± 2.5 | - | 0.43 |
| Sex (M/F) | 64/120 | 61/74 | 7/15 | - | 0.13 | 71/107 | 15/18 | 2/1 | - | 0.55 | 56/73 | 33/40 | 3/6 | - | 0.79 |
| z-score BMI | 2.31 ± 0.37 | 2.31 ± 0.37 | 2.25 ± 0.28 | - | 0.79 | 2.43 ± 0.39 | 2.48 ± 0.35 | 2.60 ± 0.25 | - | 0.61 | 2.37 ± 0.33 | 2.34 ± 0.40 | 2.09 ± 0.37 | - | 0.13 |
| †F. glucose (mg/dl) | 89.9 ± 6.6 | 91.0 ± 8.8 | 92.5 ± 11.3 | 1.97 (0.69) | 0.005 | 91.4 ± 8.2 | 94.0 ± 9.3 | 102.2 ± 3.8 | 3.27 (1.37) | 0.09 | 91.1 ± 7.4 | 95.2 ± 8.0 | 96.1 ± 8.3 | 3.59 (0.90) | 0.0001 |
| †2-h glucose (mg/dl) | 120.2 ± 26.6 | 123.5 ± 25.9 | 119.5 ± 25.9 | 1.69 (2.27) | 0.66 | 120.0 ± 26.6 | 122.7 ± 26.6 | 139.0 ± 26.2 | 5.20 (4.26) | 0.18 | 118.2 ± 22.6 | 124.7 ± 20 | 133.3 ± 31 | 7.20 (2.61) | 0.06 |
| †HbA1c (%) | 5.36 ± 0.30 | 5.42 ± 0.31 | 5.44 ± 0.29 | 0.05 (0.03) | 0.29 | 5.59 ± 0.37 | 5.59 ± 0.34 | 5.93 ± 0.32 | 0.04 (0.07) | 0.66 | 5.43 ± 0.31 | 5.51 ± 0.30 | 5.40 ± 0.46 | 0.06 (0.04) | 0.28 |
| †Insulin (µU/mL) | 34.2 ± 18.1 | 30.8 ± 17.0 | 31.1 ± 18.8 | −1.44(1.41) | 0.08 | 35.5 ± 20.3 | 37.2 ± 18.5 | 44.7 ± 34.3 | 1.35 (2.88) | 0.80 | 33.6 ± 18.9 | 33.8 ± 16.4 | 28.8 ± 3.8 | −0.24 (2.12) | 0.39 |
| †WBISI (l2/mg/µU) | 1.89 ± 1.25 | 1.86 ± 0.94 | 1.92 ± 1.19 | −0.14(0.09) | 0.61 | 1.86 ± 1.21 | 1.58 ± 0.96 | 1.44 ± 0.57 | −0.29 (0.19) | 0.36 | 1.78 ± 0.96 | 1.81 ± 1.20 | 1.51 ± 0.39 | 0.028 (0.1) | 0.54 |
| †IGI (µU/mg) | 4.32 ± 3.86 | 4.26 ± 3.81 | 3.79 ± 2.67 | −0.002(0.3) | 0.72 | 5.56 ± 4.15 | 6.32 ± 3.85 | 5.69 ± 5.49 | −0.11 (0.91) | 0.74 | 5.03 ± 4.14 | 3.69 ± 2.40 | 3.26 ± 1.34 | −1.18 (0.43) | 0.01 |
| †DI (l2/mg2) | 5.87 ± 3.59 | 6.37 ± 4.86 | 5.97 ± 3.97 | 0.10 (0.52) | 0.34 | 8.66 ± 5.83 | 8.40 ± 6.16 | 5.97 ± 3.57 | 0.67 (1.09) | 0.47 | 6.88 ± 3.96 | 5.87 ± 3.81 | 4.22 ± 2.47 | −1.52 (0.63) | 0.005 |
| Oral minimal model | |||||||||||||||
| N | 128 | 91 | 14 | 114 | 21 | 1 | 89 | 48 | 7 | ||||||
| †Φb (10−9 min−1) | 15.8 ± 6.5 | 13.5 ± 5.8 | 15.7 ± 5.5 | −0.59(0.61) | 0.36 | 12.4 ± 6.0 | 15.2 ± 5.0 | 14.0 | 2.24 (1.14) | 0.48 | 14.6 ± 5.3 | 12.3 ± 4.6 | 14.1 ± 3.0 | −1.53 (0.63) | 0.02 |
| †Φd (10−9 min−1) | 1585 ± 600 | 1358 ± 819 | 1205 ± 636 | −188 (97.5) | 0.04 | 1158 ± 106 | 1089 ± 238 | 1221 | −12.6 (206) | 0.39 | 1786 ± 600 | 1275 ± 796 | 1249 ± 475 | −431 (326) | .0009 |
| †Φs(10−9 min−1) | 74.6 ± 42.4 | 75.9 ± 40.5 | 85.1 ± 31.9 | 3.95 (4.55) | 0.19 | 80.2 ± 57.3 | 65.7 ± 38.3 | 47.8 | −14.2 (11.9) | 0.29 | 88.7 ± 56.5 | 72.1 ± 58.4 | 78.9 ± 21.5 | −14.4 (7.7) | 0.02 |
| †Φtotal (10−9 min−1) | 92.8 ± 47.4 | 95.6 ± 56.2 | 101.3 ± 39.4 | 4.89 (5.51) | 0.27 | 102.6 ± 62.3 | 88.5 ± 46.8 | 59.2 | −14.6 (12.9) | 0.17 | 109.0 ± 61.3 | 90.5 ± 71.4 | 92.6 ± 25.7 | −16.1 (8.8) | 0.01 |
| †SI (10-5dL·kg-1·min-1/pmol·L-1) | 21.6 ± 16.2 | 21.6 ± 15.1 | 15.1 ± 12.6 | −1.40 (1.9) | 0.17 | 24.8 ± 19.6 | 16.0 ± 14.6 | 6.23 | −7.96 (3.73) | 0.02 | 19.2 ± 12.2 | 25.2 ± 15.4 | 23.2 ± 1.8 | 3.24 (2.37) | 0.07 |
| †DI total (10−14 dL·kg1·min−1/pmol·L−1) | 3206 ± 2837 | 3403 ± 3330 | 2909 ± 3854 | 7.80 (383) | 0.36 | 3891 ± 3791 | 1524 ± 762 | 610 | −1912 (863) | 0.002 | 3928 ± 5422 | 4760 ± 8166 | 3559 ± 2272 | 190 (926) | 0.99 |
- a The data have been inverse normal transformed before running the analyses and the P values are adjusted for age, sex, Tanner stage, and z-score BMI. Beta and standard error (SE) values are based on raw phenotype values adjusted for age, sex, Tanner stage, and z-score BMI. The data are shown as mean and standard deviation.
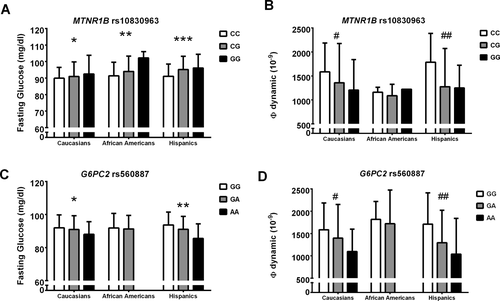
Fasting glucose and (Φ) dynamic according to the genotypes in the three ethnic groups.
(1A) Fasting glucose levels (mg/dl) according to the MTNR1B rs10830963 genotype. * P = 0.005, ** P = 0.090 and ***P = 0.0001. (1B) (Φ) dynamic (10−9) according to the MTNR1B rs10830963 genotype. # P = 0.040, ## P = 0.0009. (1C) Fasting glucose levels (mg/dl) according to the G6PC2 rs560887 genotype. *P = 0.008, **P = 0.011. (1D) (Φ) dynamic (10−9) according to the G6PC2 rs560887 genotype. # P = 0.013, ## P = 0.044.
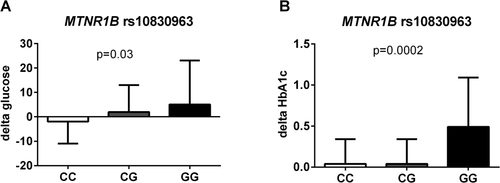
Changes over time of fasting glucose and HbA1c%.
The figure shows the delta fasting glucose (A) and delta HbA1c (B) according to the MTNR1B rs10830963 genotype. The minor allele of the MTNR1B rs10830963 was associated with a statistically significant higher delta fasting glucose (P = 0.039) and delta HbA1c% (P = 0.0002) independent of age, gender, ethnicity, z-score BMI at baseline and changes in z-score BMI, follow-up time, and baseline levels of fasting and 2-h glucose.
Association between the MTNR1B rs10830963 and the other categories of glucose tolerance
In Caucasians, the MTNR1B rs10830963 was associated with a higher risk of showing IFG and IGT together (OR = 3.118; 95% CI: 1.413-6.883, P = 0.0049), and there was a trend toward an association with the risk of isolated IGT (OR = 1.456; 95% CI: 0.989-2.537, P = 0.08). In African–Americans, the MTNR1B rs10830963 was also associated with a higher risk of showing isolated IGT (OR = 3.286; 95% CI: 1.203-8.980, P = 0.020), but there was no association between the MTNR1B variant and the IFG + IGT in African–Americans (OR = 2.140; 95% CI: 0.656-6.982, P = 0.20), and Hispanics (OR = 1.301; 95% CI: 0.595-2.846, P = 0. 51).
Association between the G6PC2 rs560887 and fasting glucose, insulin secretion and categories of glucose tolerance
The frequency of the G6PC2 rs560887 minor allele (T) was 0.276 in Caucasians, 0.066 in African–Americans, and 0.165 in Hispanics (P< 0.0001). The allele frequencies were consistent with those shown in similar ethnic groups in HAPMAP (http://hapmap.ncbi.nlm.nih.gov/). Within each ethnic group, there was no evidence against the null hypothesis that the genotype distribution was in Hardy–Weinberg equilibrium (all P > 0.05). The clinical features of the study population according to the G6PC2 rs560887 genotype are shown in Table 2. For the G6PC2 rs560887, the risk allele was the major allele that was significantly associated with higher fasting glucose levels in Caucasians (P = 0.008) and Hispanics (P = 0.011) independent of age, gender, and BMI (Figure 1C). The HbA1c was slightly increased in subjects carrying the risk allele in Caucasians and Hispanics (P = 0.075 and P = 0.0073, respectively). Further, subjects carrying the risk allele for the G6PC2 rs560887 showed increased IGI and DI in Caucasians (P = 0.048 and P = 0.021, respectively) and Hispanics (P = 0.041 and P = 0.071, respectively), independent of age, pubertal stage, sex, and z-score BMI (Table 2). The G6PC2 rs560887 risk allele was associated with an increased Φd in Caucasians and Hispanics (P = 0.013 and 0.044, respectively) (Figure 1D) and an increased Φs (P = 0.0013) in Hispanics (Table 2). There was no association between the G6PC2 rs560887 and isolated IFG or IFG + IGT or isolated IGT in any ethnic groups (all P > 0.05). To assess whether the lack of association between the G6PC2 SNP and IFG might be due to the sample size, we calculated the power to detect the association in the whole population. We assumed a risk allele frequency of 80%, a case–control ratio of 10:1, and a prevalence of 10%. A multiplicative model was used where the heterozygous genotype was assumed to have a relative disease risk of 1.2, whereas a homozygous individual was assumed to have a relative disease risk of 1.44. At the statistical significance level of 0.05, 735 cases and 7350 controls would be required to have a power of 80%. With a sample size of 50 cases and 450 controls, the power is 34% to detect a relative risk of 1.5, and 68% to detect a relative risk of 2.
| Caucasians | African-Americans | Hispanics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | Beta (SE) | p | CC | CT/TT | Beta (SE) | P | CC | CT | TT | Beta (SE) | P | |
| N | 181 | 124 | 31 | 184 | 26/1 | 142 | 58 | 5 | ||||||
| Age (years) | 13.6 ± 3.4 | 14.2 ± 3.9 | 14.8 ± 4.2 | - | 0.11 | 13.5 ± 3.8 | 12.8 ± 2.7 | - | 0.30 | 12.6 ± 3.2 | 12.5 ± 37 | 13.1 ± 3.7 | - | 0.94 |
| Sex (M/F) | 69/112 | 51/73 | 10/21 | - | 0.64 | 73/111 | 13/14 | - | 0.39 | 65/77 | 24/34 | 5/0 | - | 0.01 |
| z-score BMI | 2.33 ± 0.34 | 2.28 ± 0.40 | 2.29 ± 0.38 | - | 0.44 | 2.44 ± 0.39 | 2.45 ± 0.34 | - | 0.87 | 2.35 ± 0.35 | 2.34 ± 0.38 | 2.58 ± 0.26 | - | 0.89 |
| ‡F. glucose (mg/dl) | 92.0 ± 7.8 | 91 ± 8.3 | 88 ± 7.7 | 1.46 (0.65) | 0.008 | 91.9 ± 8.8 | 91.3 ± 8.2 | −0.51 (1.67) | 0.88 | 93.7 ± 7.8 | 91.1 ± 7.8 | 85.5 ± 8.9 | 2.59 (1.03) | 0.01 |
| ‡2-h glucose (mg/dl) | 121.6 ± 26.7 | 122.7 ± 26.4 | 118.8 ± 25.8 | −0.34 (2.15) | 0.79 | 120.2 ± 27.0 | 120.9 ± 24.5 | −0.22 (5.19) | 0.71 | 121.2 ± 22.1 | 121.5 ± 23.9 | 113.6 ± 27.4 | 0.60 (2.98) | 0.74 |
| ‡HbA1c (%) | 5.41 ± 0.31 | 5.38 ± 0.32 | 5.31 ± 0.28 | 0.03 (0.02) | 0.075 | 5.60 ± 0.37 | 5.61 ± 0.31 | 0.003 (0.07) | 0.83 | 5.49 ± 0.34 | 5.37 ± 0.25 | 5.30 ± 0.24 | 0.10 (0.04) | 0.007 |
| ‡ Insulin (µU/mL) | 33.8 ± 16.9 | 31.4 ± 17.3 | 39.0 ± 24.0 | −0.84 (1.34) | 0.69 | 35.3 ± 20.6 | 39.3 ± 16.6 | −1.11 (3.49) | 0.46 | 33.6 ± 16.9 | 33.2 ± 18.9 | 25.3 ± 6.6 | −3.89 (3.27) | 0.62 |
| ‡WBISI (l2/mg/µU) | 1.83 ± 1.18 | 1.97 ± 1.11 | 1.67 ± 0.89 | 0.004 (0.09) | 0.94 | 1.87 ± 1.19 | 1.55 ± 1.00 | 0.22 (0.23) | 0.25 | 1.77 ± 1.07 | 1.78 ± 0.93 | 2.06 ± 0.54 | 0.059 (0.14) | 0.91 |
| ‡IGI (µU/mg) | 4.79 ± 4.37 | 3.63 ± 2.76 | 3.41 ± 3.15 | 0.67 (0.30) | 0.048 | 5.78 ± 4.23 | 6.08 ± 3.34 | 0.34 (1.10) | 0.92 | 4.80 ± 3.78 | 4.00 ± 3.21 | 2.64 ± 1.59 | 0.63 (0.34) | 0.04 |
| ‡DI (l2/mg2) | 7.07 ± 6.00 | 5.57 ± 3.08 | 4.89 ± 2.73 | 1.39 (0.48) | 0.021 | 8.72 ± 6.05 | 7.99 ± 4.46 | 0.90 (1.33) | 0.70 | 7.26 ± 5.36 | 5.93 ± 4.43 | 4.83 ± 2.68 | 0.82 (0.73) | 0.07 |
| Oral minimal model | ||||||||||||||
| N | 124 | 92 | 19 | 119 | 17/0 | 99 | 41 | 4 | ||||||
| ‡Φb (10−9 min−1) | 15.4 ± 6.9 | 13.8 ± 5.2 | 16.8 ± 6.4 | 0.12 (0.58) | 0.65 | 12.4 ± 6.0 | 15.2 ± 5.0 | 0.80 (1.38) | 0.43 | 14.2 ± 4.8 | 12.3 ± 4.9 | 13.6 ± 3.3 | 1.16 (0.68) | 0.09 |
| ‡Φd (10−9 min−1) | 1585 ± 600 | 1398 ± 754 | 1099 ± 500 | 196.8 (91.8) | 0.01 | 1158 ± 106 | 1089 ± 238 | 14.9 (249) | 0.68 | 1711 ± 700 | 1294 ± 728 | 1040 ± 801 | 267.2 (149.0) | 0.04 |
| ‡Φs(10−9 min−1) | 79.8 ± 45.7 | 71.6 ± 36.5 | 70.0 ± 26.9 | 6.53 (4.29) | 0.13 | 80.2 ± 57.3 | 65.7 ± 38.3 | −2.85 (15.0) | 0.52 | 93.3 ± 65.2 | 63.2 ± 27.3 | 53.9 ± 15.1 | 25.9 (8.9) | 0.001 |
| ‡Φtotal (10−9 min−1) | 95.4 ± 50.1 | 95.2 ± 53.6 | 82.5 ± 32.0 | 2.96 (5.22) | 0.26 | 102.6 ± 62.3 | 88.5 ± 46.8 | 3.69 (15.7) | 0.67 | 116.2 ± 72.9 | 73.6 ± 30.3 | 64.1 ± 22.5 | 32.3 (10.1) | 0.0004 |
| ‡SI (10-5dL·kg-1·min-1/pmol·L-1) | 24.3 ± 20.7 | 25.9 ± 19.8 | 18.8 ± 10.7 | 0.89 (1.77) | 0.55 | 24.8 ± 19.6 | 16.0 ± 14.6 | 4.37 (4.62) | 0.58 | 23.7 ± 17.8 | 25.9 ± 17.9 | 28.6 ± 22.2 | −1.32 (2.64) | 0.64 |
| ‡DI total (10−14 dL·kg1·min−1/pmol·L−1) | 3642 ± 3574 | 4001 ± 3572 | 2281 ± 1502 | 272 (354) | 0.90 | 3891 ± 3791 | 1524 ± 762 | 696 (1080) | 0.59 | 5371 ± 7603 | 2897 ± 2364 | 4404 ± 2996 | 2039 (1054) | 0.09 |
- a The data have been inverse normal transformed before running the analyses and the P values are adjusted for age, sex, Tanner stage, and z-score BMI. Beta and standard error (SE) values are based on raw phenotype values adjusted for age, sex, Tanner stage, and z-score BMI. The data are shown as mean and standard deviation.
Follow-up cohort
The cohort of 274 subjects followed up did not differ from the main cohort for age, gender, ethnicity, and z-score BMI. Among them, 142 showed NGT, 28 isolated IFG, 38 IGT + IFG, and 66 isolated IGT. The three ethnic groups did not differ for fasting, 2-h glucose or disposition index at baseline. The main baseline clinical characteristics of the subjects according to the genotypes who performed the longitudinal follow-up are reported in Supporting Information Table 1.
For the MTNR1B rs10830963 variant, 175 subjects were CC, 91 CG, and eight were GG. The three groups of genotype did not differ for follow-up time (CC = 3.14 ± 2.29, CG = 3.21 ± 2.63, GG = 3.27 ± 1.75 P = 0.95). The minor allele of the MTNR1B rs10830963 was associated with a statistically significant higher delta fasting glucose (CC = −1.92 ± 9.58, CG = −1.96 ± 11.9, GG = 5.06 ± 18.1 P = 0.032) and delta HbA1c% (CC = 0.04 ± 0.32, CG = 0.04 ± 0.31, GG = 0.49 ± 0.62 P = 0.0002) independent of age, gender, ethnicity, z-score BMI at baseline and changes in z-score BMI, follow-up time, and baseline levels of fasting and 2-h glucose (Figure 2). It is to be noted that the MTNR1B variant showed a trend toward higher risk of transitioning from NGT to isolated IFG (HR = 2.131; 95% CI: 0.903-5.079 P = 0.067) and was associated with a significant higher risk to develop IFG + IGT, IGT, or T2D (HR = 2.595; 95% CI: 1.125-5.449, P = 0.013) independent of age, gender, ethnicity, pubertal stage, z-score BMI, baseline fasting plasma glucose, delta z-score BMI. Concerning the G6PC2 rs560887 SNP, 198 subjects were CC, 65 were CT, and 11 were TT. The G6PC2 variant was not associated with any significant changes in fasting glucose and HbA1c over time (P = 0.17 and P = 0.43, respectively). Furthermore, NGT subjects homozygous for the rs560887 risk allele did not show a higher risk of developing pre-diabetes at follow-up (HR = 1.004; 95% CI: 0.376-2.528, P = 0.87).
Discussion
The current study provides, for the first time, insights into the genetic predisposition to IFG of obese adolescents. Herein, we show that the risk alleles of the rs10830963 in the MTNR1B gene and of the rs560887 in the G6PC2 gene are associated with increased levels of fasting glucose, but while the risk allele of the MTNR1B rs10830963 is associated with a lower dynamic beta-cell responsivity, the G6PC2 rs560887 risk allele is associated with an increased dynamic beta-cell responsivity. Further, while the MTNR1B rs10830963 SNP carries an increased risk of showing and developing IFG, this is not observed for the G6PC2 rs560887. These data suggest that (1) the two glucose raising alleles might lead to high fasting glucose levels via different mechanisms and that (2) the association between the two risk alleles and the fasting glucose have a different clinical relevance, with the MTNR1B variant being a potential trigger for prediabetes in obese youth.
The MTNR1B rs10830963 is a single-nucleotide polymorphism (SNP) located in the single intron of the MTNR1B gene, which is a two exons gene in the long arm of chromosome 11 (11q21-q22). The MTNR1B gene encodes one of the two known human melatonin receptors, the MT2, and it is highly expressed in the beta cells. Previous studies have shown that the MTNR1B allele is involved in the regulation of insulin secretion; in fact, animal and in vitro experiments showed that the treatment of pancreatic beta cells with melatonin attenuates insulin secretion and that exogenously administered melatonin inhibits insulin secretion in rodents. (27) Given these observations, some authors have hypothesized that the risk allele of the rs10830963 variant may affect insulin secretion by causing an increased expression of the MT2 on the beta-cell membrane, (22) which in turn results in a decreased cAMP-dependent signaling and ultimately in a reduced insulin secretion. Moreover, consistent with previous studies (22, 28-30) herein, we observed that subjects carrying the MTNR1B rs10830963 minor allele tend to have a lower Φd, which is a measure of the dynamic beta-cell responsivity, whose impairment is a key feature of IFG, along with hepatic insulin resistance. (6)
As we observed quite a large effect size for the association between the MTNR1B SNP and fasting glucose, we reviewed some previous studies aimed at assessing the same association to see how different our observation is from those. Interestingly, studies in obese children (31) and adults with European ancestries including also subjects with type 2 diabetes (22) showed an effect size larger than that observed in our population of obese youth of European ancestries. In fact, the effect size observed in our group of obese individuals of European ancestries (after converting the glucose in mmol/L to compare our results with those obtained in previous studies) is beta (β) = 0.10 and standard error (SE) = 0.038, similar to that observed in obese children (β = 0.20; SE 0.038) (31) or in adults (β = 0.16; SE 0.022). (22) When we analyzed the studies dealing with the association between the MTNR1B and fasting glucose in African–American and Hispanics, the effect size was much larger in the group of African–American and Hispanic youth in our study than in adults. (17, 32) This is interesting, because obese kids belonging to these two ethnic groups are at high risk of developing prediabetes and type 2 diabetes. (33)
The mechanisms underlying the association between the G6PC2 variant and fasting glucose, so far, are poorly understood. In fact, consistent with previous studies (19, 34), we observed that the G6PC2 rs560887 risk allele is associated with higher glucose levels and higher rate of insulin secretion. This paradox is quite difficult to explain, because any increase in insulin secretion should lead to a decrease in glucose levels unless the insulin-targeted tissues are more resistant or the insulin molecule is less active. In our cohort, insulin sensitivity was similar among the G6PC2 genotypes in each ethnic group (Table 2), but the OGTT derived WBISI, used to assess whole body insulin sensitivity, does not allow to discriminate the degree of insulin resistance in different tissues, and therefore, we cannot exclude an effect of the G6PC2 variant on hepatic insulin resistance. In a previous study in adults, though, the authors showed that subjects carrying the risk genotype have a higher hepatic glucose production, (35) but the hypothesis of an impairment of hepatic insulin sensitivity is not consistent with the fact that the G6PC2 gene is expressed exclusively in the beta cell. In particular, the G6PC2 gene encodes for the glucose-6-phosphatase, which catalyzes the hydrolysis of glucose-6-phosphate to glucose and inorganic phosphate thereby reducing the glycolytic flux and opposing the action of the glucokinase. In the beta cell, this effect translates in a negative regulation of the glucose stimulated insulin secretion. (16, 36, 37) The major allele (C) of the rs560887 variant, which is located within the third intron of the gene, has been demonstrated to lead to an increase in the G6PC2 expression, (23) which should result in a reduction of glycolytic flux and a reduced insulin secretion with a consequent increase of glucose levels, but this hypothesized scenario contrasts with the phenotype observed in subjects carrying the risk allele. Therefore, it is possible that this gene variant impairs insulin synthesis and/or function, or is in linkage disequilibrium with another locus modulating glucose levels by affecting hepatic insulin sensitivity. Moreover, we did not observe an association between the G6PC2 variant and prediabetes, and this might mean that the effect of the rising glucose genotype is not strong enough to confer susceptibility to prediabetes, as it has been suggested also by studies in adults showing no effect of the G6PC2 rs560887 glucose rising allele on the development of type 2 diabetes. (38, 39) The lack of an association between the G6PC2 variant and the IFG in our study might be due to the fact that our study is underpowered to detect the association between the G6PC2 variant and the IFG; in fact, we calculated that to detect the association between the G6PC2 variant and IFG in our cohort a sample size of about 8000 subjects would be needed. The need of such a large samples size just corroborates the fact that the effect size of the G6PC2 risk allele on the risk of showing IFG might be quite low.
We compared the effect size for the association between the G6PC2 rs560887 and fasting glucose observed in our study with the ones observed in previous studies done in adults and children. Interestingly, the effect size of the association between the G6PC2 SNP and fasting glucose in our group of obese youth of European ancestries was comparable to that found in other pediatric groups, in fact, our effect size, after converting glucose in mmol/L was β = 0.073; 95% CI: 0.058-0.088 and was similar to that found in the study by Barker et al. (21) The effect size observed in adults of European ancestries was β = 0.076. (40) Also, the effect size of the association between G6PC2 SNP and fasting glucose was higher in our group of Hispanic patient (β = −2.63; SE = 1.059) compared to that seen Hispanic adults (β = −1.60; SE = 0.61). (18) Although we cannot make any definitive statement, it is possible that the association between the G6PC2 rs560887 and fasting glucose is stronger in children than in adults. Furthermore, it is interesting to note that in our study the group of Hispanic children showed a larger effect size than African–Americans and Caucasians for the association between fasting glucose and some of the insulin secretion indexes for both MTNR1B and G6PC2 genotypes. Despite that, we did not find any statistically significant interaction, meaning that the effect sizes are not statistically different among the three ethnic groups; however, this negative result could be due the small sample size.
We acknowledge that this study has some limitations. In particular, the major limitation is the samples size, but it has to be kept in mind that metabolic studies are very expensive for the investigators and demanding for the children. In fact, children undergoing the studies lose a day of school and the parents a day of work, and also, the children have to be fasting for more than 12 h before the study. In light of our sample size, we acknowledge that our data need to be interpreted with caution, in particular some associations for the nominal significance of G6PC2 variant, could be a false-positive association caused by multiple testing for more than 10 traits tested in three different populations.
The strengths of our study are the young age of our patients, the assessment of the dynamic measures of insulin secretion evaluated by the oral minimal model (OMM), and the follow-up component.
In conclusion, this is the first study showing in obese children and adolescents that the MTNR1B rs10830963 and the G6PC2 rs560887 variants are associated with alterations of fasting glucose levels and insulin secretion and that they probably have a different clinical relevance. In fact, while the MTNR1B rs10830963 variant is associated with the risk of developing prediabetes, the effect of the G6PC2 rs560887 on the risk of prediabetes in obese youth might be negligible, but further studies in larger cohorts of obese youth are needed to confirm these findings.





