Glomerular filtration rate and cardiometabolic risk in an outpatient pediatric population with high prevalence of obesity
DISCLOSURE: The authors have no competing interests.
Abstract
Objective
To evaluate the relationship between estimated glomerular filtration rate (eGFR) and cardiometabolic risk factors (CMRF) in an outpatient pediatric population with high prevalence of obesity.
Design and Methods
eGFR was evaluated in 901 children unselected for chronic kidney disease of whom 694 were overweight/obese (6-16 years) and 207 were age- and sex-matched normal weight (NW). We generated three categories of eGFR: mild-low eGFR (< 20th percentile), high eGFR (>80th percentile) and intermediate eGFR (20-80th percentile), considered as the reference category
Results
Children with either mild-low or high eGFR category showed a 2-4 fold higher Odds ratio of high blood pressure, left ventricular hypertrophy, and microalbuminuria compared with children of the intermediate eGFR category. In addition, children with mild-low eGFR levels showed a 1.5-2 fold higher Odds ratio of impaired fasting glucose and high white blood cell count compared with children with intermediate eGFR levels.
Conclusions
In outpatient children with high prevalence of obesity, children with either mildly reduced or high eGFR have an increased burden of CMRF. Children with eGFR < 97 mL/min/1.73 m2 show a worse CMR profile. This finding supports the usefulness to assess eGFR to identify children with unfavorable CMR profile.
Introduction
The introduction of reliable and low-cost methods to assess glomerular function through specific equations has enabled us to evaluate glomerular function in large cohorts of patients as well as in population studies. Growing interest is focused on the estimated glomerular filtration rate (eGFR) as this measure proved a useful tool for estimating renal function and an accurate indicator of cardiovascular (CV) risk (1). In adults, low eGFR develops as a consequence of pathological conditions, including diabetes (2), hypertension (3), and obesity (4), and is an independent predictor of end-stage renal disease (ESRD) and cardiovascular disease both in diabetic and nondiabetic subjects (5). Interestingly, the association between eGFR and some cardiometabolic risk (CMR) factors appears nonlinear, as either low or high eGFR are associated with increased risk of metabolic diseases and all-cause mortality (5-7).
Limited information is available on the relationship between eGFR and CMR factors (CMRF) in the pediatric population. Previous studies demonstrated a positive relation between body mass index and high eGFR as expression of hyperfiltration (8), whereas other studies did not observe any differences in eGFR levels between obese and nonobese children (9, 10). However, previous studies overlooked the possibility that the association between eGFR and CMR could not be linear, as recently documented in adults (5-7). On the basis of these premises, we evaluated whether high or mild-low eGFR cluster with unfavorable CMR profile in children with high prevalence of overweight/obesity.
Methods
Participants and methods
A total of 901 children and adolescents, of whom 207 were normal weight, 129 overweight, and 565 obese (age range 6-16 years) were consecutively enrolled into the study at the Outpatient Unit of the Pediatric Department of Pozzuoli Hospital between 2004 and 2013. All children had been referred to our Unit by their General Practitioners because of allergy problems, suspect gastro-esophageal reflux, overweight, or obesity as elsewhere described (11). All subjects were healthy, none had history of diabetes, hypertension, thyroid, adrenal, and pituitary (GH) disorders, nor had any evidence of liver, renal, urinary, and infectious diseases. None of them were or had been under pharmacological treatment.
Children were visited in the morning after an overnight fast (≥12 hours) in the Outpatient Unit and anthropometric and laboratory data were collected by trained personnel. Weight was determined to the nearest 0.1 kg on a medical scale, height was measured to the nearest 0.1 cm with a wall-mounted stadiometer. Waist circumference was measured using a flexible tape at the high point of the iliac crest at minimal respiration when the participant was in a standing position as elsewhere described (11). Sexual maturity was evaluated by Tanner's stage for pubic hair (stages I-V). Blood pressure (BP) was measured according to standard methods; briefly, BP was measured at the right arm in the supine position after a 5-min rest, using a mercury sphygmomanometer with an appropriately sized cuff, and a stethoscope placed over the brachial artery pulse; three readings were taken two minutes apart and the average of the two last values was used in the analyses. The mercury sphygmomanometer was calibrated at least once a year.
Measurements
All biochemical analyses were performed in the centralized laboratory of Pozzuoli Hospital. Fasting plasma glucose (FPG), insulin, and lipid profile were measured by standard methods. Creatinine was measured by kinetic colorimetric compensated Jaffe technique using a ROCHE analyzer (Modular Analytics Serum Work Area, 68298 Mannheim, Ger). The assay was isotope dilution mass spectrometry standardized, traceable to National Institute of Standards and Technology creatinine standard reference material (SRM 914 and SRM 967). This method is highly correlated with the enzymatic method, as previously demonstrated (12). eGFR was calculated using updated Schwartz's formula: 0.413 × height (cm)/serum creatinine (mg/dl) (13). Glycosylated haemoglobin (HbA1c) was measured by high-performance liquid chromatography and standardized according to the DCCT method. Insulin resistance was evaluated by the homeostasis model assessment of insulin resistance (HOMA-IR) index using the following standard formula: fasting insulin (U/L) × fasting glucose (mmol/L) divided by 22.5. Urinary albumin was determined by the kinetic nephelometric method (IMAGE system, Beckman Coulter, Fullerton, CA, USA) in 584 subjects who did not differ from the whole population for age, BMI, and sex distribution.
Echocardiography
Echocardiographic examination was performed in a randomly selected sub-sample of 275 children who (Table 2) were similar for age, gender distribution to the whole population. Two-dimensional, M-mode and Doppler echocardiograms were recorded with a standard method by a commercially available echocardiographic system (Artida/Aplio; Toshiba), as elsewhere described (11). All measurements were analyzed according to the recommendations of the American Society of Echocardiography. LV mass was calculated according to the Penn convention and indexed for height2.7. Relative wall thickness (RWT) was calculated from posterior wall thickness (PWT), interventricular septum thickness (IVST), and left ventricular diastolic diameter (LVDD) using the following formula: (PWT + IVST)/LVDD. RWT was normalized for age by the following equation: RWT − 0.005 × (age - 10) (14). Transmitral peak rapid filling velocity (E), peak atrial filling velocity (A), E/A ratio, and isovolumic relaxation time (IRT) were obtained as measures of diastolic function. All readings were made on-line by the same investigator who was blinded to the metabolic status of the children.
Definitions
As only 70 children showed eGFR < 90 mL/min/1.73 m2 and no definition of hyperfiltration is currently available, we generated three categories of eGFR: mild-low eGFR (< 20th percentile), high eGFR (>80th percentile), and intermediate eGFR (20-80th percentile), considered as the reference category. The definition of normal weight, overweight, and obesity was based on International Obesity Task Force criteria (15). Prepubertal stage was defined by Tanner stage I. High blood pressure levels were defined by systolic or diastolic blood pressure (BP) ≥90th percentile for gender, age, and height according to the Fourth Report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents (16). Impaired fasting glucose (IFG) was defined by FPG ≥100 mg/dl. High white blood cells (WBC) count was defined by a value ≥9.0 (103/l), corresponding to the 80th percentile of our population (11). Microalbuminuria was defined as 24-h urinary albumin excretion (UAE) rate ≥30 and ≤300 mg/24h (17). LV hypertrophy (LVH) was defined by LV mass index ≥95th percentile for age and gender (18). The study was approved by the Local Ethics Committee, and informed consent was obtained from the parents of all participants.
Statistical analysis
Data are expressed as Mean ± SD, median, and interquartile range or number (%). Given the skewed distribution of HOMA-IR, triglycerides, plasma creatinine, UAE, the statistical analysis of these variables was applied after log-transformation. Means were compared using ANOVA. Chi-square or Fisher's exact test, as appropriate, were used to compare proportions.
The Odds Ratio (OR) of CMR factors associated with mild-low and the high eGFR category were compared with the intermediate (reference) category and analyzed by multiple logistic regression analysis adjusted for major confounders. A two-sided P value < 0.05 was considered statistically significant. The statistical analysis was performed with SPSS for Windows, version 13.0 (SPSS, Chicago, USA).
Results
The anthropometric and clinical characteristics of participants according to eGFR categories are reported in Table 1. A statistically significant difference across categories of eGFR was found in age (P = 0.014), FPG (P < 0.0001), triglycerides (P = 0.03), WBC count (P = 0.027), diastolic blood pressure (P = 0.009) and UAE (P = 0.006). With regard to echocardiographic evaluation, a statistically significant difference across categories of eGFR was found in LVM index (P = 0.037), whereas no difference was observed for both systolic and diastolic function (Table 2).
| Mild-low eGFR | Intermediate eGFR | High eGFR | P value | |
|---|---|---|---|---|
| n =901 | 179 | 544 | 178 | |
| eGFR (mL/min/1.73 m2) | 70-96 | 97-126 | 127-183 | --- |
| Age (years) | 11 ± 3 | 10 ± 3 | 10 ± 3 | 0.014 |
| Boys (%) | 83 (46) | 274 (50) | 86 (48) | 0.629 |
| Prepubertal stage (%) | 42 (24) | 132 (24) | 52 (29) | 0.357 |
| Obese (%) | 114 (64) | 336 (62) | 115 (65) | 0.758 |
| Body mass index (kg/m2) | 26 ± 6 | 25 ± 6 | 26 ± 6 | 0.096 |
| Waist circumference (cm) | 84 ± 15 | 82 ± 17 | 81 ± 17 | 0.165 |
| Fasting plasma glucose (mg/dl) | 87 ± 8 | 86 ± 7 | 83 ± 7 | 0.0001 |
| HbA1c (%) | 5.4 ± 0.3 | 5.4 ± 0.3 | 5.4 ± 0.3 | 0.781 |
| HOMA-IR | 3.1 ± 2.8 | 2.7 ± 2.1 | 2.7 ± 2.2 | 0.134 |
| Cholesterol (mg/dl) | 157 ± 37 | 157 ± 32 | 157 ± 32 | 0.975 |
| HDL-Cholesterol (mg/dl) | 53 ± 11 | 52 ± 12 | 51 ± 11 | 0.156 |
| Triglycerides (mg/dl) | 89 ± 41 | 87 ± 43 | 79 ± 35 | 0.030 |
| White blood cell count (103/l) | 7.9 ± 2.2 | 7.4 ± 2.2 | 7.4 ± 2.2 | 0.027 |
| Systolic BP (mmHg) | 107 ± 12 | 106 ± 12 | 106 ± 12 | 0.465 |
| Diastolic BP (mmHg) | 62 ± 9 | 59 ± 9 | 60 ± 10 | 0.009 |
| UAE (mg/24 h) | 5.6 (3.5-9.5) | 4.5 (2.9-7.5) | 6.0 (3.0-10.0) | 0.006 |
- Data are expressed as Mean ± SD, n (%), range, median (interquartile range) UAE: urinary albumun excretion.
| Mild-low eGFR | Intermediate eGFR | High eGFR | P value | |
|---|---|---|---|---|
| n | 51 | 161 | 63 | |
| Obese (%) | 37 (73) | 118 (73) | 46 (73) | 0.994 |
| LVM (g) | 104 ± 36 | 93 ± 37 | 100 ± 37 | 0.09 |
| LVM index (g/h2.7) | 40 ± 12 | 35 ± 11 | 38 ± 11 | 0.037 |
| RWT | 0.353 ± 0.06 | 0.354 ± 0.05 | 0.367 ± 0.07 | 0.257 |
| Heart rate (b/min) | 83 ± 12 | 85 ± 13 | 85 ± 13 | 0.646 |
| Ejection fraction (%) | 64 ± 6 | 64 ± 6 | 65 ± 5 | 0.572 |
| IRT (ms) | 65 ± 10 | 66 ± 9 | 67 ± 11 | 0.394 |
| E/A ratio | 2.2 ± 0.9 | 2.0 ± 0.6 | 2.1 ± 0.6 | 0.350 |
- Data are expressed as Mean ± SD, n (%)
- E/A, early peak flow velocity/atrial peak flow velocity; IRT, isovolumic relaxation time; LVM, left ventricular mass; RWT, relative wall thickness.
CMRF and categories of eGFR
Analyzing CMR factors as categorical variables, a greater prevalence of high BP, LV hypertrophy, and microalbuminuria was found either in mild-low or in high eGFR compared with the reference category. In addition, children with mild-low eGFR showed an increased prevalence of IFG and high WBC count (Figure 1). In Table 3, the OR of CMR factors among categories of eGFR is reported. Children with mild-low eGFR had an increased OR of high BP (P < 0.05), LV hypertrophy (P < 0.01), microalbuminuria (P < 0.025), IFG (P < 0.025) and high WBC count (P < 0.025) compared with the reference group. Children with high eGFR had an increased OR of high BP (P < 0.025), LV hypertrophy (P < 0.025) and microalbuminuria (P < 0.025), compared with the reference category, independently of prepubertal stage and BMI (Table 3).
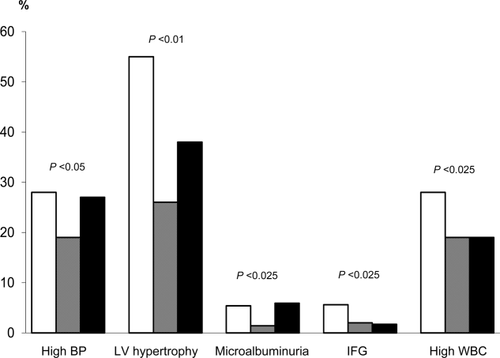
Prevalence of high blood pressure (BP), LV hypertrophy, microalbuminuria, impaired fasting glucose (IFG), and high white blood cell count (WBC) among mild-low (white bar), intermediate (grey bar), and high (black bar) eGFR.
| CMRF | Mild-low eGFR | Intermediate eGFR | High eGFR |
|---|---|---|---|
| High blood pressurea | 1.52 (1.03-2.29)b | 1.00 | 1.67 (1.10-2.53)c |
| Left ventricular hypertrophyd | 3.62 (1.78-7.36)e | 1.00 | 2.22 (1.13-4.35)c |
| Microalbuminuriaa | 4.26 (1.27-14.35)c | 1.00 | 4.45 (1.38-14.40)c |
| Impaired fasting glucosea | 2.73 (1.14-6.57)c | 1.00 | 0.86 (0.24-3.11) |
| High white blood cell counta | 1.62 (1.08-2.42)c | 1.00 | 0.97 (0.62-1.52) |
- a Adjusted for prepubertal stage, and BMI.
- b P < 0.05.
- c P < 0.025.
- d Adjusted for prepubertal stage, BMI, systolic BP, heart rate.
- e P < 0.01.
Discussion
This study demonstrates that in an outpatient pediatric population with high prevalence of obesity, unselected for chronic kidney disease (CKD), the two extremes of eGFR distribution have an increased burden of CMR factors. In particular, high BP, LV hypertrophy and microalbuminuria are more prevalent in either mild-low or high eGFR category showing a “U” shaped distribution, whereas IFG and high WBC count are more prevalent in children with mildly reduced eGFR levels. Globally, children with mild-low eGFR levels exhibit a worse CMR profile than the other groups.
To date, few studies have examined the relationship between eGFR and CMR factors in the pediatric population without CKD and the results are quite conflicting. Kouloridis et al. (8) reported a positive relation between BMI and eGFR as expression of hyperfiltration; in contrast, Savino et al. (9) and Fadrowsky et al. (10) did not observe any difference in eGFR between obese and nonobese children. This finding is likely to explain why, at variance with adults, so far pediatricians have paid little attention to eGFR as a possible marker of CMR.
Several studies have pointed out the alarming increase in the prevalence of CMR factors not only in obese children (19) but also in the general pediatric population (20, 21). The current data provide the novel finding that assessment of eGFR may be helpful in clinical practice to stratify obesity-associated CMR and identify children with early signs of renal and cardiac damage.
Being cross-sectional, our study cannot elucidate the mechanisms underlying the association between eGFR and CMR factors; however, some considerations are worthwhile. Interestingly, we found that microalbuminuria and LV hypertrophy were more prevalent in children at both extremes of eGFR distribution; this finding is consistent with recent prospective studies in adults demonstrating that either high or low eGFR levels are associated with increased metabolic and CV risk (5-7). In particular, in middle-aged adults of the Atherosclerosis Risk in Communities (ARIC) study, Matsushida et al. demonstrated that either hyperfiltration (eGFR ≥120 mL/min/1.73 m2) or low filtration (< 90 mL/min/1.73 m2) were associated with an increased risk of ESRD and all-cause mortality compared with normal eGFR (5). Our data underscore the early appearance of an unfavorable CMR profile together with preclinical signs of organ damage in young people with eGFR deviation from the normal range and support the need to monitor children with either mildly reduced (< 97 mL/min/1.73 m2) or high (>126 mL/min/1.73 m2) eGFR. Noteworthy is the finding that the risk of microalbuminuria was increased both in the highest and in the lowest eGFR categories. Although the mechanism underlying this association remains unsettled, this finding is clinically relevant as microalbuminuria is a predictor of kidney disease and a marker of CV disease. In our study, we also observed that either mildly decreased or high eGFR levels were associated with a 3-4 folds greater probability of LV hypertrophy compared with normal eGFR. This observation is particularly important in the light of the very young age of our population and also considering that in adults LV hypertrophy is a strong independent predictor of CV events (22). Analysis of LV geometry suggests specific mechanisms. Children with high eGFR showed a tendency, although not statistically significant, toward increased RWT, in accordance with a concentric LV remodeling, which is likely consequent to increased afterload. In contrast, children in the mild-low eGFR category tended toward an eccentric geometry, which suggests volume overload probably because of neurohormonal and metabolic changes. Obviously, this hypothesis needs to be confirmed in a larger sample. In our population a mild reduction in eGFR is associated with a higher probability of glucose dysregulation and inflammation; noteworthy, this phenotype was found in children with manifest CKD (23), suggesting that these abnormalities may precede the progression toward CKD. In any case, the presence of several CMR factors and preclinical signs of organ damage in children with clinically normal renal function raises interesting perspectives about the usefulness of eGFR to identify children with increased burden of CMRF.
The strength of our study is the large sample in an age range poorly investigated and the analysis of a broad range of eGFR levels in relation to several CMR factors, included LV mass. Some limitations should be acknowledged. First, the cross-sectional design prevents conclusions about the causal relationship between eGFR and CMR factors. Second, we measured creatinine concentration by kinetic colorimetric compensated technique, whereas in the updated Schwartz's formula it was determined by an enzymatic method (13). However, the two methods are highly correlated (12); furthermore, updated Schwartz's formula based on colorimetric compensated Jaffe technique has been widely applied in the pediatric population (10) and in cohort (11, 12, 24, 25) studies. Finally, our analysis refers to a population with high prevalence of obesity, which may limit the applicability of these results to the general pediatric population. In conclusion, in a pediatric population with high prevalence of overweight/obesity, either mild-low or high eGFR levels are associated with an increased burden of CMR factors. Children with eGFR < 97 mL/min/1.73 m2 show a worse CMR profile. Our study suggests that eGFR is a good tool to identify children with unfavorable CMR profile who warrant a closer monitoring. The prognostic importance of eGFR with regard to future cardiovascular and renal diseases needs to be defined in prospective studies.





