Redefining metabolic syndrome as a fat storage condition based on studies of comparative physiology†‡
Disclosure: Dr Johnson is listed as an inventor on patent applications with the University of Florida related to lowering uric acid as a means for preventing or treating the metabolic syndrome. Dr Johnson and Dr Lanaspa are listed as inventors on patent applications from the University of Colorado on blocking fructose metabolism in the treatment of metabolic syndrome in response to carbohydrates. Finally, Dr. Johnson has a lay book, The Fat Switch (2012, Mercola.com) that discusses the role of fructose in metabolic syndrome in more detail. All other investigators have no conflicts.
Funding agencies: NIH RO-1 HL-68607 and NIH grants RC4 DK090859 provided support for this article.
Disclosure: Dr Johnson is listed as an inventor on patent applications with the University of Florida related to lowering uric acid as a means for preventing or treating the metabolic syndrome. Dr Johnson and Dr Lanaspa are listed as inventors on patent applications from the University of Colorado on blocking fructose metabolism in the treatment of metabolic syndrome in response to carbohydrates. Finally, Dr. Johnson has a lay book, The Fat Switch (2012, Mercola.com) that discusses the role of fructose in metabolic syndrome in more detail. All other investigators have no conflicts.
Abstract
Objective: The metabolic syndrome refers to a constellation of signs including abdominal obesity, elevated serum triglycerides, low HDL-cholesterol, elevated blood pressure, and insulin resistance. Today approximately one third of the adult population has the metabolic syndrome. While there is little doubt that the signs constituting the metabolic syndrome frequently cluster, much controversy exists over the definition, pathogenesis, or clinical utility.
Design and Methods: Here we present evidence from the field of comparative physiology that the metabolic syndrome is similar to the biological process that animals engage to store fat in preparation for periods of food shortage.
Results: We propose that the metabolic syndrome be changed to fat storage condition to more clearly align with its etiology. Obesity in humans is likely the consequences of both genetic predisposition (driven in part by thrifty genes) and environment. Recent studies suggest that the loss of the uricase gene may be one factor that predisposes humans to obesity today.
Conclusion: Understanding the process animals engage to switch from a lean insulin-sensitive to an obese insulin-resistant state may provide novel insights into the cause of obesity and diabetes in humans, and unique opportunities for reversing their pathology.
Introduction
In 1923 the Swedish physician, Eskil Kylin, noted that obese subjects not uncommonly have elevated glucose levels, elevated blood pressure, and hyperuricemia ((1)). Subsequently others also observed a clustering of signs, including obesity, glucose intolerance, hypertriglyceridemia, and hyperuricemia or gout in subjects at risk for developing diabetes ((2-7)). In 1989 Reaven elaborated on these cluster of signs which he called syndrome X and suggested that insulin resistance was the underlying pathophysiological mechanism ((8)). Shortly thereafter the cluster became known as the metabolic syndrome, and was given various definitions, for which one of the most popular is the one provided by the Adult Treatment Panel (ATP) III of the National Cholesterol Education Program (NCEP-ATPIII). According to the NCEP-ATPIII definition, an individual must have three of the following five characteristics: ((1)) abdominal obesity determined by waist circumference (Men > 102 cm; Women > 88 cm); ((2)) triglycerides ≥ 150 mg dL−1; ((3)) Low HDL cholesterol levels (Men < 40 mg dL−1; Women < 50 mg dL−1); ((4)) Blood pressure≥130/≥ 85 mmHg; and ((5)) Fasting glucose ≥ 110 mg dL−1 ((9)). On the basis of this definition, ∼34% of the US ((10)) and 27% of the southern European ((11)) adult population have metabolic syndrome.
Controversy exists, however, on the use of the term, metabolic syndrome ((12), (13)). While the frequent clustering of the signs are not denied, the concept that insulin resistance may be the underlying mechanism has also been challenged ((14)). Agreement on definitions and cut-off levels have been difficult ((14)). There are also other findings frequently associated with metabolic syndrome but which are not included in the definition, such as fatty liver, microalbuminuria, sleep problems, hyperuricemia, inflammation, loss of muscle and bone mass, kidney stones, and evidence of oxidative stress and endothelial dysfunction. The predictive value of metabolic syndrome as a risk factor for cardiovascular disease also does not appear any better than its individual components. This has led to some societies to recommend abandonment of the term metabolic syndrome ((14)). Even Gerald Reaven, whose paper brought the concept to national attention in the 1990s, has questioned whether it retains any useful value ((15)). It has been stated that until a better understanding of the underlying cause of metabolic syndrome is provided, the entity will remain controversial ((12)).
In this article we extend previous arguments ((16)) to propose that the metabolic syndrome has many similarities with the normal biological processes by which animals store fat in preparation for periods of food shortage. The primary difference is that humans with metabolic syndrome continue to accumulate fat. As such, we propose to rename the metabolic syndrome “fat storage syndrome” to better characterize its underlying physiological basis.
Accumulating Fat is a Normal Survival Process
A longstanding tenet in the medical literature is that the development of insulin resistance and other features of metabolic syndrome is a pathophysiological process, meaning that it is either caused by a disease or represents a dysregulation of normal physiological mechanisms ((8), (12)). However, we would argue that it instead represents a normal physiological process, as it is engaged throughout the animal kingdom as a survival mechanism to avert starvation.
One of the basic concepts of Darwinian evolutionary theory is survival of the fittest, in which it was proposed that those individuals who could best adapt to the changing environment would survive to successfully reproduce and thereby be the ones to pass their genes to subsequent generations ((17)). Evolution has been considered a natural experimental process to select animal design characteristics with a survival advantage and one of the most challenging aspects of life is to be able to survive during times of food shortage. While some animals have learned to do this by storing foods, others have developed intricate ways to store fat (triglycerides) in their tissues, especially the white adipose tissues, the liver, and the blood. In the setting of food shortage, survival may not truly favor those who are lean and fit, but rather those with the greatest fat stores, and hence a more appropriate term than “survival of the fittest” might be “survival of the fattest.” Indeed, an understanding of the particular adaptations of different species in nature may lead to novel interventions for the prevention and treatment of human diseases, such as metabolic syndrome. As discussed by Singer ((18)) this approach underlies the principles of biomimicry, which is the science that studies nature's models and, inspired by these designs and processes, try to solve human health problems.
There are numerous examples in the zoological literature of the importance of fat in survival of different animal species. Insect larvae store fat in their fat bodies which they use to support their life when in the pupal stage ((19)). Adult insects also rely on their fat stores to provide energy when food is not available. One striking example relates to the work of Weis-Fogh, who performed energy balance studies in African desert locusts ((20)). Weis-Fogh determined that the locusts use fat for more than 85% of their energy needs when they are in flight. He further demonstrated that locusts with 10% fat could last only 12 h in flight, whereas those with 15% fat would last 20 h. As such, he calculated that when the swarms of locusts would make the 600 mile trek from southern Morocco to Portugal, many individuals would perish during the flight from exhaustion of their fat stores ((20)).
Some fish store fat for their survival. Lungfish live in freshwater lakes in Africa, but during the heat of the summer the water can evaporate and the lungfish must burrow into the mud to survive by burning stored fat until the waters return, which may be a year or more (termed estivation) ((21)). Another example is the salmon, who stop eating when they migrate up fresh water rivers to spawn. During these migrations they can consume up to 60-80% of their fat, and if the fat stores become depleted, the fish will die ((22), (23)).
One of the best examples of storing fat as a means for survival is the freshwater Pacu fish (Piaractus mesopotamicus), which lives in the Amazon and Orinoco River. Every year these rivers flood during the tropical rains, and the river levels may rise 6-18 m, flooding 70,000 to 300,000 km2 of rain forest. During this time of flooding the Pacu enters into the flooded jungle to feast on ripe fruit that has fallen into the river. The Pacu rapidly becomes fat, and its fat content can reach 28%. When the waters recede, the Pacu quits eating and lives off its fat stores for up to 6 months; during this time its fat content dwindles down to 10% or less ((24), (25)).
Yet another example is the water holding desert frog (Cyclorana platycephalus) that lives in the deserts of Australia where it prepares for droughts by storing fat in pads in its feet. Some frogs accumulate up to one-fifth of their weight in these food pads. When drought occurs, they burrow into the sand and use their fat stores, where they may survive for years without food. However, the amount of fat is critical for survival. Indeed, only 10% of frogs can survive a drought of 5 years duration, and it is those frogs who were able to store 20% or more of their overall weight as fat in their feet ((26)).
Birds also exploit stored fat for their survival. As an example, Emperor Penguins double their weight in fat to help them survive without any food for 2-4 months during the Antarctic winter while they are nesting inland ((27), (28)). Long-distance migrating birds, such as the godwit, also accumulate large amounts of fat prior to their migrations ((29)).
Hibernating mammals, such as the 13-lined ground squirrel double their weight in the late summer in preparation for winter. The animals emerge lean from hibernation in spring, having burned these fat stores to survive the winter without eating ((30)). The gray whale increases its fat stores markedly prior to migrating to Baja, Mexico where it breeds. During the 6 months it travels to Baja and back it will fast and lose as much as 30% of its weight ((31), (32)).
Classical Sites of Fat Storage
Some animals store fat in unusual places, such as the footpad in the water holding desert frog, the tail of the lizard, and the neck of the horse. However, for most animals, the storage of fat is similar to that observed in humans, and occurs primarily in the blood (as serum triglycerides), in the liver (steatosis), or in the fat tissues themselves (adipocytes). Birds in particular store large amounts of fat in the liver. For example, the hummingbird, which ingests a large amount of nectar during the day can develop such an extreme fatty liver that this organ is actually creamy white by the end of the day ((33)). However, because of their extremely high metabolism, hummingbirds may actually deplete their fat stores during the night, forcing them to enter torpor until the morning comes when they can feed on nectar again ((34)). Other animals, such as the Minke whale, can have such high serum triglycerides that the serum appears lipemic ((35)). Hibernating squirrels are known to increase their serum and liver triglyceride content during the summer and then use these lipid stores as their energy source during hibernation ((36)). Regardless, the fact that animals purposefully develop fatty liver, elevated triglycerides and visceral fat stores is reminiscent of features observed in patients with metabolic syndrome.
Insulin Resistance is a Feature of Fat Storage Syndrome
An important feature of fat storage is that animals also may become insulin resistant. For example, hibernating mammals, such as the marmot and squirrel, develop hyperinsulinemia and insulin resistance while they are accumulating fat in late summer ((16), (37)). Likewise, the European Garden Warbler increases its fat content in its liver, fat tissues and blood and becomes insulin resistant prior to migrating across the Sahara to subtropical Africa ((38)). The desert gerbil (Psammomys obesus), which is so predisposed to becoming fat that it is also called the fat sand rat, will become diabetic shortly after being placed on a high protein diet, also suggesting it may be prediabetic in the wild ((39)). Horses are also at risk for developing obesity and insulin resistance ((40)).
Should We Reappraise the Metabolic Syndrome Based on Comparative Physiology?
Most of the studies in the field of medicine focus on classical fields such as physiology, genetics, and molecular biology. Comparative physiology is often not at the top of the list. As such, the observation of a clustering of signs such as insulin resistance, hypertriglyceridemia, elevated blood pressure, abdominal obesity, and fatty liver may appear as a dysregulation of normal physiology. However, studies in a wide variety of animals support the concept that these signs may represent an adaptive form of fat storage (Figure 1). The fact that there is a continuum from a state of leanness to marked fat storage, as well as variability among and within species as to the degree with which fat is stored can also explain the difficulty in establishing strict definitions for metabolic syndrome or fat storage condition in humans. In some respects, the variations in manifestation of fat storage condition may not be dissimilar from systemic lupus erythematosus, in which diagnosis is made by the presence of four of eleven criteria; yet in the latter everyone agrees that the condition exists. By recognizing that fat storage condition is a normal biological process found in many animals to protect them from food shortage, we believe that we should focus not so much on the reality of this condition, or on its predictive components, but rather on the fundamental mechanisms that trigger the change back and forth between a lean, insulin sensitive animal to an obese, insulin resistant animal. One significant difference between humans and animals in the wild, of course, is that many animals become obese prior to a period of fasting, such as during hibernation, long distance migration, or nesting. According to this reasoning, our predisposition to obesity may simply be due to the continued availability of food.
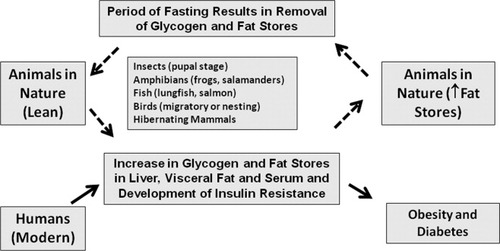
The metabolic syndrome as a disorder of fat storage. Many animals develop features consistent with metabolic syndrome as part of the normal physiological processes involved in fat storage (shown as dotted lines). This suggests that metabolic syndrome may represent a form of fat storage. However, most animals undergo a period of fasting that brings the animal back to their regular weight, whereas many humans will progressively increase their fat stores until they become frankly obese or diabetic (solid lines).
Relationship to the Thrifty Gene Hypothesis
It is also possible that humans may have acquired (or lost) genes in our past that may predispose us to obesity today. Over 50 year ago James Neel proposed the thrifty gene hypothesis, in which he suggested that the epidemic of obesity and diabetes today are due to the acquisition of genes that occurred during periods of famine in our past that would facilitate fat stores and insulin resistance ((41)). This hypothesis has been controversial, with some papers supporting ((42-44)) and others criticizing ((45-47)) the hypothesis. One of the problems is that until recently, no thrifty genes had been identified. However, there is now evidence that it was not the gain of a gene, but rather the loss of the gene, uricase, that may have functioned to stimulate the thrifty phenotype ((48)).
Uricase is a hepatic enzyme that degrades uric acid, eventually generating allantoin. It is present in all mammals except for the apes and humans. For the latter species, uricase was lost due to parallel mutations affecting the great apes and humans (occurring around 15 million years ago) and the lesser apes (occurring around 10 million years ago) (Figure 2) ((49), (50)). As such, humans and apes have higher serum uric acid levels than other mammals.
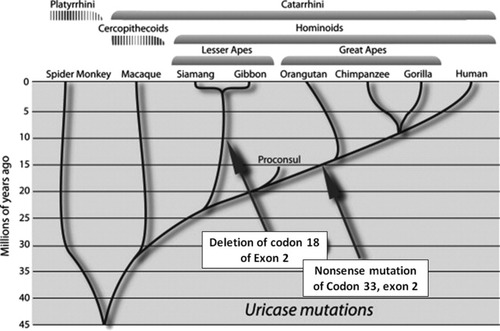
Parallel mutations in uricase occurred during the evolution of hominoids in the miocene. Adapted from Johnson et al. ((50)) with permission of Elsevier.
The fact that there were parallel mutations of uricase in two lineages of apes suggests that there was an evolutionary benefit to the uricase mutation to apes in the mid Miocene. It is thus important to understand the paleoenvironment of this period. In the early Miocene (∼20 million years ago), all apes lived in Africa, where their diet consisted almost entirely of fruit. Then global cooling began, possibly initiated by volcanic activity in the Rift Valley, and leading to an expansion of the polar ice caps and a fall in the sea level. A land bridge that connected Africa with Eurasia appeared around 17 million years ago, and numerous species exited Africa, including giraffes, anteaters and apes. Shortly thereafter ape species can be found throughout Turkey and Europe.
Initially the apes in Europe and Turkey lived in tropical rain forests similar to those present in Africa. As global cooling continued, there was a loss of tropical rain forests in Europe with the replacement by deciduous trees. The climate became more seasonal, and the ape populations retreated to isolated colonies, where there is evidence for intermittent starvation (based on dental studies) ((51)). By 8-9 million years ago, all apes had become extinct in Europe. In contrast, the apes in Africa were able to continue with their regular diet of tropical fruit throughout the year, for while cooling also occurred here, it resulted primarily in a retraction of the tropical rain forest rather than their replacement as occurred in Europe.
While the extinction of apes in Europe may appear like a biological dead-end, there is increasing evidence from the fossil record that the ancestors to humans and modern apes came from a European ape that returned to Africa prior to the final extinctions. Both Kenyapithecus and Dryopithecus have been proposed as our candidate ancestors to have made the exodus “Back to Africa” ((52), (53)). It was during this period that the uricase mutation occurred in our ancestors. On the basis of the increasing evidence that the loss of uricase can increase fat stores and enhance the effects of fructose (see below), Johnson and Andrews hypothesized that the uricase mutation likely occurred in Europe as it would have provided a survival advantage under the conditions of seasonality and dwindling fruit availability ((48), (54)).
The effect of the uricase mutation on fat storage and metabolic syndrome has only recently been appreciated. While initial studies focused on the potential benefit of uric acid as an antioxidant in the circulation, more recent studies show that an elevated serum uric acid likely has a direct role to increase fat stores and induce features of metabolic syndrome. Indeed, lowering serum uric acid has been found to reduce fatty liver, serum triglycerides, blood pressure and improve insulin resistance in a variety of animal models ((55-57)). Epidemiological studies show unequivocally that hyperuricemia is a consistent independent risk factor for obesity, fatty liver, insulin resistance and hypertension ((58-61)). Some genetic studies have linked polymorphisms in uric acid transport with hypertension, obesity and metabolic syndrome ((62), (63)) whereas others have not ((64), (65)). Early clinical studies are also suggestive of a benefit of lowering serum uric acid on metabolic syndrome ((66), (67)), although larger, randomized studies are needed before any definitive conclusions can be made.
One of the more important interactions of uric acid is with fructose. Fructose is distinct from glucose in that it raises intracellular uric acid, due to the rapid and unchecked phosphorylation of fructose with ATP depletion and consequent degradation of nucleotides. This reaction is driven by fructokinase C, and has a critical role in driving the metabolic phenotype in response to fructose ((68)). We have recently found that the intracellular uric acid has an important role in fat accumulation from fructose by causing both direct effects on mitochondria as well as increasing the expression and activity of fructokinase itself (69,70). Consistent with this finding, the inhibition of uricase amplifies the effects of fructose to induce insulin resistance, raise blood pressure and increase serum triglycerides in rats ((69)). Furthermore, we have recently been able to study the ancestral primate uricase and have shown that hepatocytes (HepG2 cells) exposed to fructose show a blunted triglyceride response (Lanaspa and Gaucher et al., unpublished). Consistent with the evidence that uric acid regulates fructokinase, a recent study showed that subjects with an elevated serum uric acid show a greater ATP depletion in response to fructose ((70)).
The uricase mutation that occurred in the Miocene amplified the biological effects of fructose from fruits to enhance fat storage, and was likely of great benefit to apes during this period of famine. However, with this type of diet, serum uric acid levels were only modestly higher than that of uricase-expressing primates. Indeed, in a study we conducted at the San Diego Zoo, the uricase-expressing primates have serum uric acid levels in the 1-2 mg dl−1 range whereas the apes lacking uricase have uric acid levels in the 3 mg dl−1 range ((71)). This latter level of uric acid is also similar to what is observed in tribes living on Paleolithic diets, such as the Yanomamö Indians ((72)). However, the introduction of sugarcane into the western diet led to a dramatic increase in fructose intake (Figure 3). The rise in sugar intake has closely paralleled the rise in serum uric acid levels, obesity and diabetes throughout the world ((73), (74)). Sugary soft drinks, a major source of added sugars, predict the development of obesity, insulin resistance and diabetes ((77-80)). In addition to stimulating fatty liver, elevated triglycerides and insulin resistance, fructose also induces leptin resistance that blunts satiety, resulting in enhanced intake of foods with high fat content ((81)).
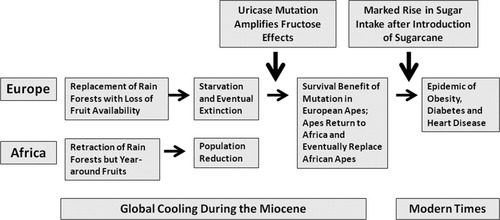
The thrifty gene hypothesis and uricase. The uricase mutation occurred during global cooling in the mid Miocene and has been postulated to have provided a survival advantage to European apes who were undergoing periodic starvation due to the loss of fruit availability in the cooler seasonal months. Specifically, the mutation amplified the effects of fructose in fruits to increase fat stores. Later the introduction of sugar led to a major increase in fructose availability and likely has a role in the epidemic rise in obesity and diabetes ((54)).
These recent studies help explain various controversies related to the thrifty gene hypothesis. Because the uricase mutation increases the susceptibility to fructose-induced metabolic syndrome, it explains why the mutation is present in everyone, yet obesity is not. Hence, obesity is a consequence of both environmental factors (such as fructose intake) coupled with an underlying genetic mutation (such as mutation in uricase). The argument that the thrifty gene hypothesis is unlikely to have occurred because humans have only been present for a million years or less is also not valid, for our studies suggest that the mutation occurred much earlier (during the Miocene). Indeed, we have also postulated that the loss of vitamin C due to a mutation in L-gulono-gamma-lactone oxidase nearly 50 million years ago may have represented another genetic change that would enhance the thrifty phenotype ((50)).
Conclusion
In conclusion, metabolic syndrome in humans can be considered a type of fat storage condition. Increasing evidence suggests that metabolic syndrome today represents an interaction between genetic changes that we acquired to protect us during food shortage, coupled with changes in our environment. One of the genetic changes appears to be the loss of the uricase gene in the Miocene.
Acknowledgements
This article represents a publication of the Colorado Obesity Research Initiative.





