Osteocyte lacuno-canalicular microstructure across the mid-shaft femur in adult males from medieval England
Abstract
Archaeological human bone histology can reveal well-preserved osteocyte lacunae, which are indicators of bone remodelling activity. Analyses of these lacunae can be useful when reconstructing past human mechanical loading histories or metabolic fluctuations from bone microstructure. However, the relationship between osteocyte lacunae density and morphology, and bone anatomical variation within archaeological samples is largely unknown. We examined osteocyte lacuno-canalicular network morphology in medieval human femora to test if osteocyte lacunae change with anatomical site location. Osteocyte lacunae density (Ot.Dn) data were analysed statistically in 10 middle-aged (35–50 years old) males dated to the 11th–16th century AD (Canterbury, England). A subsequent case study (n = 2) was conducted using two well-preserved samples from which canaliculi number per lacuna (Ci.N) and canaliculi-rich lacunae density (Ci.Dn) were preliminarily examined descriptively. The data were collected from cortical bone regions encompassing intracortical to subperiosteal mid-shaft femoral bone, comparing anterior, posterior, medial and lateral locations interindividually and intraindividually. Results show that Ot.Dn varied significantly between the four anatomical regions (p = 0.001), with the medial and lateral femoral regions showing the highest median Ot.Dn. The median of Ci.N was also the highest on the medial aspect, but Ci.Dn did not change greatly across the four apects of the bone. The combination of these results suggests that mid-shaft femoral anatomical location, which undergoes morphological change with biomechanical load, affects the expression of bone microstructure at the osteocyte lacuna level. This knowledge will benefit future osteoarchaeological methods that infer past behaviour from the human femur.
1 INTRODUCTION
Osteoarchaeologists typically reconstruct ancient human behaviour and lifestyle from external morphology, morphometry and robusticity of limb bones (Licata et al., 2019; Meyer, Nicklisch, Held, Fritsch, & Alt, 2011; Ruff, 2008; Ruff & Larsen, 2014; Villotte & Knüsel, 2013; Wanner, Sosa, Alt, & Blos, 2007). However, when internal bone structures are well preserved, behavioural inferences can also be achieved through histological methods (e.g., Miszkiewicz & Mahoney, 2016; Robling & Stout, 2003; Stout, 1978). Microscopic indicators of bone remodelling can reflect the way living bone adapts to mechanical stimuli (Miszkiewicz, 2016; Robling, Castillo, & Turner, 2006). Histological features typically examined in archaeological human bone include Haversian canals and secondary osteons (e.g., Miszkiewicz & Mahoney, 2016; Pfeiffer, Crowder, Harrington, & Brown, 2006; Robling & Stout, 2003), which can provide insight into strenuous physical activities associated with variation in mechanical load (van Oers, Ruimerman, van Rietbergen, Hilbers, & Huiskes, 2008). Osteocyte lacunae, cavities that house osteocytes in live bone, have been less studied in osteoarchaeology despite their broad application in the palaeobiology of fossil bone form and function (e.g., Cullen, Evans, Ryan, Currie, & Kobayashi, 2014; Grunmeier & D'Emic, 2019; Miszkiewicz et al., 2020).
Studies of modern human bone have reported that osteocyte lacunae densities (Ot.Dn) reflect metabolism and body mass (Bromage et al., 2009, 2016). Human and animal experimental research has found that filopodia are the central mechanosensory components of osteocytes and are distributed within bone according to nutrient accessibility and biomechanical load (Bonewald, 2011; Kerschnitzki et al., 2013; Thi, Suadicani, Schaffler, Weinbaum, & Spray, 2013; Verbruggen, Vaughan, & McNamara, 2014). However, our understanding of variation in osteocyte lacunae, and the number of osteocyte filopodia across different regions of bone, remains poorly understood overall, particularly for archaeological human samples. Improving this understanding is important to assist with osteoarchaeological inferences of bone functional adaptation (Crowder & Stout, 2011).
1.1 Osteocytes and the osteocyte lacuno-canalicular network
In living bone, metabolic activity is essentially governed by osteocytes and executed by osteoblasts and osteoclasts. These cells play a key role in regulating the internal adaptability of bone to biomechanical load (Burger, Klein-Nulend, & Smit, 2003; Qiu, Rao, Fyhrie, Palnitkar, & Parfitt, 2005; Sims & Vrahnas, 2014). During bone remodelling, osteoclasts absorb bone matrix, osteoblasts synthesize and secrete new matrix, and osteocytes become embedded within the matrix to perform communicatory and regulatory roles (Bonewald, 2011; Burger, Klein-Nulend, & Smit, 2003; Qiu, Rao, Fyhrie, Palnitkar, & Parfitt, 2005; Sims & Vrahnas, 2014). External stimuli and demand for bone adaptation activate signals in the osteoblast and transform its circular shape into a smaller, stellate morphology. This transformed cell is then labelled an osteocyte (Bonewald, 2011). Osteocytes in living tissue comprise a cell body, single nucleus, organelles (Golgi apparatus, free ribosomes, endoplasmic reticulum and mitochondria) and projections called filopodia (Heckman & Plummer, 2013; Sugawara et al., 2008; Uda, Azab, Sun, Shi, & Pajevic, 2017). The osteocyte cell body is maintained in a cavity called a lacuna, and its filopodia project into long canals called canaliculi (Marotti, Ferretti, Remaggi, & Palumbo, 1995). These lacunae and canaliculi connect with other neighbouring lacuna–canaliculi complexes to form the osteocyte lacuno-canalicular network—a communication system as complex as the neuronal network in the human brain (Buenzli & Sims, 2015; Franz-Odendaal, Hall, & Witten, 2006). Current literature suggests that, outside of transporting nutrients and maintaining bone homeostasis (Bonewald, 2011; Kerschnitzki et al., 2013; Marotti, Ferretti, Remaggi, & Palumbo, 1995), the network is responsible for stimulus recognition and response (Judex et al., 2010).
1.2 Osteocyte lacuno-canalicular network and behaviour
Previous studies considering the lacuno-canalicular network focused on its micromorphological trends and quantification in cell lines, animal models and fresh human bone (Buenzli & Sims, 2015; Kartsogiannis & Ng, 2004; Zhang, Bakker, Klein-Nulend, & Bravenboer, 2019). For example, it has been suggested that osteocyte cells are spatiotemporally distributed according to nutrient accessibility and response demand (Kerschnitzki et al., 2013; Marotti, Ferretti, Remaggi, & Palumbo, 1995). Marotti, Ferretti, Remaggi, and Palumbo (1995) noted that this was also a trend for osteocyte lacunae, whereby canaliculi presence was non-uniform within bone and directionality correlated to nearby nutrient reservoirs. Kerschnitzki et al. (2013) suggested that the network may therefore sense stimulus and transport nutrients to sites with high bone remodelling demand.
Biomechanical load is recognized through changes in fluid pressure, sheer stress and hydrostatic pressure in a process called mechanosensation (Judex et al., 2010; Uda, Azab, Sun, Shi, & Pajevic, 2017). It has been suggested that the majority of the mechanosensation occurs with polarity at the filopodia of the osteocyte (Thi, Suadicani, Schaffler, Weinbaum, & Spray, 2013; Verbruggen, Vaughan, & McNamara, 2014). Ongoing research suspects that the filopodia form gap junctions, which transform a signal into a biological cue in a process called mechanotransduction (Heckman & Plummer, 2013; Uda, Azab, Sun, Shi, & Pajevic, 2017). The mechanosensation and mechanotransduction processes are also known to vary between skeletal elements and different species (Van Hove et al., 2009). For example, the human femur experiences a different pattern of biomechanical load compared with the human tibia due to its central weight-bearing role (Drapeau & Streeter, 2006; Miszkiewicz, 2016; Van Hove et al., 2009). Rudman, Aspden, and Meakin (2006) explored this and noted that high modulus was present in areas of high bone density and allowed for the development of an optimized strain pattern that is characteristic only of the human femur. Tayton, Evans, and O'Doherty (2010) supported this finding and noted that strain also varied at different bone sites.
Although the principle of bone remodelling and redistribution as part of bone functional adaptation is the premise of osteoarcheological research into behaviour (see Ruff, Holt, & Trinkaus, 2006, for review), osteocyte lacunae are yet to be studied properly within archaeological human assemblages. The osteocyte relationship to load has already been shown using ovine, dog, extant birds and dinosaur samples, confirming different loading patterns, osteocyte distributions and bone turnover rates in comparison with humans (Canè et al., 1982; Cullen, Evans, Ryan, Currie, & Kobayashi, 2014; Grunmeier & D'Emic, 2019; Kerschnitzki et al., 2013). Therefore, where access to femoral cross sections in archaeological human samples is available, even when osteocytes themselves do not preserve, their lacunae are evidence of osteocyte existence and thus can shed light on localized bone functional adaptation and anatomical location (Bromage et al., 2009; Miszkiewicz & Mahoney, 2016, 2019).
Given the relationships between biomechanical load and the osteocyte lacuno-canalicular network, we can predict that osteocyte lacunae and their canaliculi should vary between different anatomical regions (anterior, posterior, medial and lateral) of a single bone as per stress distribution of the bone shaft. Therefore, we examined osteocyte lacuno-canalicular network morphology in pre-prepared histological slides of medieval human mid-shaft femora (Miszkiewicz, 2016; Miszkiewicz & Mahoney, 2012, 2016) to address if density of osteocyte lacunae (Ot.Dn) could indicate whether or not the mechanosensory cells of bone are uniformly distributed throughout the bone cross section. We also conducted a subsequent ‘case study’ using this sample to test whether the number of osteocyte canaliculi per lacuna (Ci.N), and the density of canaliculi-rich osteocyte lacunae (Ci.Dn), also changes with anatomical region. This could provide a preliminary insight into osteocyte role in sensing and responding to varying biomechanical load and should explain the distribution and preservation of the communication network within bone (Rolvien et al., 2018).
2 MATERIALS AND METHODS
The human mid-shaft femoral samples analysed in this study were randomly selected from the Hard Tissue Histology collection of thin sections housed as part of the Biological Anthropology Collection at the School of Archaeology and Anthropology (Australian National University, Canberra). The samples are dated to the 11th–16th century AD and represent a larger British osteological collection curated at the Skeletal Biology Research Centre (University of Kent, UK) (see Miszkiewicz & Mahoney, 2017). There were 10 individuals represented in the study, each having four anatomical regions available for analysis (medial, lateral, anterior and posterior). As previously reported by Miszkiewicz and Mahoney (2012), following standard anthropological methods (Buikstra & Ubelaker, 1994), each individual was estimated to be a middle-aged (35–50 years old) male (Miszkiewicz & Mahoney, 2012). The femora were previously cross-sectioned in the transverse plane into 1 ± 0.2-cm femoral segments, which were later processed into thin sections (~100 μm) following standard methods (Miszkiewicz & Mahoney, 2017). This involved embedding the femoral samples in epoxy resin, sectioning on a Buehler IsoMet 1000 precision saw, grinding, polishing, dehydrating in ethanol baths, clearing in Histoclear® and covering with glass microscope slide covers.
2.1 Histomorphometric analysis
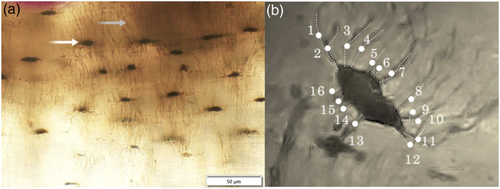
The thin sections were analysed using an Olympus BX53 high-powered microscope with a DP74 camera. From the thin sections, images representing regions of interest (ROIs) were multilayer captured using the Olympus CELL® Live Biology Imaging software that allows z-plane stacking while live imaging. These were taken from the midpoint of each sample mainly within the subperiosteal area of bone (Figure 1). However, in some cases, the ROIs crossed into the intracortical space where overlapping of ROIs in the subperiosteal region was unavoidable. All ROIs contained at least an approximate 50% of one secondary osteon captured at 40× magnification (Figure 1). For reference purposes and to avoid repeated capture, 10× or 20× captures were also taken for each anatomical region that contained the associated ROIs. In the case where extensive network was evident, higher magnifications, such as 60×, were used to aid in the microstructural analysis. The ‘multi-point’ tool of ImageJ® (Vol. 1.52) software was used to manually count the osteocyte lacunae and their canaliculi in each capture for an area of ~0.13 mm2, so that Ot.Dn, Ci.N and Ci.Dn could be computed. On the basis of the overall preservation within the sample, a threshold for inclusion in the count of ‘canaliculi-rich’ lacunae was five primary projecting canaliculi. Where ambiguity was apparent, additional tools were employed to aid in the analysis of the captures at 40×. For example, increased magnification at 60× was used to clarify whether each canaliculus was primary or secondary and also whether they projected from a neighbouring lacuna. The extent of branching was further confirmed when the sample was viewed in the z-plane stacking function. All ambiguous, branching or neighbour-originating canaliculi were excluded from the manual counts. Figure 2b provides an example of the final canaliculi count for a single isolated lacuna.
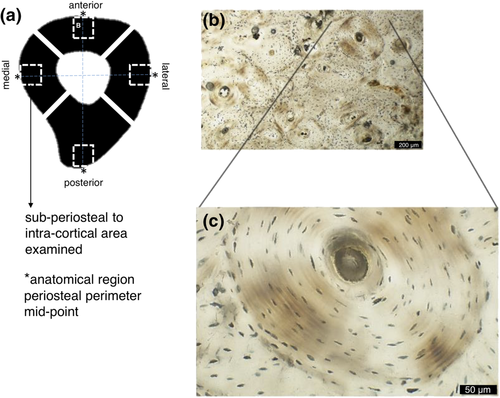
For the main goal of the study, a minimum of five ROIs were captured (aiming for 20 ROIs per individual = 200 in total) to compute Ot.Dn in each anatomical region. However, two ROIs showing poorly identifiable osteocyte lacunae on the posterior aspect were evident in two different individuals. This reduced the total ROI number to 198 from which data could be collected. The densities were calculated as number of lacunae per image area, that is, per ~0.13 mm2 (Miszkiewicz, 2016; Miszkiewicz & Mahoney, 2019). Any osteocyte lacuna in the capture with the appropriate morphology based on that outlined by Bonewald (2011) and Marotti, Ferretti, Remaggi, and Palumbo (1995) was included. Lacunae on the image borders were also counted.
For the subsequent case study, we undertook qualitative observations of the osteocyte lacunae occurrence subperiosteally and manual counts of extremely minute canaliculi (previously reported diameter range is 0.13–0.39 μm, You, Weinbaum, Cowin, & Schaffler, 2004). This meant we could only examine samples that were of pristine preservation at the osteocyte lacuna level. The two best preserved samples (Individuals SK2 and SK9) were selected for Ci.N and Ci.Dn analyses (Figure 2). Ci.N was the number of all visible primary canaliculi protruding directly out of each lacuna, whereas Ci.Dn was the number of osteocyte lacunae with rich canaliculi divided by image area. ‘Canaliculi-rich lacunae’ were defined as those with more than five extending canaliculi. For the Ci.N and Ci.Dn calculations, we aimed to capture six ROIs per anatomical region (anterior, posterior, medial and lateral). SK2 had six ROIs measured for Ci.N counts but five ROIs for Ci.Dn because six single ROIs of each anatomical region could not be clearly examined because of the close proximity of the limited accessible canaliculi-rich lacunae. Furthermore, the calculation of Ci.Dn was not possible in two ROIs on the anterior aspect in SK9 because of a lack of identifiable canaliculi; that is, Ci.N and Ci.Dn were zero. We could not find all six ROIs with suitably preserved lacunae, so only five ROIs were included in the analysis of Ci.N for SK9.
2.2 Statistical procedures
The statistical analysis was conducted in IBM SPSS® statistical software (2019). The sample size warranted the use of non-parametric inferential tests, but this was only applicable to the main goal of the study where all 10 individuals were examined. For the purpose of repeatability checks, intraobserver and interobserver tests were performed on randomly selected 20% of the images for Ot.Dn and 23% of the images for Ci.N. The Ot.Dn and Ci.N data were recounted by two co-authors (E. R. D. and J. J. M.) and then compared with the original counts using a Wilcoxon signed-rank test.
The descriptive analysis of all data was conducted by reporting the median, minimum, maximum and interquartile range (Q1 at 25% and Q2 at 75%) for each anatomical region for all three parameters (Ot.Dn, Ci.N and Ci.Dn). For the intraindividual descriptives, we only report the median, minimum and maximum as the interquartile ranges are not truly meaningful per individual. The case study also includes qualitative comments on histological observations.
To address the main goal of the study using inferential statistics, Ot.Dn was analysed using a non-parametric analysis of variance (ANOVA) (related-samples Friedman's two-way ANOVA) with a related-samples Wilcoxon signed-rank post hoc test to determine if the median distribution was equal. If the reported p was <0.05, the data were deemed statistically significant. The four anatomical regions were compared across all 10 individuals first. This was followed by an intraindividual analysis where Ot.Dn data were compared between the anatomical regions belonging to each individual. We also performed Spearman's ρ correlations from each region to test for possible femoral side mutual relationships in an increase or decrease of Ot.Dn. In addition to p < 0.05, the strength of correlations was interpreted from the ρ value (ρ > 0.68 = strong correlation, see Miszkiewicz, 2016).
3 RESULTS
The repeatability tests of osteocyte lacunae identification returned statistically insignificant differences between the original and repeated counts (intraobserver: p = 0.180, W = 12; interobserver: p = 0.343, W = 15). No statistically significant differences in the counts of canaliculi were identified either (intraobserver: p = 0.317, W = 0; interobserver: p = 0.564, W = 4).
3.1 Osteocyte lacunae densities
There was a statistically significant difference in Ot.Dn values between anterior, posterior, medial and lateral femoral regions in the entire sample (p = 0.001) (Table 1 and Figure 3a). Descriptively, the medial and lateral aspects of the bone had the highest median Ot.Dn, whereas the anterior and posterior aspects had relatively lower median values. This was further supported statistically. Post hoc comparisons indicated that the medial and lateral Ot.Dn consistently differed significantly from the anterior and posterior bone aspects (p < 0.05). The medial and lateral sections of the femoral mid-shaft also had the highest values in the 25% (Q1) and 75% (Q2) quartiles, despite the maximum lateral data point being the lowest when compared with the remaining anatomical locations. Spearman's ρ correlations also demonstrated a strong positive relationship between Ot.Dn from the medial and lateral aspects (ρ = 0.715, p < 0.001, n = 50), but a weak one when anterior and posterior regions were considered (ρ = 0.380, p = 0.008, n = 48) (Figure 3b).
| Femoral region | N ROIs | Min. | Max. | Median | Q1 | Q2 |
| Anterior (A) | 50 | 46.15 | 1830.77 | 211.54 | 121.15 | 465.38 |
| Posterior (P) | 48a | 23.08 | 1861.54 | 242.31 | 130.77 | 782.692 |
| Medial (M) | 50 | 53.85 | 2023.08 | 373.08 | 165.38 | 1194.23 |
| Lateral (L) | 50 | 15.38 | 1538.46 | 473.08 | 146.16 | 996.15 |
| Related-samples Friedman's two-way ANOVA | N ROIs | χ2 | df | p | ||
| A, P, M, L (n = 48) | 48 | 16.111 | 3 | 0.001* | ||
| Related-samples Wilcoxon signed-rank test | Test statistic | Std. test statistic | p | |||
| A, L (n = 50) | 926.000 | 2.785 | 0.005* | |||
| A, M (n = 50) | 1112.500 | 4.585 | <0.001* | |||
| P, M (n = 48) | 836.500 | 2.549 | 0.011* | |||
| P, L (n = 50) | 763.000 | 2.106 | 0.035* | |||
- Abbreviations: ANOVA, analysis of variance; df, degrees of freedom; max., maximum; min., minimum; N ROIs, number of regions of interest; Q1, lower quartile; Q2, upper quartile; std., standardized.
- a Excludes two ROIs with no Ot.Dn data due to poor preservation.
- * Statistically significant p < 0.05.
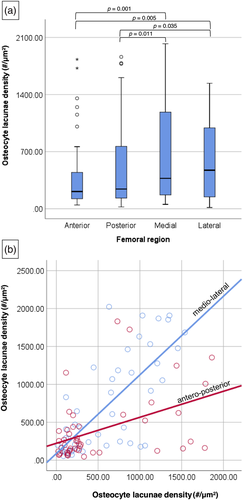
The density of osteocyte lacunae also differed significantly across the anterior, posterior, medial and lateral femoral mid-shaft sections in all individuals when considered intraindividually (p < 0.05) (Tables 2 and 3 and Figure 4), except for one individual (SK5). However, there was inconsistency in which pairs of mid-shaft locations differed from each other. For example, some individuals had similar data across almost all anatomical location comparisons, except for only one pair being statistically significantly different (e.g., medio-lateral in SK1 and antero-posterior in SK4). Out of all the anatomical pairs tested, the antero-posterior and medio-lateral comparisons were the most consistently statistically significantly different with six and five individuals having p < 0.05, respectively. Overall, it is apparent that the majority of our individuals showed variation in Ot.Dn between the anterior, posterior, medial and lateral bone regions.
| ID | Femoral region | N ROIs | Min. | Max. | Median | Q1 | Q2 |
|---|---|---|---|---|---|---|---|
| SK1 | Anterior | 5 | 153.85 | 384.62 | 253.85 | 203.85 | 338.46 |
| Posterior | 4 | 30.77 | 253.85 | 126.92 | 30.77 | 246.15 | |
| Medial | 5 | 269.23 | 469.23 | 292.31 | 276.92 | 438.46 | |
| Lateral | 5 | 115.38 | 161.54 | 138.46 | 115.38 | 153.85 | |
| SK2 | Anterior | 5 | 161.54 | 761.54 | 600.00 | 342.31 | 692.31 |
| Posterior | 5 | 800.00 | 1776.92 | 1138.46 | 930.77 | 1619.23 | |
| Medial | 5 | 915.38 | 1461.54 | 1069.23 | 984.62 | 1323.08 | |
| Lateral | 5 | 669.23 | 1269.23 | 853.85 | 703.85 | 1200.00 | |
| SK3 | Anterior | 5 | 69.23 | 115.38 | 92.31 | 73.08 | 115.38 |
| Posterior | 4 | 23.08 | 46.15 | 34.62 | 25.00 | 44.23 | |
| Medial | 5 | 53.85 | 169.23 | 69.23 | 57.69 | 157.69 | |
| Lateral | 5 | 15.38 | 184.62 | 84.62 | 38.46 | 161.54 | |
| SK4 | Anterior | 5 | 46.15 | 123.08 | 84.62 | 53.85 | 111.54 |
| Posterior | 5 | 138.46 | 438.46 | 153.85 | 138.46 | 384.62 | |
| Medial | 5 | 92.31 | 200.00 | 123.08 | 100.00 | 169.23 | |
| Lateral | 5 | 69.23 | 146.15 | 107.69 | 84.62 | 130.77 | |
| SK5 | Anterior | 5 | 253.85 | 446.15 | 346.15 | 261.54 | 442.31 |
| Posterior | 5 | 92.31 | 269.23 | 200.00 | 123.08 | 253.85 | |
| Medial | 5 | 192.31 | 2023.08 | 646.15 | 311.54 | 1426.92 | |
| Lateral | 5 | 223.08 | 630.77 | 284.62 | 234.62 | 615.38 | |
| SK6 | Anterior | 5 | 292.31 | 1153.85 | 592.31 | 346.15 | 896.15 |
| Posterior | 5 | 115.38 | 246.15 | 153.85 | 123.08 | 223.08 | |
| Medial | 5 | 338.46 | 1223.08 | 607.69 | 384.62 | 1057.69 | |
| Lateral | 5 | 446.15 | 807.69 | 653.85 | 473.08 | 738.46 | |
| SK7 | Anterior | 5 | 1007.69 | 1830.77 | 1353.85 | 1126.92 | 1776.92 |
| Posterior | 5 | 730.77 | 1861.54 | 1438.46 | 803.85 | 1823.08 | |
| Medial | 5 | 1530.77 | 1907.69 | 1869.23 | 1607.69 | 1907.69 | |
| Lateral | 5 | 1030.77 | 1538.46 | 1223.08 | 1076.92 | 1446.15 | |
| SK8 | Anterior | 5 | 69.23 | 215.38 | 153.85 | 84.62 | 200.00 |
| Posterior | 5 | 130.77 | 284.62 | 169.23 | 130.77 | 276.92 | |
| Medial | 5 | 115.38 | 207.69 | 130.77 | 115.38 | 180.77 | |
| Lateral | 5 | 184.62 | 315.38 | 223.08 | 184.62 | 292.31 | |
| SK9 | Anterior | 5 | 100.00 | 246.15 | 138.46 | 111.54 | 200.00 |
| Posterior | 5 | 246.15 | 1607.69 | 1207.69 | 380.77 | 1561.54 | |
| Medial | 5 | 1153.85 | 1853.85 | 1476.92 | 1215.38 | 1742.31 | |
| Lateral | 5 | 900.00 | 1453.85 | 1207.69 | 946.15 | 1396.15 | |
| SK10 | Anterior | 5 | 130.77 | 207.69 | 161.54 | 130.77 | 203.85 |
| Posterior | 5 | 61.54 | 292.31 | 161.54 | 80.77 | 280.77 | |
| Medial | 5 | 176.92 | 246.15 | 223.08 | 184.62 | 242.31 | |
| Lateral | 5 | 384.62 | 1061.54 | 884.62 | 573.08 | 1034.62 |
- Abbreviations: df, degrees of freedom; max., maximum data point; min., minimum data point; N ROIs, number of regions of interest; Q1, lower quartile; Q2, upper quartile.
| Related-samples Friedman two-way ANOVA | N ROIs | χ2 | df | p | ||
| SK1 | 20 | 8.132 | 3 | 0.043* | ||
| SK2 | 25 | 10.680 | 3 | 0.014* | ||
| SK3 | 20 | 8.100 | 3 | 0.044* | ||
| SK4 | 25 | 10.680 | 3 | 0.014* | ||
| SK5 | 25 | 5.880 | 3 | 0.118 | ||
| SK6 | 25 | 9.720 | 3 | 0.021* | ||
| SK7 | 25 | 6.840 | 3 | 0.077 | ||
| SK8 | 25 | 7.800 | 3 | 0.050 | ||
| SK9 | 25 | 10.680 | 3 | 0.014* | ||
| SK10 | 25 | 11.160 | 3 | 0.011* | ||
| Related-samples Wilcoxon signed-rank test | AP | AM | AL | PM | PL | ML |
| SK1 | 0.285 | 0.104 | 0.080 | 0.068 | 1.000 | <0.05* |
| SK2 | <0.05* | <0.05* | <0.05* | 0.225 | 0.225 | 0.225 |
| SK3 | <0.05* | 0.080 | 0.686 | <0.05* | <0.05* | 0.345 |
| SK4 | <0.05* | 0.225 | 0.893 | 0.080 | 0.138 | 0.225 |
| SK5 | 0.066 | 0.893 | 0.225 | 0.068 | 0.068 | 0.500 |
| SK6 | <0.05* | 0.225 | 0.893 | <0.05* | <0.05* | 0.893 |
| SK7 | <0.05* | 0.686 | 0.078 | 0.225 | 0.345 | <0.05* |
| SK8 | 0.686 | <0.05* | 0.345 | 0.225 | 0.686 | <0.05* |
| SK9 | <0.05* | <0.05* | <0.05* | 0.225 | 0.500 | <0.05* |
| SK10 | 0.893 | <0.05* | <0.05* | 0.500 | <0.05* | <0.05* |
- Abbreviations: A, anterior; ANOVA, analysis of variance; df, degrees of freedom; L, lateral; M, medial; N ROIs, number of regions of interest (minimum five per anatomical location); P, posterior.
- * Statistically significant p < 0.05.
3.2 Canaliculi number per osteocyte lacuna and canaliculi-rich osteocyte lacunae density case study
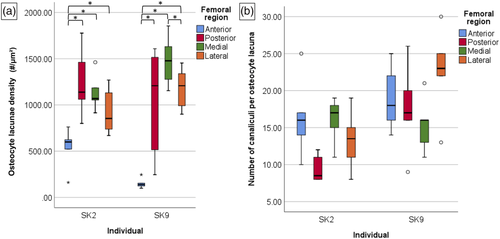
Descriptively, the median of Ci.N was highest on the medial but lowest on the posterior anatomical region in SK2 (Figure 4). The Ci.N data were almost consistent across the femoral regions in individual SK9, except in the case of the lateral region. In SK9, the highest median Ci.N was observed in the lateral region, and the lowest median was apparent in the medial region. Thus, in a similar manner to the Ot.Dn results presented for the entire sample (Tables 1 and 2 and Figure 3a), these data suggest that one individual showed variation in Ci.N, whereas the other did not, though a larger sample size will validate these findings in the future. Descriptively, the highest median Ci.Dn was observed in the medial followed by the anterior regions and the lowest in the posterior region in individual SK2. In SK 9, Ci.Dn was the largest on the lateral side, though we cannot make a more comprehensive comment as the anterior side only had three ROIs examined (Table 4). Visually, it appeared that osteocyte lacunae located immediately closely to the periosteal border were somewhat richer in their canaliculi numbers.
| ID | Femoral region | N ROIs | Min. | Max. | Median | |
|---|---|---|---|---|---|---|
| SK2 | A | Ci.N | 6 | 10.00 | 25.00 | 16.00 |
| Ci.Dn | 5 | 61.54 | 807.69 | 192.31 | ||
| P | Ci.N | 6 | 8.00 | 12.00 | 8.50 | |
| Ci.Dn | 5 | 23.08 | 100.00 | 38.46 | ||
| M | Ci.N | 6 | 11.00 | 19.00 | 17.00 | |
| Ci.Dn | 5 | 100.00 | 738.46 | 192.31 | ||
| L | Ci.N | 6 | 8.00 | 19.00 | 13.50 | |
| Ci.Dn | 5 | 61.54 | 738.46 | 130.77 | ||
| SK9 | A | Ci.N | 5 | 14.00 | 25.00 | 18.00 |
| Ci.Dn | 3 | 7.69 | 23.08 | 7.69 | ||
| P | Ci.N | 5 | 9.00 | 26.00 | 17.00 | |
| Ci.Dn | 5 | 7.69 | 84.62 | 38.46 | ||
| M | Ci.N | 5 | 11.00 | 21.00 | 16.00 | |
| Ci.Dn | 5 | 23.08 | 76.92 | 61.54 | ||
| L | Ci.N | 5 | 13.00 | 30.00 | 23.00 | |
| Ci.Dn | 5 | 46.15 | 184.62 | 161.54 | ||
- Abbreviations: A, anterior; L, lateral; M, medial; Max., maximum data point; Min., minimum data point; N ROIs, number of regions of interest; P, posterior.
4 DISCUSSION
Limited prior osteoarcheological research has considered osteocyte lacunae and linked them to behaviour. One example includes 11th–16th century medieval English samples (also the subjects of our study, Miszkiewicz, 2016). Osteocyte lacuna characteristics were also previously examined in an Iron Age (3rd–5th century BC, Alfedena and Sulmona, Italy) sample, describing diagenesis at the microscopic level (Capasso & Tota, 1993). Results from our study indicate that the medieval individuals had statistically significant variation in osteocyte lacunae among the anterior, posterior, medial and lateral anatomical regions. Thus, our results expand the limited data currently available in the osteoarchaeological literature, benefiting future studies that infer past behaviour from the human femur.
4.1 Behavioural links
Previous research on this sample indicated femoral morphology to range from gracile to robust, with some associations to histology, hinting at bone structure hierarchical effects of functional adaptation in this sample (Miszkiewicz & Mahoney, 2012). When comparing the bone histology with anatomical region, we found that there were more consistent regions for trends in Ot.Dn, namely, the medial and lateral regions. Both had the highest median osteocyte lacunae, and both differed significantly from the posterior and anterior aspects, where the osteocyte lacunae median was lower. This suggests that there is less paired variation in Ot.Dn among individuals in these portions of the femoral mid-shaft. These results suggest that mid-shaft femoral anatomical location, which undergoes morphological changes with biomechanical load, affects the expression of bone microstructure at the osteocyte level.
Our main finding that osteocyte lacunae increase on both the lateral and medial aspects of the femur, whereas the anterior and posterior sides of the bone are not as influenced by this increase, is consistent with conclusions drawn in the literature where fresh human bone has been studied. For example, a cadaveric human histological study by Gocha and Agnew (2015) exploring osteon population density variation in the human femoral mid-shaft determined that the lateral and antero-lateral regions of the femoral transverse cross sections experience the highest strain magnitude and tensile strain resulting in higher presence of secondary osteons in these regions. Their finding is further supported by previously reported positive correlations between secondary osteon population densities and osteocyte lacunae densities (Miszkiewicz, 2016), implying that these microstructural variables express localized increase or decrease of remodelling activity stimulated by behaviour. A study by Stigler et al. (2019) explored the distribution of osteocytes across cranial, axial and limb areas of the skeleton and reported that cortical bone showed variation among anatomical sites, whereas trabecular bone did not. This was consistent with what was discovered in our study; however, our degree of variation among anatomical sites was not always statistically significant.
The case study results indicating that SK2 and SK9 show some differences in canaliculi-rich lacunae suggest that individual behaviour could possibly influence the morphological expression of osteocyte lacunae. This would be worth exploring further in individual human cases with documented behaviour, where known biomechanical loading histories can be potentially matched to femoral macromorphology and micromorphology. We also observed some variation in the distribution of canaliculi-rich osteocyte lacunae, whereby the more outer (closer to the subperiosteal bone) regions of the femur had somewhat higher canaliculi counts. This may indicate the osteocyte needs for more nutrients, communication and/or bone remodelling (Bonewald, 2011) (Figure 5). Previously, observations of canaliculi identified anatomical sites responsible for the generation of strain potentials (Cowin, Weinbaum, & Zeng, 1995). Rolvien et al. (2018) suggested that there was a reduction in both canaliculi density and number per lacuna with age in the femur. Marotti, Ferretti, Remaggi, and Palumbo (1995), examining tibiae, also noted that canaliculi density was not significantly variable within secondary osteons but instead found that there was a strong constitutive negative regulation of osteoclasts and positive regulation of osteoblasts by osteocytes through their canaliculi. We propose that further studies using fresh or post-mortem bone could test whether more canaliculi-rich osteocyte lacunae are indeed also situated at the periosteal border (Figure 5), possibly linked to increased biomechanically induced remodelling demands.
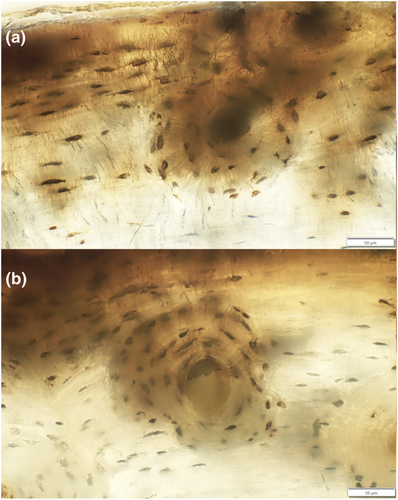
4.2 Remarks on limitations and multidimensional visualization of osteocyte morphology
One limitation of our study was not measuring bone porosity, which is a variable that has been previously positively correlated with osteocyte lacunae densities (Dong et al., 2013). Therefore, abnormal bone porosity, which occurs with age and/or disease, may associate with increased osteocyte lacunae (Tiede-Lewis & Dallas, 2019). However, this would be inconsistent with what is known about bone pathologies such as osteoporosis, osteoarthritis, osteomalacia, osteopenia and osteopetrosis, which are associated with deteriorating bone quality and related histological structures (Oliveira et al., 2016; Tatsumi et al., 2007).
Mullender, van der Meer, Huiskes, and Lips (1996) also suggest that osteocyte density decreases with age, likely through micropetrosis. A study by Tiede-Lewis and Dallas (2019) supplements these conclusions by reporting that although osteocyte density does not follow a particular pattern throughout bone, the lacuno-canalicular network of osteocytes does show variation with ageing. Specifically, they identified that canaliculi reduced in densities and lacunae deformed and deteriorated with age (Tiede-Lewis & Dallas, 2019).
Another limitation of our study is the sample size of 10. We also relied on the assumption that one canaliculus contained one filopodia, which is a necessary methodological oversimplification, though it is likely that more than one filopodia may have projected into the canaliculus (Marotti, Ferretti, Remaggi, & Palumbo, 1995). Future research should combine two-dimensional (2D) thin sectioning with a three-dimensional (3D) approach such as micro-computed tomography (CT) or laser confocal scanning, as this will allow consideration of the lacunae shape and connectivity between individual osteocytes (Andronowski, Crowder, & Soto, 2018; Dallas & Moore, 2020). Our 2D approach only provides information on a single orientation of the lacuno-canalicular complex, which might have particularly underlied some differences in Ci.Dn and Ci.N data between SK2 and SK9 in our case study. Where possible, if access to macroscopic information is available (e.g., bone robusticity or exterior morphology), it may help to improve understanding of the microscopic variation with femoral size (see Miszkiewicz & Mahoney, 2019). Additionally, future studies would benefit from testing the distribution of canaliculi-rich lacunae statistically to determine whether location within the mid-shaft femoral cross section affects Ci.Dn and Ci.N.
5 CONCLUSION
This study reported intraindividual and interindividual variation in the osteocyte lacuno-canalicular network in the human femoral mid-shaft in a sample of medieval males. The results showed that the medial and lateral femoral regions had the highest densities of osteocyte lacunae when compared with anterior and posterior femoral aspects. The results correspond to what is known in literature in other species, as well as in fresh human bone. The data also agree with the preservation of biomechanical loading patterns in humans, as well as the lacuno-canalicular network, which changes in morphology with age, disease and/or behaviour. This suggests that the reconstruction of past human behaviour within osteoarchaeology could incorporate osteocyte lacunae analyses into their microscopic sampling and analysis protocols.
Not only can this be used as a complementary method to the bone exterior shape and size data, but histological analyses can also be applied to fragmented human remains where the external anatomy is compromised (Crescimanno & Stout, 2012; Cuijpers, 2006; Cummaudo et al., 2019; Haas & Storå, 2015; Lemmers, Gonçalves, Cunha, Vassalo, & Appleby, 2020). On the basis of our data, future researchers may be able to estimate which anatomical region a mid-shaft femoral fragment derives from.
ACKNOWLEDGEMENTS
The authors would like to thank the University of Kent for facilitating access to the medieval osteological collection; David McGregor (Australian National University [ANU]) for technical support; ANU College of Arts and Social Sciences (CASS) and the Australian Research Council (DE190100068) for funding; Hannah Miles (ANU) for research assistance and discussions; and the reviewers and Editor Debra Martin for their constructive contributions to our manuscript. The thin sections examined in this study were created under a 2010–2014 GTA PhD studentship at the University of Kent (awarded to Miszkiewicz).
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.




