A palaeoepidemiological investigation of osteomata, with reference to medieval Poland
Abstract
The osteoma, among other forms of benign neoplastic disease, has received little palaeopathological or palaeoepidemiological interest largely because of its asymptomatic nature. This is problematic because these tumours are regarded as common occurrences in bioarchaeological contexts, despite there being scant data to support these claims. This investigation presents a palaeoepidemiological enquiry into osteomata. Five hundred ninety individuals from six skeletal assemblages from Poland, dating from the 9th to 17th century, were macroscopically surveyed for osteomata. This was followed by a palaeoepidemiological analysis, looking at sex- and age-specific prevalence. Ninety-three osteomata were observed in 67 individuals. The sex-specific prevalence was 13.5% (95% confidence interval [CI]: 9.7–18.1) for males and 11.6% (95% CI 7.9–16.2) for females. The age-specific prevalence for middle adults was 2.1% (95% CI: 0.6–5.2) and 5.3% (95% CI: 2.5–9.8) for mature adults. The results indicated the prevalence of benign tumours was similar between males and females and seemed to increase with age. This case study adds to a sparse area of palaeo-oncological research and calls for further future investigation.
1 INTRODUCTION
Benign tumours are noninvasive neoplasms, which typically arise during childhood or adolescence and continue to slowly grow throughout life (Dorfman et al., 2002; Hakim, Pelly, Kulendran, & Caris, 2015; Vlychou & Athanasou, 2008). In palaeopathology, benign neoplasms have received little attention, unlike their malignant counterparts, and of the vast array of benign skeletal neoplasms, the osteoma has seemed to receive the least amount of enquiry. For example, although the osteoma has been described as a common occurrence in archaeological skeletal remains (Giuffra et al., 2019; Ortner, 2003; Roberts & Manchester, 2010), there has been scant research regarding the prevalence of this lesion, confirming the above assertion. The lack of palaeopathological and palaeoepidemiological investigation into osteomata and other forms of benign neoplasms is problematic to an overall understanding of neoplastic disease in bioarchaeological contexts (Siek, 2019).
Clinically, the osteoma is a round-shaped, slow-growing osteoblastic lesion (Campillo, 1998). It is comprised of compact bone with well-defined borders and no surface irregularities (Hakim et al., 2015). Osteomata typically arise from the bone (homoplastic) or can arise in other tissues (heteroplastic), such as a choroidal osteoma of the eye (Hakim et al., 2015; Shields, Shields, & Augsburger, 1988). In the skeleton, osteomata most commonly occur on the outer table of the cranium, particularly on the frontal and parietal bones, and in the frontal and paranasal sinuses (Greenspan, Jundt, & Remagen, 2007). They may also occur on the mandible and seldomly on the long and short tubular bones, but such cases are rare, representing <0.03% of biopsied primary bone lesions (Peyser et al., 1996). Osteomata affect males and females equally and are most often reported between the ages of 30 and 50 years (Greenspan et al., 2007).
Osteomata are typically asymptomatic and incidental findings; however, they may have adverse secondary effects, depending on their size and location. For instance, in the paranasal sinuses, osteomata may block nasal ducts, leading to headaches, pain and the possible loss of sense of smell (Greenspan, 1993). An osteoma may also develop and encroach in the orbit, resulting in possible exophthalmos and vision loss, if it presses against the optic nerve (Greenspan, 1993). If it is an endocranial lesion, it may press against the brain causing neurological problems (Greenspan, 1993). In cases such as these, surgical intervention may be required to alleviate swelling, nerve compression and cosmetic issues (Eyesan, Idowu, Obalum, Nnodu, & Abdulkareem, 2011).
The occurrence of multiple osteomata has been linked to the autosomal disorder Gardner's syndrome, which is a phenotypic variant of familial adenomatous polyposis (Greenspan, 1993; Hakim et al., 2015; Koh, Park, & Kim, 2016). Clinically, Gardner's syndrome is marked by multiple osteomata, commonly occurring on the mandible and long bones (Bilkay et al., 2004; Chimenos-Küstner, Pascual, Blanco, & Finestres, 2005; Koh et al., 2016; Panjawani, Bagewadi, Keluskar, & Arora, 2011). Of patients with Gardner's syndrome, 68% to 82% present with multiple osteomata (Chimenos-Küstner et al., 2005; Giuffra et al., 2019).
There is some debate in the clinical and palaeopathological literature if osteomata can be considered true neoplasms (Eshed et al., 2002; Gundewar, Kothari, Mokal, & Ghalme, 2013). For the purpose of this investigation, we consider the osteoma to be a benign neoplasm. Considering that benign neoplasms are composed of differentiated and mature tissues and their structure does not differ significantly from surrounding normal tissues, osteomata meet these criteria (Pierce & Damjanov, 2006). Although the mechanisms of osteomata formation are not clear, whichever factor (e.g., trauma or genetic predispositions) induces the process of their growth, it leads to a local overgrowth of bony tissue (Hakim et al., 2015). Osteomata are therefore benign neoplasms, which take the form of a tumour. It should be also noted that there are many neoplasms that do not form soft tissue tumours, such as leukaemia. By contrast, there are also tumours and tumour-like lesions that are often included in clinical and palaeopathological surveys, such as giant cell tumours and simple bone cysts (Greenspan et al., 2007; Marques, 2019).
2 MATERIALS AND METHODS
The skeletal assemblages examined in this investigation were sourced from the osteological collection of the Department of Anthropology, Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences (HIIET-PAS), in Wrocław, Poland (Figure 1). For this investigation, six human skeletal assemblages were examined. These assemblages were from medieval Polish cemeteries dating from the 9th to17th century (Table 1).
| Skeletal assemblage | Polish region/province | Chronological date | Number of individuals | Reference |
|---|---|---|---|---|
| Tomice | Lower Silesia | 9–12th century | 22 | Miszkiewicz, 1968a; Romanow, Miszkiewicz, & Wachowski, 1973 |
| Sandomierz | Holy Cross | 10–12th century | 25 | Sarama, 1957; Zoll-Adamikowa, 1966 |
| Wrocław (Ołbin, pl. Dominikański, św. Elżbieta, św. Jakub) | Lower Silesia | 12–15th century | 188 | Kwiatkowska & Gronkiewicz, 2003; Kwiatkowska, 2005 |
| Milicz | Lower Silesia | 12–13th century | 233 | Miszkiewicz & Gronkiewicz, 1986 |
| Gródek nad Bugiem | Masovia | 13–17th century | 73 | Belniak, Krupińksi, Magnuszewicz, Rauhut, & Szczotkowa, 1961 |
| Pawłów Trzebnicki | Lower Silesia | 15–16th century | 49 | Miszkiewicz, 1968b |
Clearly defined inclusion criteria were established for the HIIET-PAS assemblage data collection to ensure standardization and to form the final denominators for the palaeoepidemiological analyses. The skeletal material included in this investigation incorporated individuals regardless of sex or age. The skeletal elements examined in each individual included the cranium and mandible as these are the regions where osteomata are most likely to develop (Greenspan et al., 2007). Although osteomata occurring on the long bones are rare, the femora, tibiae and humeri were also examined.
The examination of the skeletal material began with assessing the sex of each individual through standard anthropological methods, using the morphology of the ossa coxae and cranium (Buikstra & Ubelaker, 1994; Phenice, 1969). The age-at-death of the individuals was determined by assessing the morphology of the pubic and auricular surfaces (Brooks & Suchey, 1990; Buikstra & Ubelaker, 1994; Lovejoy, Meindel, Przybeck, & Mensforth, 1985; Todd, 1921). The individuals were sorted into standard age categories: subadult (≤19 years), young adult (20–34 years), middle adult (35–49 years) and mature adult (≥50 years) (Buikstra & Ubelaker, 1994).
The skeletal material was then analysed macroscopically based on the established palaeopathological guidelines for neoplasms. This included identifying if the lesion was osteoblastic or osteolytic, if it had clear or ill-defined borders and if it was solitary or multiple. (Nerlich, Zink, & Löhrs, 1997; Ragsdale, Campbell, & Kirkpatrick, 2018). The operative diagnostic definition for an osteoma was a round-shaped lesion that consisted of well-differentiated mature bone tissue with a predominantly lamellar surface (Greenspan et al., 2007).
The identified osteomata were then recorded and photographed. All the lesions were measured using standard, 0.1-cm spreading digital callipers. In cases where the lesions were located in areas that were deemed too fragile or difficult to access with callipers, a soft plastic measuring tape was used. A hand-held endoscopic light was used to examine the interior of complete crania, cranial sinuses and bones where postmortem damage allowed access.
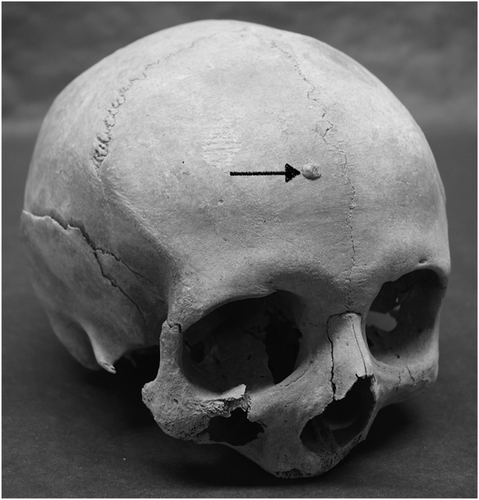
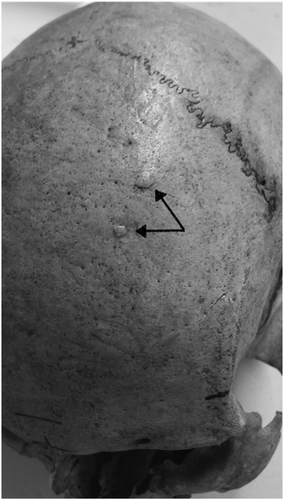
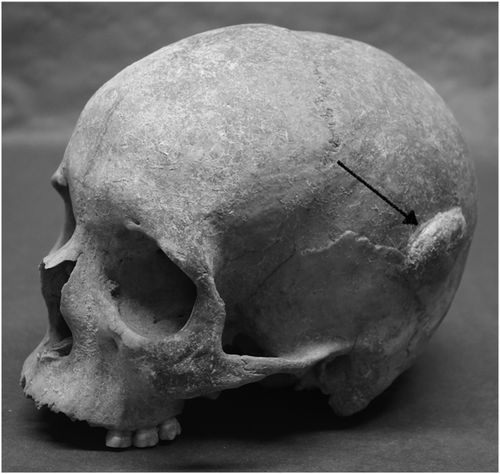
The typology developed by Eshed et al. (2002) was applied to all osteomata that were observed. This included three size categories and four distribution patterns. The size categories were small (1–2 mm), medium (3–5 mm) and large (≥6 mm). The first distribution pattern was 'button osteoma' consisting of a solitary, circular ivory-like exostosis with sharply defined margins. The next pattern was ‘satellite osteoma’ where flat, small, ivory-like roundels are located in close proximity to a large lesion. This was followed by ‘nested osteoma’ where two or more small osteomata are uniform in size and in close proximity with each other. The fourth pattern was ‘disseminated osteoma’ characterized by three or more lesions distributed over a large area of the cranium (Eshed et al., 2002).
Since all palaeoepidemiological investigations are cross-sectional in nature, the only measure of disease frequency that can be calculated is prevalence, which is the proportion of a defined group having a disease condition at a single point in time (Waldron, 2007). An odds ratio was calculated to assess the odds of benign neoplasm development for sex and age. The guidelines set forth by Sahai and Khurshid (1996) were used for further clarification regarding the interpretation of the odds ratio. Ninety-five percent confidence intervals were calculated to indicate the precision or imprecision of the prevalence proportions and odds ratios (Gardner & Altman, 2000). All calculations were performed using the open-source statistics software, MedCalc. The software calculates the “exact” Clopper–Pearson confidence interval for prevalence (Clopper & Pearson, 1934; Fleiss, Levin, & Paik, 2003) and calculated the odds ratio and its confidence interval according to Altman (1991).
3 RESULTS
From the six skeletal assemblages at HIIET-PAS, a total of 590 individuals were examined (Table 2). The largest age category was middle adult (n = 195); the sex ratio was almost even with 282 males and 251 females. Osteoma was present in at least one individual from each of the six cemetery assemblages (Figures 2-4), and in total, 93 osteomata were observed in 67 individuals. All the osteomata presented as round, almost circular, growths of smooth cortical bone. They were limited to the cranium, with the frontal bone being the most frequent location, but some were also found on the parietal and occipital bones. Half of the observed osteomata were in the medium-size group, and the average size was 5.9 × 5.1 mm. The majority were classified to the button osteoma distribution pattern (Table 3).
| Assemblage | Male | Female | Undefined | Subadult | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young adult | Middle adult | Mature adult | Undefined | Young adult | Middle adult | Mature adult | Undefined | Young adult | Middle adult | Mature adult | Undefined | |||
| Tomice | 1 | 5 | 2 | 0 | 1 | 6 | 5 | 1 | 0 | 0 | 0 | 0 | 1 | 22 |
| Sandomierz | 0 | 5 | 10 | 0 | 0 | 2 | 2 | 2 | 0 | 1 | 1 | 1 | 1 | 25 |
| Wrocław | 0 | 41 | 33 | 10 | 1 | 42 | 23 | 6 | 3 | 9 | 1 | 15 | 4 | 188 |
| Milicz | 3 | 34 | 22 | 55 | 6 | 16 | 11 | 72 | 1 | 4 | 0 | 5 | 4 | 233 |
| Gródek nad Bugiem | 0 | 7 | 19 | 15 | 2 | 9 | 14 | 6 | 0 | 0 | 0 | 0 | 1 | 73 |
| Pawłów Trzebnicki | 0 | 9 | 10 | 1 | 2 | 5 | 17 | 0 | 0 | 0 | 0 | 2 | 3 | 49 |
| Total | 4 | 101 | 96 | 81 | 12 | 80 | 72 | 87 | 4 | 14 | 2 | 23 | 14 | 590 |
| Position | |
|---|---|
| Cranial position | Number of osteomata |
| Frontal | 57 |
| Parietal | 32 |
| Occipital | 3 |
| Bregma | 1 |
| Size | |
|---|---|
| Size category | Number of osteomata |
| Small | 15 |
| Medium | 47 |
| Large | 31 |
| Distribution | |
|---|---|
| Distribution pattern | Number of individuals |
| Button osteoma | 57 |
| Satellite osteoma | 5 |
| Nested osteoma | 1 |
| Disseminated osteoma | 4 |
Among the assemblages at the HIIET-PAS, 67 of 590 individuals exhibited at least one osteoma; therefore, the crude prevalence for the entire HIIET-PAS assemblage (Table 4) was 11.4% with a 95% confidence interval (CI) of 8.9 to 14.2. The sex-specific prevalence for males was 13.5% (95% CI: 9.7–18.1) and 11.6% (95% CI: 7.9–16.2) for females. The age-specific prevalence for the middle adult group was 2.1% (95% CI: 0.6–5.2) and 5.3% (95% CI: 2.5–9.8) for the mature adult group (Table 5). The confidence intervals have the expected inverse relationship with the numbers in the assemblage, that is, the larger the assemblage, the narrower the confidence intervals tend to be. Where the confidence interval is narrow (e.g., the mature adults in Table 5), this suggests that the precision of the estimate of the prevalence is high (Szumilas, 2010). The odds ratio comparing males versus females was 0.9 (95% CI: 0.5–1.4) suggesting that females were slightly, but not significantly, less likely to have a benign neoplasm than males. With regard to age, the odds ratio comparing middle and mature adults was 2.6 (95% CI: 0.8–8.5), which suggests that mature adults were more likely to have a benign neoplasm than the younger age group.
| Assemblage | Cases | Assemblage total | Prevalence (%) | 95% CI range | |
|---|---|---|---|---|---|
| Tomice | 1 | 22 | 4.5 | 0.8–21.8 | |
| Sandomierz | 2 | 25 | 8.0 | 2.2–25 | |
| Wrocław | 6 | 188 | 3.2 | 1.5–6.8 | |
| Milicz | 44 | 233 | 18.9 | 14.1–24.5 | |
| Gródek nad Bugiem | 10 | 73 | 13.7 | 7.6–23.4 | |
| Pawłów Trzebnicki | 4 | 49 | 8.2 | 3.2–19.2 | |
| Total | 67 | 590 | 11.4 | 8.9–14.2 | |
| Cases | Assemblage total | Prevalence (%) | 95% CI range | |
|---|---|---|---|---|
| Male | 38 | 282 | 13.5 | 9.7–18.1 |
| Female | 29 | 251 | 11.6 | 7.9–16.2 |
| Subadult | 0 | 14 | 0.0 | - |
| Young adult | 0 | 21 | 0.0 | - |
| Middle adult | 4 | 195 | 2.1 | 0.6–5.2 |
| Mature adult | 9 | 170 | 5.3 | 2.5–9.8 |
4 DISCUSSION
In palaeopathology, research regarding osteomata has typically been approached on a case-by-case basis (Bartelink & Wright, 2011; Castro et al., 2019; Giuffra et al., 2019; Galassi, Varotto, Angelici, & Picchi, 2020; Premužić & Šikanjic, 2013; Smith, 2010). In regard to palaeoepidemiology, the prevalence of osteomata is typically reported in general terms. For instance, in Greece, a large number of osteomata were observed in several skeletons from Thasos (Agelarakis, 2002), as well as Thrace and other Aegean islands (Bourbou, 2003), but no other details, such as an exact number, anatomical location or size, were given by the researchers. In the investigation of a 5th century CE skeletal assemblage from the Czech Republic, the prevalence of osteoma was reported to be 3.9%, but there was no further discussion regarding these tumours (Vargová et al., 2016). Pálfi (1989) reported osteoma in six individuals from a Hungarian skeletal assemblage but focused his discussion on the prevalence of the observed malignant tumours. The prevalence of osteomata has also been alluded to when reviewing the palaeoepidemiology of neoplastic disease as a whole. For example, Gładykowska-Rzeczyska (1991) and Farkas, Józsa, Paja, and Molnár (2007) noted osteomata in their palaeopathological surveys, but prevalence was only reported for benign tumours as a single group, rather than individual tumour types, and there was no consideration of age- or sex-specific prevalence. Only two studies directly related to the palaeoepidemiology of osteomata were found in the palaeopathological literature. The first investigated the anatomical location of osteomata in regard to age and sex (Antona Montoro, Rodríguez González, Campo Martín, n.d.). The study showed that the majority of osteomata were observed among the young adult age group (20–34 years). Males had a higher frequency of osteomata on the frontal and parietal bones, and females were observed to have a higher frequency of osteomata on the occipital bone (Antona Montoro et al., n.d.). The second investigation compared the frequency of osteomata in modern and archaeological samples (Eshed et al., 2002). The results showed a prevalence of 41% in the archaeological sample and 37.6% in the modern sample, with no difference between sex or ancestry (Eshed et al., 2002).
The results of this palaeoepidemiological investigation indicate that the prevalence of osteomata was similar between males and females but seemed to increase with age. This trend is similar to that reported by Eshed et al. (2002) in their study on modern osteomata in the Hamann–Todd collection. Similar to Antona Montoro et al. (n.d.), the majority of the observed osteomata were on the frontal and parietal bones. The lack of observable osteomata in subadults and young adults is possibly due to their slow growth characteristics. Although these neoplasms tend to begin developing during childhood and adolescence, their slow growth may result in the tumours not becoming macroscopically visible until adulthood (Greenspan et al., 2007). As these tumours may occur in any region of the skull and sinuses, searching for them microscopically would be highly difficult and impractical. Another possibility is that an individual died from other causes before the tumour had yet reached a macroscopically visible size. The large number of osteomata is notable as this form of tumour is thought to be underrepresented in modern clinical literature because of its asymptomatic nature and incidental detection (Giuffra et al., 2019).
Although most clinical sources include osteoma in their overviews of osteogenic, benign tumours (El-Mofty, 2009; Greenspan et al., 2007; Hogendoorn & de Andrea, 2019; Wei & Stevens, 2014), there has been some debate whether this lesion can be considered a true neoplasm, and no definite conclusion has been reached either clinically or palaeopathologically (Gundewar et al., 2013; Odes et al., 2018). Kaplan, Calderon, and Buchner (1994) argued against mandibular osteoma as neoplastic because the lesion's growth potential and growth rate are limited, recurrence after surgical excision is rare and there have been no clinical reports of malignant transformation. Instead, it is possible that mandibular osteoma may be a response to trauma. The proposed mechanism was that trauma caused subperiosteal bleeding that would elevate the periosteum and initiate an osteogenic response (Kaplan et al., 1994). Capasso (1997) classified osteoma as a form of circumscribed idiopathic hyperostosis. These forms of hyperostosis are of unknown aetiology and are similar to neoplasms, such as having a slow unlimited growth, but are not pathological. Although the rational of Kaplan et al., (1994) can also be applied to cranial osteomata, Eshed et al. (2002) suggested that, based on their histological features, these lesions be reclassified as hamartoma, a malformation that resembles a neoplasm but results from faulty development. In palaeopathological case reports of osteomata, these lesions are referred to as tumours, and the debate regarding their status as a neoplasm is either not mentioned or not discussed (Blau, 2006; Castro et al., 2019; Dąbrowski et al., 2015; Galassi et al., 2020; Giuffra et al., 2019; Licata, Borgo, Armocida, Nicosia, & Ferioli, 2016; Piombino-Mascali, Zink, & Panzer, 2017; Premužić & Šikanjic, 2013; Odes et al., 2018; Smith, 2010; Zias, 2006). Bartelink and Wright (2011) seem to be the only researchers who did note this debate in their report of a mandibular tumour from Guatemala, dated to the 6th to 9th century CE. The lesion was described as a dense, solitary outgrowth of the bone on the mandibular corpus with clear demarcations from the surrounding bone. It was classified as a benign lesion because of its localized growth, its well-defined borders, its density suggesting slow growth and its lack of spicules or osteolysis. In their differential diagnosis, Bartelink and Wright (2011) included neoplastic lesions, such as osteoma, and hyperplastic lesions, such as hamartoma.
The present investigation adds to the almost nonexistent palaeoepidemiology of benign tumours, which certainly existed in antiquity, with numerous cases having been found in prehistoric animals. The earliest fossil evidence of an osteoma was found in a Phanerosteon mirabile, a fish from the Lower Carboniferous period, approximately 300 million years ago (Capasso, 2005; Moodie, 1927). Osteoma was exhibited by hominins such as Homo naledi, one of which displayed a mandibular osteoma (Odes et al., 2018). Despite these examples, along with numerous palaeopathological case reports, there has never been an in-depth investigation into the prevalence of benign tumours. They are considered to be a common occurrence in palaeopathology, but this has never been empirically confirmed (Giuffra et al., 2019; Siek, 2019). This parallels modern clinical literature where the frequency of benign tumours is debated and unclear (Hakim et al., 2015). The apparent apathy towards benign tumours has also created a circular argument within palaeopathology. This argument is as follows: The supposedly common and asymptomatic nature of benign tumours, which precludes them from meaningful investigation, has resulted in a lack of data; however, as these tumours are largely asymptomatic and common, there is no great need to research their aetiology or palaeoepidemiology.
5 CONCLUSION
Utilizing Polish human skeletal material from six medieval assemblages, age- and sex-specific prevalence was calculated for observed osteomata. The results indicated that although females were slightly less likely to have a neoplasm than males, sex was not a significant factor. By contrast, mature adults were much more likely to have an osteoma. The lack of osteomata among young adults may be due to their slow growth and the inability to detect them on a macroscopic level, similar to modern clinical contexts. This investigation adds to the sparse literature in palaeopathology regarding benign osteomata. Despite being regarded as common, they remain enigmatic and warrant further future investigation.




