Cerebellar activation during a motor task in conversion disorder with motor paralysis: A case report and fMRI study
Abstract
Background
Motor conversion disorders are characterized by movement symptoms without a neurological cause. A psychogenic etiology is presumed for these disorders, but little is known about their underlying neural mechanisms. Functional magnetic resonance imaging (fMRI) has been utilized to understand the mechanisms associated with unexplained motor symptoms. Here, we used fMRI to investigate the cerebral response to motor stimulation in a patient with conversion disorder with motor paralysis to determine the underlying neural mechanisms of this disorder.
Methods
Brain activation induced by movements of the bilateral ankle joints (repeated plantar flexion and dorsiflexion) was recorded using fMRI in a patient with conversion disorder with unexplained motor paralysis. We acquired 2 types of imaging data: (i) data obtained while motor paralysis remained present and (ii) data obtained after motor paralysis had completely improved. We used a within-subject fMRI block design to compare the patient's brain activities during the motor task and at rest.
Results
Cerebral motor areas were significantly activated during the motor task relative to at rest, both when motor paralysis remained present and when paralysis had improved (FWE-corrected P < .05), although there was greater activation in motor areas when motor paralysis had improved than when motor paralysis remained. Notably, activation in the cerebellum posterior lobe during the motor task when motor paralysis remained (FWE-corrected P < .05) disappeared after motor paralysis had completely improved.
Conclusions
The cerebellum is a region that is closely associated with voluntary motion. We suggest that complementary abnormal function in the cerebellum might be associated with the neural basis of conversion disorder with motor paralysis.
1 CASE PRESENTATION
A 22-year-old woman with complete motor paralysis below the neck visited the Department of Emergency Medicine at Kanazawa Medical University. She had obtained her first job after graduating from the university and was employed as a member of the clerical staff at Firework Company. However, the firm's workload became busy in the early summer. She was required to help with her company's work as a skilled pyrotechnist, particularly in the manufacture and use of fireworks. After 1 week of this dramatically increased workload, she suddenly experienced complete motor paralysis below the neck one morning and was brought to our hospital's Department of Emergency Medicine. She had also lost her voice that morning, but that symptom had resolved by the time she arrived at the hospital.
She did not have any past history or family history. Her height and weight were 160 cm and 50 kg, respectively. Her blood pressure, heart rate, pulse oximetry saturation, and body temperature were 106/64 mm Hg, 60 beats/min, 99% (room air), and 36.5°C, respectively. Her hematological and biochemical findings were within normal ranges. Because medical staff at the Department of Emergency Medicine doubted that the patient had suffered a stroke, a neurologist performed a medical examination. Magnetic resonance imaging (MRI) revealed no signs of cerebral infarction, bleeding, or a tumor. The neurologist suspected pseudoneurologic symptoms and performed a drop test. When the neurologist dropped a “paralyzed” arm over the patient's face, the “paralyzed” arm did not strike her face. No other notable neurological findings were observed. We considered the possibility that the onset of the patient's illness might have been associated with psychosocial stress from her job. The patient was diagnosed with conversion disorder with motor paralysis by trained psychiatrists in accordance with criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). On the same day, she was admitted to a psychiatric inpatient unit to treat her pseudoparalysis.
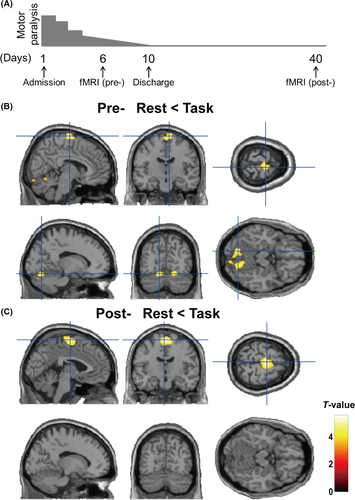
After the patient was admitted to that unit, her motor paralysis gradually improved because the psychosocial stress caused by her busy workload was largely eliminated. Impairment of her upper limbs steadily improved, whereas impairment of her lower limbs remained present. Motor paralysis of her lower limbs gradually decreased each day, without medication. The patient's limb motor paralysis of limb had completely resolved, and she was discharged from the hospital on the tenth day. We used functional MRI (fMRI) to investigate the differences in her brain activation during motor stimulation. We acquired 2 types of imaging data: (i) data obtained while motor paralysis remained (on the sixth day) and (ii) data obtained after motor paralysis had completely resolved (on the 40th day) (Figure 1A). Written informed consent was obtained from the patient after the study procedures had been fully explained. This study was performed in accordance with the World Medical Association's Declaration of Helsinki. Here, we used fMRI to investigate cerebral responses to motor stimulation in a patient with conversion disorder with motor paralysis to determine the underlying neural mechanisms of this disorder.
2 METHODS
2.1 fMRI data acquisition
The subject underwent functional imaging on a research-dedicated MRI scanner operated at 3.0 T (Siemens, Erlangen, Germany) using a standard birdcage head coil. Functional images were acquired using a T2*-weighted single-shot echo-planar imaging (EPI) sequence that covered the whole brain (TE, 30 ms; TR, 3000 ms; flip angle, 90°; slice thickness, 3 mm without a gap; matrix, 64 × 64 mm; FOV, 192 mm) to obtain blood oxygen level-dependent (BOLD) contrast. For each scanning run, a total of 60 EPI volume images were acquired along the anterior commissure and the posterior commissure plane. The total scan time was 3 minutes. The first 2 volumes were discarded due to magnetization instability. We thus obtained a total of 58 EPI volumes for analysis.
2.2 Motor task
The motor task was presented during the acquisition of fMRI data.1, 2 This task involved a block design (30 seconds without stimulation and 30 seconds with stimulation, repeated 3 times) and 3-minute scans. The stimulation was as follows: dorsiflexion and plantar flexion of the bilateral ankle joints were repeatedly alternated. The patient was at rest during periods without stimulation.
2.3 fMRI data analysis
fMRI images were processed using the voxel-based morphometry (VBM) toolbox VBM8 in Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) running on MATLAB R2012b (MathWorks, Natick, MA, USA) in accordance with guidelines in the SPM8 manual (http://www.fil.ion.ucl.ac.uk/spm/doc/spm8_manual.pdf). The patient's functional scans were realigned to correct for subject motion.3 The scans were normalized to the Montreal Neurological Institute (MNI) template image and spatially smoothed by convolution with an 8 mm full-width at half-maximum Gaussian kernel. The smoothed data were analyzed using the general linear model framework implemented in SPM8. After the first 2 images of the first session (from a 30 seconds prestimulus interval) were discarded to allow for saturation effects of the BOLD signal, the remaining images were realigned as previously reported.4 After preprocessing, individual analysis was performed using a general linear regression model. To calculate cerebral activation during dorsiflexion and plantar flexion of the bilateral ankle joints, the motor task and rest states were contrasted. This contrast yielded a statistical parametric map of the t statistic (SPM t). We applied a voxel-level height threshold of P < .001 (uncorrected for multiple comparisons); clusters of more than 100 contiguous voxels were considered for exploratory VBM analyses. Next, we applied a family-wise error (FWE) correction for multiple testing to avoid type I errors at the whole-brain level. Eventually, a significance threshold of P < .05 (FWE-corrected) was established.5
3 RESULTS
As shown in Figure 1A, we obtained imaging data at 2 points: (i) when motor paralysis remained present and (ii) after motor paralysis had completely resolved. We investigated brain activation at rest and during the motor task at these 2 points. We have summarized the activated brain regions observed before and after improvement in motor paralysis (Table 1). The right medial frontal gyrus (FWE-corrected P = .014; peak voxel: MNI coordinates x, y, z = 8, −14, 76; cluster size = 373) and the left posterior lobe of the cerebellum (FWE-corrected P = .023, x, y, z = −14, −72, −16; cluster size = 240) were significantly activated during the motor task relative to at rest when motor paralysis remained present (Figure 1B). In contrast, only cerebral motor areas were significantly activated during the motor task relative to those at rest after motor paralysis had completely resolved (Figure 1C; FWE-corrected P = .037; x, y, z = −2, −12, 66; cluster size = 785). No significant activation of the cerebellum region was found during the motor task after motor paralysis had improved (uncorrected P < .05). No other significant cerebral activations (indicated by greater activity during the motor task than at rest) were found before or after improvement in motor paralysis (uncorrected P < .001).
| Brain regions | R/L | BA | CS | P values (peak) | MNI coordinates | |||
|---|---|---|---|---|---|---|---|---|
| T | FWE | x | y | z | ||||
| Preimprovement: during task > At rest | ||||||||
| Medial frontal gyrus | R | 6 | 373 | 5.47 | .014a | 8 | −14 | 76 |
| Cerebellum posterior lobe (Declive) | L | NA | 240 | 5.30 | .023a | −14 | −72 | −16 |
| Cerebellum posterior lobe (Declive) | R | NA | 275 | 4.86 | .082 | 16 | −70 | −16 |
| Postimprovement: during task > At rest | ||||||||
| Medial frontal gyrus | L | 6 | 785 | 5.38 | .037a | −2 | −12 | 66 |
- R, right; L, left; BA, Brodmann area; CS, cluster size; FWE, family-wise error; NA, not available.
- Significant activated regions (FWE-corrected P < .05).
In this study, we do not have findings during the motor task for healthy controls or other patients to which to compare our findings in this patient. Further study is needed to investigate whether our findings in the patient are a common pathophysiology of conversion disorder with motor paralysis. A previous study has suggested that an increased connectivity between the supplementary motor area and cerebellum may occur due to compensation for dysfunction of the primary motor cortex in patients with stroke.6 Our findings suggest that complementary abnormal function in the cerebellum might be associated with the neural basis of conversion disorder with motor paralysis, as with cases of stroke, but the function might not be a disease-specific response.
ACKNOWLEDGMENT
We would like to thank the individual who participated in this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest for this article. [Correction added on 5 March 2018, after first online publication: The conflict of interest statement has been amended from ‘None.’ to the present statement.]
DATA REPOSITORY
The entirety of the patient's data cannot be made publicly available as data sharing was not included in the consent.
INFORMED CONSENT
Written informed consent was obtained from the patient after the study procedures had been fully explained. This study was performed in accordance with the World Medical Association's Declaration of Helsinki.