Widespread Pain Moderates the Response to Centrally-Acting Therapies in an Observational Cohort of Patients With Urologic Chronic Pelvic Pain Syndrome: A MAPP Research Network Study
Andrew Schrepf and Kenneth Locke are contributed equally to the study.
ABSTRACT
Purpose
Urologic Chronic Pelvic Pain Syndrome (UCPPS) impacts millions of people in the United States but treatment options remain largely unsatisfying. A large number of neurobiological studies from the Multidisciplinary Approach to the study of chronic Pelvic Pain (MAPP) research network and others point to aberrant pain mechanisms in patients with UCPPS and widespread pain, but the clinical significance of widespread pain has been speculative.
Materials and Methods
In the current exploratory study we investigated whether pain and urologic symptoms responded to centrally-directed therapies (tricyclic antidepressants/gabapentinoids) versus peripherally-directed (pelvic floor physical therapy/hydrodistension) therapies depending on the presence or absence of widespread pain when the new treatment was initiated.
Results and Conclusions
Forty UCPPS patients (n = 19 widespread) underwent an evaluation of UCPPS symptoms before and after twelve weeks of either centrally-directed (n = 16) or peripherally-directed therapy. Participants were stratified post hoc into widespread (two or more non-pelvic pain sites + pelvic pain) and localized pain categories. General linear models were used to test the group X treatment interaction effect, adjusting for age, sex, and baseline outcome levels. On average, patients with widespread pain receiving centrally-directed therapies improved more than six points on the 0–28 Pelvic Pain Severity scale, while those with localized pain showed no average improvement (interaction p = 0.005). Similar effects were observed for the bladder symptom impact score (interaction p = 0.011) but not urologic symptom severity (interaction p = 0.72). While these findings are exploratory, they provide preliminary evidence for phenotype X treatment interactions in UCPPS and should be followed by confirmatory studies.
Trial Registration
ClinicalTrials.gov Identifier: NCT02514265—MAPP Research Network: Trans-MAPP Study of Urologic Chronic Pelvic Pain: Symptom Patterns Study (SPS). ClinicalTrials.gov Identifier: NCT02898220—Trans-MAPP Study of Urologic Chronic Pelvic Pain: Control Study Protocol.xs.
1 Introduction
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) and Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) are debilitating urologic pain disorders affecting millions of Americans [1, 2]. Together, these conditions can be described under the umbrella term, Urologic Chronic Pelvic Pain Syndrome (UCPPS). Few therapeutic advances have been made in the past 25 years and treatment for the wide spectrum of pain and urologic symptoms is often based on trial and error. Past clinical trials for approved pharmacological treatments for IC/BPS in the United States that directly or indirectly target the bladder have shown variable effectiveness. Guidance for both disorders is currently focused on multimodal therapy and self-management [3-5].
The Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network was initiated more than a decade ago to advance our understanding of UCPPS and inform the development of new, improved clinical trials [6, 7]. Importantly, Network study results have revealed distinct clinical phenotypes based on the distribution of pain, specifically pelvic-only and widespread pain subgroups. The widespread pain phenotype further showed higher rates of co-morbid Chronic Overlapping Pain Conditions (COPCs) [8]. Neurobiological studies within the MAPP clearly demonstrated altered brain structure and function, enhanced sensitivity to experimental pain, and heightened ex-vivo immunoreactivity in the widespread pain phenotype [9-12]. In addition to this neurobiological evidence, a recent reanalysis of randomized clinical trials for IC/BPS demonstrated that the widespread pain phenotype is associated with treatment efficacy—participants with localized pain showed a better response to pentosan polysulfate and hydroxyzine than participants with widespread pain [13]. Taken together, these findings suggest that widespread pain may be a critical factor to consider when selecting amongst available treatments. To further explore the clinical significance of widespread versus localized pain, the MAPP Network's second phase observational study, the MAPP Symptom Pattern Study (SPS), assessed if the degree of localized versus widespread symptoms in participants correlates with differential response to commonly used therapies.
In the present study we present findings from the SPS-embedded Analysis of Therapies during the Longitudinal Assessment of Symptoms (ATLAS) module [14]. The SPS was a 36-month observational study in which male and female UCPPS participants were assessed through a variety of integrated, urologic and non-urologic measures. The optional ATLAS sub-study provided additional phenotypic characterization for UCPPS participants at the time of initiating one of a group of selected interventions as part of standard care, and again after 12 weeks of treatment. This allowed a direct examination of the response for phenotypic subtypes identified through various pain and urologic symptom measures to different categories of treatments. We hypothesized that participants with widespread pain (and by inference more CNS involvement) would respond preferentially to centrally-directed medication therapies for both pain and urologic outcomes while participants with pain solely localized to the pelvis would respond preferentially to treatments designed to address peripheral pain drivers, such as pelvic floor physical therapy or cystoscopy with hydrodistention.
2 Methods
2.1 MAPP Network SPS ATLAS Sub-Study
The MAPP Network SPS study (ClinicalTrials.gov Identifier: NCT02514265) was an observational cohort study in which participants were followed for 36 months while receiving standard of care from their treating physician. It was predicted that during this 3-year study some study participants would initiate new treatments as a natural part of their medical management. To leverage these events and to measure participants' responses to new therapy, an optional ATLAS protocol was included in the clinical protocol, wherein participants initiating selected new therapies could provide urologic and non-urologic symptom assessments before and after 12 weeks of the new treatment course.
The initiation of one of two broad classes of centrally-directed medications commonly used to treat urologic chronic pelvic pain triggered an invitation to participate in the ATLAS sub-study: Tricyclic antidepressants (e.g., amitriptyline HCl, doxepin HCl, nortriptyline HCl) and gabapentinoids (e.g., gabapentin, pregabalin). In addition, initiation of one of two commonly used peripherally-directed therapies, pelvic floor physical therapy and cystoscopy with hydrodistention of the bladder, also triggered optional participation in the ATLAS sub-study [14].
All procedures were approved by Institutional Review Boards at participating institutions with informed consent.
2.1.1 Measures
Patient age and gender were captured by self-report at study entry.
The 74-site Collaborative Health Outcomes Information Registry (CHOIR) body map [15] was collapsed by the MAPP Network into 13 body regions (one pelvic, 12 non-pelvic) [16]. Participants were dichotomized into two groups utilizing a median split; participants who self-reported pelvic-localized pain plus zero or one additional body region were considered to have “localized” (i.e., pelvic pain only) or “low widespread” pain. Those who reported pelvic-localized pain plus two or more additional pain regions were designated as “high widespread” pain.
Pelvic pain severity (PPS) and urinary symptom severity (USS, including urinary frequency and urgency) were measured using previously described instruments [17]. PPS Scores range between 0 and 28 and USS scores between 0 and 25.
Impact of Symptoms. The Bladder Symptom Impact (BSI) scale was used to assess impact/interference of bladder symptoms on daily life [18]. This six-item scale asks about symptom impact on home responsibilities, social life, self-worth, interest in life, energy, and mood. Scores range between 0 and 42.
2.2 Statistical Analyses
The ATLAS cohort was compared to the larger MAPP SPS cohort based on age, sex, proportion experiencing widespread pain, and the outcome measures listed above by ANOVA and Chi-squared difference test for continuous and categorical variables, respectively.
Generalized linear models were used to test the hypothesis that widespread pain status moderates treatment response for centrally-directed versus peripherally-directed therapies. For the PPS, USS, and BSI, we modeled the change in outcome 12 weeks following initiation of the new treatment with multiple regression. Predictors for all models included a treatment effect for centrally-directed therapies and widespread pain effects for centrally-directed and peripherally-directed therapies. All treatment and widespread pain effects were adjusted for age, sex, and outcomes assessed at baseline.
The treatment effect for the centrally-directed therapy represents the difference in outcome compared to peripherally-directed treatment for patients with low widespread pain. The two widespread pain effects represent the difference in outcome for patients with high widespread pain compared to low within respective treatment categories. To test for interaction between treatment and widespread pain status, we tested for equality of the two widespread pain treatment effects. All statistical analyses were conducted in SAS 9.4.
3 Results
3.1 Demographic Information and Comparison of the ATLAS Cohort to the Larger MAPP SPS Cohort
Demographic and pre-treatment clinical information is shown in Table 1 with comparisons to the remainder of the MAPP SPS cohort. Patients were 41 years old on average and 80% of the sample was female. There were no significant differences between the ATLAS cohort and the rest of the MAPP SPS participants on age, sex, or the outcome measures of interest (all p > 0.05).
| MAPP II SPS Cohort | ATLAS Cohort | p value | |||
|---|---|---|---|---|---|
| n | 538 | 40 | |||
| Age (mean (SD)) | 45.14 (15.68) | 40.52 (15.00) | 0.072 | ||
| Female/male (%) | 353 (65.6) | 185 (34.3) | 32 (80.0) | 8 (20.0) | 0.091 |
| Widespread pain/local pain (%) | 201 (37.3) | 337 (62.6) | 19 (47.5) | 21 (52.5) | 0.311 |
| PPS Baseline (Mean [SD]) | 14.21 (5.64) | 14.30 (4.90) | 0.917 | ||
| USS Baseline (Mean [SD]) | 11.54 (6.21) | 12.20 (5.62) | 0.511 | ||
| Bladder Symptom Impact (Mean [SD]) | 16.43 (10.97) | 18.10 (11.90) | 0.358 | ||
3.2 Widespread Pain Moderates Effects of Peripherally Versus Centrally-Directed Treatment
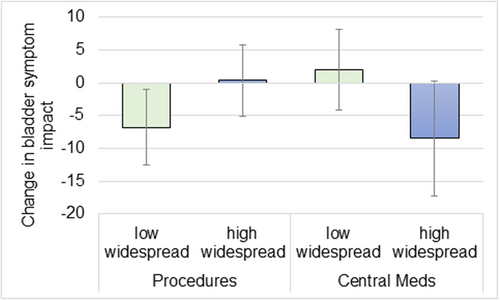
There was a significant interaction of widespread pain status on outcomes by treatment category for PPS (pain) and BSI (symptom interference) (Table 2). Specifically, the change in PPS from baseline to 12 weeks post-treatment was greater in the high widespread pain group when receiving centrally-directed therapies (−6.21) compared to peripherally-directed therapies (1.25). This equates to a greater than 6-point improvement for the widespread group on the 0–28 PPS scale with centrally-directed therapy (95% CI, −10.39, −2.03), compared to roughly 1 point worsening for this sub-group when receiving peripherally-directed therapies (95% CI, −1.59, 4.09). This average drop is greater than the minimal clinically important difference in PPS (4 points), and represents a more than 40% reduction from pre-treatment levels [19]. The 12 week change in BSI was greater in the low widespread pain group when receiving peripherally-directed therapies (−6.79) compared to centrally-directed therapies (2.01; both p < 0.05). Therefore, the low widespread group receiving peripherally-directed therapies experienced a nearly seven point improvement (i.e., reduction in the BPI score) in bladder interference (95% CI, −12.55, −1.04) on the 0−42 point BPI scale, compared to a roughly 2-point worsening when receiving centrally-directed therapies (95% CI, −4.16, 8.18). This roughly 7-point improvement represents a more than 35% reduction from pre-treatment levels. Figure 1 shows levels of improvement for the BSI by widespread pain status and treatment type. Conversely, there were no significant effects of widespread pain status by treatment category on USS (urologic symptoms) score over the 12 weeks (p > 0.05).
| Peripherally-directed, n = 24 | Centrally-directed, n = 16 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low widespread | High widespread | Low widespread | High widespread | ||||||||||
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Interaction p value | |||||
| Δ PPS* | −2.22 | −5.28 | 0.85 | 1.25 | −1.59 | 4.09 | 0.34 | −2.97 | 3.66 | −6.21 | −10.39 | −2.03 | 0.005 |
| Δ USS* | −1.18 | −3.67 | 1.30 | −1.18 | −3.44 | 1.08 | −0.08 | −2.72 | 2.55 | 0.86 | −2.55 | 4.28 | 0.723 |
| Δ BSI† | −6.79 | −12.55 | −1.04 | 0.34 | −5.06 | 5.74 | 2.01 | −4.16 | 8.18 | −8.46 | −17.21 | 0.30 | 0.011 |
- * Calculated at average age = 40.52, proportion of females = 0.8 and average baseline outcome
- † Calculated at average age = 40.53, proportion of females = 0.816 and average baseline outcome
4 Discussion
The present study provides a proof of concept that the degree of widespread pain in UCPPS (i.e., IC/BPS and CP/CPPS) is associated with differential response to commonly used treatments. Participants with widespread pain showed the most improvement in genitourinary pain when receiving centrally-directed therapies, while participants with localized pain showed the greatest improvement in genitourinary pain and interference symptoms when receiving peripherally-directed therapies. The MAPP Network has demonstrated that widespread pain in UCPPS is strongly associated with the concept of central sensitization/nociplastic pain and these participants are more likely to have COPCs and more severe painful and non-painful symptoms [20], as well as neurobiological changes that suggest entrenched CNS mechanisms promoting pain (discussed further below). These neurobiological differences are likely at the root of why centrally-acting therapies appear to be more beneficial in participants with significant pain outside the pelvic region. These findings support further characterization of widespread/comorbid pain in future clinical trials and research studies to determine if treatment response in UCPPS can be improved by “matching” patient phenotype to appropriate therapies. Indeed, the distinction between localized versus widespread pain may be even more clinically relevant than previously considered, as roughly three quarters of MAPP Network study participants showed substantial degrees of pain outside the pelvic area [20].
The current findings are supported by a recent reanalysis of randomized controlled trials for IC/BPS that considered the potential impact of widespread pain on oral pentosan polysulfate + hydroxyzine, intravesical Bacillus Calmette-Guerin, and oral amitriptyline treatments. This study showed that localized pain responded preferentially to oral PPS + hydroxyzine compared to placebo, while widespread pain did not; intravesical BCG responses were equivalent, and widespread pain responded preferentially to oral amitriptyline versus placebo, while localized pain did not (the low widespread pain group showed a pronounced response to placebo so this finding should be interpreted cautiously) [13]. While this was not a prospective study, the findings support that incorporating widespread pain into clinical decision-making could increase clinical response rates to available treatments.
MAPP Network studies have also revealed neurobiological differences that may underly observed treatment effects. In a MAPP Network brain neuroimaging study participants demonstrated increases in gray matter volume in the supplementary motor area and primary somatosensory (S1) and motor (M1) cortices as a function of the degree of widespread pain [11]. These areas encompass the hip/trunk/pelvic representation in S1/M1 suggesting structural reorganization with potential pathologic consequences. The left S1/M1 area was shown to exhibit higher functional connectivity with the brain's salience network, a constellation of regions involved in determining the importance of stimuli, including those arising within the body. Importantly, this finding was also shown in a cohort of fibromyalgia patients, a condition characterized by widespread pain that has been shown to respond to tricyclic antidepressants and gabapentinoids [11]. As an example, pregabalin has been shown to reduce concentrations of excitatory neurotransmitter in the posterior insula and patterns of functional connectivity associated with clinical pain in fibromyalgia patients [21].
5 Conclusions
The current study, while exploratory, provides critical new support for the importance of targeting therapy to specific symptom profiles for the improvement of treatment effects (i.e., personalized medicine). Here we assessed interventions with commonly accepted localized versus centralized sites of action to test the hypothesis that such therapies would show different efficacy for urologic pelvic pain phenotypes. These findings are consistent with the hypothesis that urologic pelvic pain involves different mechanistic subtypes that can be exploited to provide desperately needed improvement in the efficacy of future trials and ultimately clinical management. It is important to note that urologic symptom outcomes did not show this interaction effect—further research is needed to determine if the apparent differential effects of centrally-acting therapies are confined to pain outcomes.
Limitations and Future Directions: the current study has a small sample size and the participants were all self-selected into treatment categories. Future clinical trials that stratify participants by the presence or degree of widespread pain for treatment selection are needed to further test the significance of these results.
Author Contributions
John Farrar, J. Richard Landis, and Daniel J. Clauw were primarily responsible for the study design. All authors contributed to the drafting and critical revision of the work. Primary analysis was performed by Andrew Schrepf, Kenneth Locke, and J. Richard Landis.
Acknowledgments
Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, and DK82316).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The MAPP SPS data will be made publicly available through the NIDDK repository.




