The impact of pregnancy and childbirth on stress urinary incontinence in women previously submitted to mid-urethral sling: A systematic review and metanalysis
Abstract
Introduction
There is no guideline or clinical consensus concerning the mid-urethral sling (MUS) operation for stress urinary incontinence (SUI) and future pregnancies. The aim of this systematic review and metanalysis is to evaluate the impact of pregnancy and of delivery on SUI in women who previously sustained a MUS surgery.
Methods
We performed a systematic review and meta-analysis, according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines, and selected seven publications for inclusion in the analysis.
Results
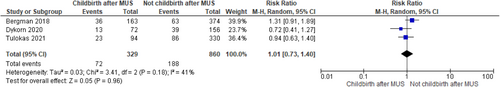
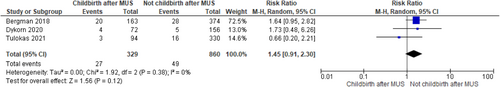
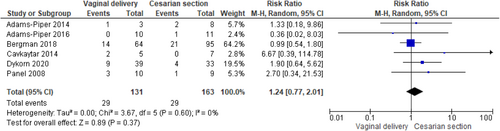
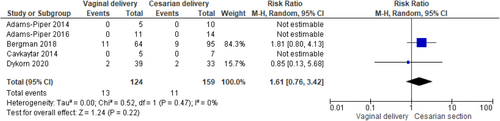
Recurrence of SUI after childbirth in women previously submitted to MUS was 22% (95% confidence interval [CI]: 18.0%−26.0%; I2 = 0%) while the reintervention rate for SUI the 5% (95% CI: 2.0%−8.0%; I2 = 47.34%) in the included studies. There was not statistically significant difference between women who delivered (both vaginally and by caesarian section) or not after MUS in SUI recurrence (RR 1.01, 95% CI 0.73−1.40; p = 0.96 and I2—test of 41% p = 0.18) and in SUI reintervention (RR 1.45, 95% CI 0.91−2.30; p = 0.12 and I2—test of 0% p = 0.38) with homogeneity among studies. There was no difference between women who delivered vaginally or by caesarian section both for recurrence of SUI (RR 1.24, 95%CI 0.77-2.01; p = 0.37 and I2—test of 0% p = 0.60) and reintervention (RR 1.61, 95% CI 0.76−3.42; p = 0.22 and I2—test of 0% p = 0.47). BMI ≥ 30 kg/m2, urinary incontinence (UI) before and during pregnancy emerged as risk factors for postpartum UI relapse.
Conclusion
Childbirth do not affect SUI relapse or reintervention in women previously submitted to MUS. In the same population of patients, no difference was highlighted concerning the mode of delivery for the outcome SUI relapse or reintervention. Previous MUS surgery may not be an appropriate indication for cesarean birth in subsequent pregnancy.
1 INTRODUCTION
Stress urinary incontinence (SUI) is a highly prevalent pelvic floor disorder, affecting approximately 46% of adult women, peaking at 50% in women 40 years and older.1 It has been estimated that 10–14% of women will have an operation for SUI during their lifetime.2, 3 Mid-urethral sling (MUS) is widely regarded as the most efficacious method for surgical treatment of SUI.4, 5 For young women of reproductive age experiencing SUI, it has been suggested to delay surgical intervention until after completing childbirth due to concerns regarding the potential for SUI recurrence or complications during pregnancy or delivery.6 Moreover, caesarian section has been commonly proposed as route of delivery in case of pregnancy after MUS surgical implantation.7 Surgical treatment for SUI is less frequent among women under 40 years old compared to older women.8 However, there is a notable prevalence of severe SUI, reported to be around 10%, among women aged 25−49 years old.1, 9
There is no guideline or clinical consensus concerning the MUS operation and future pregnancies, and the scientific evidence to substantiate any recommendation is scarce. Women who plan to become pregnant and give birth after undergoing an anti-incontinence procedure require thorough counseling regarding how pregnancy and various delivery methods can affect the likelihood of urinary incontinence (UI) recurrence. For that reason, we decided to perform a systematic review and metanalysis aiming to evaluate the impact of pregnancy and of delivery on SUI in women who previously sustained a MUS surgery.
2 METHODS
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines.10 The protocol was registered in PROSPERO (CRD42024497349).
2.1 Eligibility criteria
In our analysis we included the available studies that evaluated the efficacy and safety of MUS procedure for female SUI after delivery. The following epidemiological designs were considered suitable: randomized control trials (RCTs), observational prospective or retrospective cohort studies and case series. We excluded review articles, case reports, commentaries, editorials, meeting abstracts, non-English articles. The research was conducted without any restrictions regarding the year of publication.
2.2 Information source
A systematic literature research was performed using the PubMed/MEDLINE (Medical Literature Analysis and Retrieval System Online) and EMBASE database (last search date: December 1, 2023).
2.3 Search strategy
- −
For PubMed research:
-
“Urinary incontinence, stress” [Mesh] OR “Urinary incontinence, stress*” [tw]
-
“Pregnancy” [Mesh] OR “Delivery” [tw] OR “pregnan*” [tw] OR “childbirth” [tw]
-
“Suburethral slings”[Mesh] OR “Suburethral sling*” [tw]
- —
For Embase research:
-
“Stress Incontinence”
-
“Childbirth” OR “Pregnancy” OR “Delivery”
-
“Suburethral sling”
All pertinent articles were carefully evaluated, and their reference lists were examined to identify other manuscripts that could be retrieved in this review.
2.4 Selection process
Two independent reviewers (A. F. R. and M. L.) selected each article being considered, through titles and abstracts, and excluded unrelated studies. Discrepancies were resolved by consensus, including a third author (M. C.), who checked the eligible studies. Potential eligible studies were assessed in full text to decide whether to include them.
2.5 Data collection
Structured tables were used to extract necessary data from each eligible study. The data extracted included: authors' names, year of publication, country, study design, age of patients, parity, body mass index (BMI), type of UI, UI assessment, type of MUS surgery, failure rate, reoperation rate, interval between surgery and pregnancy, continence before and during pregnancy, mode of delivery, incontinence after delivery, complications related to surgical procedure, reoperation rate, time to follow-up.
2.6 Data items
-
MUS surgery failure rate after childbirth.
-
Reoperation for SUI after childbirth.
2.7 Study risk of bias assessment
Risk of bias and quality assessments of the included studies were determined for nonrandomized studies using the Risk of bias in Non-Randomized Studies of Intervention (ROBINS-I) tool.11
2.8 Statistical analysis
Categorical variables were expressed as absolute and relative (percentage) values. A proportional meta-analysis was performed for several outcome measures (MUS failure rate and reoperation rate for SUI recurrence) and Forest plots were used to graphically display the estimated results. A random-effects model12 was used for the statistical pooling of data. Results were reported as a pooled percentage with related 95% confidence intervals (95%-CI). Forest plots were used to display RRs and 95% CI for the analyzed outcomes. Heterogeneity between studies was based on the Higgins I2 index,13 with I2 values of >50% being considered to indicate the presence of heterogeneity.13, 14 Funnel plot for publication bias was not assessed using Egger's because articles were less than 10 as suggested by the Cochrane Manual. Metanalysis was performed using Review Menager version 5.4.1 software (Cochrane Training) and Jamovi Software version 2.3.28.0.
3 RESULTS
3.1 Study selection
We identified 334 (EMBASE) and 79 (PubMed) studies (Figure 1). After checking for double results, and title, 36 articles were selected for abstract screening and 10 for text evaluation. Among these, 7 studies were selected for inclusion in the systematic review.15-21
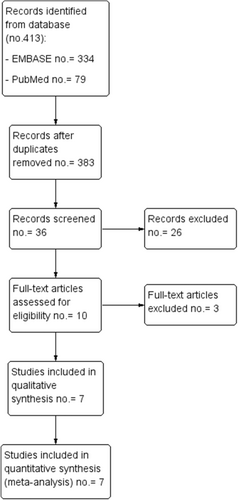
3.2 Risk of bias in studies and levels of evidence
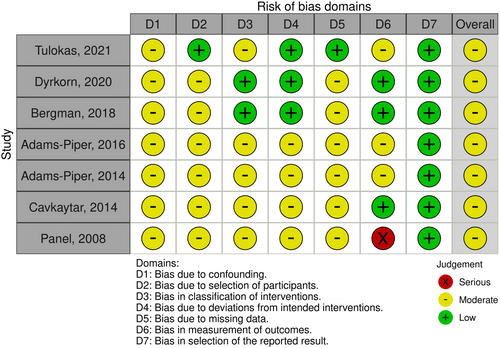
The risk of bias analysis was performed for each study ranging from low to serious and it is summarized in Figure 2. The risk of bias analysis performed for the seven nonrandomized studies, showed that all seven studies had a moderate risk of bias.
3.3 Study characteristics and study results
Seven studies including 401 women who delivered and that have been previously submitted to SUI surgical treatment were included in this systematic review and metanalysis. The study design and the characteristics of the studies are reported in Table 1. Two studies were conducted in the United States, 1 study in Turkey, 1 study in Norway, 1 study in Finland, 1 study in Sweden, and 1 study in France. Five retrospective studies and 2 case series were included. Three retrospective case-control studies compared SUI outcomes who delivered or not after the incontinence procedure. The sample size of women who delivered after MUS was variable from 12 to 163. Median age of the overall sample size ranged from 33.0 years-old to 42.0 years-old. Median BMI was reported only in 2 studies, ranging from 23.0 to 25.0 Kg/m2. Median parity was reported between 2 and 3 among studies. No study reported the how UI was assessed before surgery. Concerning the type of UI, four studies reported anti-incontinence procedure for SUI treatment, two studies for SUI, MUI, and unknown UI. One study did not report the type of UI. SUI surgery outcomes before, during and after childbirth are reported in Table 2. Interval between index surgery and pregnancy ranged between 19.0 and 108.0 months. Concerning the mode of delivery, cesarean section was adopted in the 43% up to the 66.7% of women. Overall follow-up ranged between 13.8 and 129.6 months.
| Authors and year | Country | Cases (young women submitted to incontinence surgery), n (%) | Study design | BMI, Kg/m2 | Parity median (IQR) | Type of incontinence, n (%) | Incontinence assessment | Type of surgery, n (%) | Age at surgery, years, median or mean (IQR or SD) |
|---|---|---|---|---|---|---|---|---|---|
| Tulokas et al.21 | Finland | Childbirth after MUS: 94/424 (22.2) | Retrospective case-control study | Childbirth after MUS: 23.0 (21.0-25.0) in 24/94 (30%) women | Childbirth after MUS, n (%): 0 = 9/94 (10.0%); 1-2 = 57/94 (61.0%); ≥3 = 28/94 (30.0%) |
Childbirth after MUS: SUI 81 (86.0) MUI 5 (5.0) Unknown or other 8 (9.0) |
NR | Childbirth after MUS: TVT 61 (65.0) TOT 33 (35.0) |
Childbirth after MUS: 35.0 (32.0-38.0) |
| No childbirth after MUS: 330/424 (77.8) | No childbirth after MUS: 23.0 (21.0-26.0) in 217/330 (66%) women | No childbirth after MUS, n (%): 0 = 13/330 (4.0%); 1-2 = 279/330 (85.0%); ≥3 = 38/330 (12.0%) |
No childbirth after MUS: SUI 267 (81.0) MUI 23 (7.0) Unknown or other 40 (12.0) |
No childbirth after MUS: TVT 213 (65.0) TOT 117 (35.0) |
No childbirth after MUS: 36.0 (33.0-38.0) |
||||
| Dyrkorn et al.20 | Norway | Childbirth after MUS: 72/228 (31.6) | Retrospective case-control study | Childbirth after MUS: 25.0 (19.0-37.0) | Childbirth after MUS: 2.0 (0.0-5.0) | SUI and MUI | NR | Childbirth after MUS: TVT 60 (83.3) TOT 10 (13.9) SIS 2 (2.8) |
Childbirth after MUS: 34.0 (24.0-44.0) |
| No childbirth after MUS: 156/228 (68.4) | No childbirth after MUS: 25.0 (18.0-43.0) | No childbirth after MUS: 2.0 (1.0-7.0) | No childbirth after MUS: TVT 156 (100.0) |
No childbirth after MUS: 38.0 (28.0-44.0) | |||||
| Bergman et al.19 | Sweden | Childbirth after MUS: 163/537 (30.3) | Retrospective case-control study | Childbirth after MUS: ≥30 = 19/163 (11.6) |
Childbirth after MUS: 3.0 (1.0-7.0) | NR | NR | TVT and TOT | Childbirth after MUS: 42.0 (28.0-56.0) |
| No childbirth after MUS: 374/537 (69.7) | No childbirth after MUS: ≥30 = 49 (13.1) | No childbirth after MUS: 2.0 (1.0-5.0) | No childbirth after MUS: 43.0 (28.0-57.0) | ||||||
| Adams-Piper, Buono, et al.18 | United States | 25/25 (100.0) | Case series | NR | 3.0 (3.0-4.0) | SUI | NR | TVT 16 (64.0) TOT 9 (36.0) |
35.3 (27.9-48) |
| Adams-Piper, Darbinian, et al.17 | United States | 15/15 (100.0) | Case series | NR | 3.0 (2.0-3.0) | SUI | NR | TVT 12 (80) TOT 2 (13.3) SIS 1 (6.7) |
33.0 (29.0-37.0) |
| Cavkaytar et al.16 | Turkey | 12/12 (100.0) | Case series | NR | 3.0 (2.0-5.0) | SUI | NR | TVT 8 (66.7) TOT 4 (33.3) |
33.1 ± 4.3 |
| Panel et al.15 | France | 20/20 (100.0) | Retrospective study | NR | 2.0 (1.0-3.0) | SUI | NR | MUS 20 (100.0) | 33.9 (20-42) |
- Abbreviations: BMI, body mass index; MUI, mixed urinary incontinence; MUS, mid-urethral sling; NR, not reported; RP, retropubic; SIS, single incision sling; SUI, stress urinary incontinence; TVT, tension-free vaginal tape; TOT, Transobturator tape; UI, urinary incontinence; UUI, urgency urinary incontinence.
| Authors and year | Interval between index surgery and pregnancy (months), median or mean (IQR or SD) | Incontinence before pregnancy, n (%) | Incontinence during pregnancy, n (%) | Mode of delivery, n (%) | Newborn weight (g) | OASIS, n (%) | Incontinence after delivery, n (%) | Incontinence at last follow-up, n (%) | Failure assessment | Reoperation for SUI, n (%) | Risk factors for recurrence/reoperation | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tulokas et al.21 | 31.2 (19.2-55.2) | 15 (15.9) | SUI 3 (3.0) MUI 1 (1.0) |
VD 54 (57.0) Elective CS 24 (26.0); |
NR | 0 (0) | 0 (0) | Childbirth after MUS: SUI 14 (14.9) MUI 9 (9.6) |
Re-visit for UI | Childbirth after MUS: TVT 1 (1.1) Bulking 2 (2.2) |
None of the tested variables (subsequent pregnancy, incontinence type, operation type and parity) affected the risk for SUI re-procedure | Childbirth after MUS 129.6 (86.4-166.8) |
| Urgent or emergency CS 16 (17.0); | No childbirth after MUS: SUI 57 (17.3) MUI 29 (8.8) |
No childbirth after MUS: TVT 9 (2.7) TOT 4 (1.2) Bulking 3 (0.9) |
No childbirth after MUS 127.2 (85.2-164.7) |
|||||||||
| Dyrkorn et al.20 | VD 29.0 (10.0-113.0) CS 25 (11.0-116.0) |
5 (7.0) | NR | VD 39 (54.2) CS 33 (45.8) |
VD 3672.0 (2260.0-4800.0) CS 3390.0 (2650.0-4455.0) |
14/72 (19.4) | NR | Childbirth after MUS: SUI 13 (18.0) | Norwegian validated short-form urinary disease-specific questionnaire | Childbirth after MUS 4 (5.6) | Risk factor: BMI ≥ 30 Repeated surgery for SUI |
Childbirth after MUS 120.0 (25.0-213.0) |
| No childbirth after MUS: SUI 39 (25.0) | No childbirth after MUS 5 (3.2) | Protective: Parity before MUS |
No childbirth after MUS 121.0 (22.0-215.0) | |||||||||
| Bergman et al.19 | Childbirth after MUS: 108 (24–168) | NR | NR | VD 64 (39.3) CS 95 (60.7) |
NR | NR | NR | Childbirth after MUS: SUI 36 (22.0) |
Subjective (UDI) | Childbirth after MUS 20 (12) | Risk factor: BMI ≥ 30 Repeated surgery for SUI Previous other prolapse surgery Psychiatric disorder |
Childbirth after MUS 72 (24–228) after delivery |
| No childbirth after MUS: 96 (24–180) | No childbirth after MUS: SUI 63 (17.0) | No childbirth after MUS 28 (7.5) |
No childbirth after MUS 156 (48–372) after delivery |
|||||||||
| Adams-Piper, Buono, et al.18 | 27.3 (9.3-70.4) | 5 (20.0) | 7 (28.0) | VD 11 (44.0) Elective CS 13 (52.0) Urgent CS 1 (4.0) |
NR | 0 (0) | 6 (24.0) | SUI 5 (20.0) MUI 1 (4.0) |
Subjective cure rate | 0 (0) | NR | 19.8 (1.0-51.0) following delivery |
| Adams-Piper, Darbinian, et al.17 | 19.0 (16.0-38.0) | 4 (26.7) | NR | VD 5 (33.3) Elective CS 10 (66.7) |
NR | 0 (0) | 6 (40.0) | SUI 4 (26.7) MUI 2 (13.3) |
Subjective cure rate | 0 (0) | NR | 28.0 (12.0-35.0) following delivery |
| Cavkaytar et al.16 | 30.2 ± 14.2 | NR | 3 (25.0) | VD 5 (41.7) CS 7 (58.3) |
2940.0 (2352-3452.5) | NR | 2 (16.7) | SUI 2 (16.7) | Stress test | 0 (0) | Incontinence during pregnancy | 52.0 ± 12.4 |
| Panel et al.15 | 21.6 (3.0-63.0) | NR | 2 (10.6) | VD 10 (50.0) CS 9 (45.0) Ongoing pregnancy 1 (5) |
3327.5 (3130-3633.5) | 0 (0) | SUI 4 (20.0) | SUI 4 (20.0) | Subjective cure rate | 1 (5.0) | NR | 13.8 (1.0-52.0) following delivery |
- Abbreviations: BMI, body mass index; CS, cesarian section; MUI, mixed urinary incontinence; MUS, mid-urethral sling; NR, not reported; OASIS, obstetrics anal sphincter injuries; RP, retropubic; SIS, single incision sling; SUI, stress urinary incontinence; TVT, tension-free vaginal tape; TOT, transobturator Tape; UI, urinary incontinence; UDI, urogenital distress inventory; UUI, urgency urinary incontinence; VD, vaginal delivery.
3.4 Results of synthesis
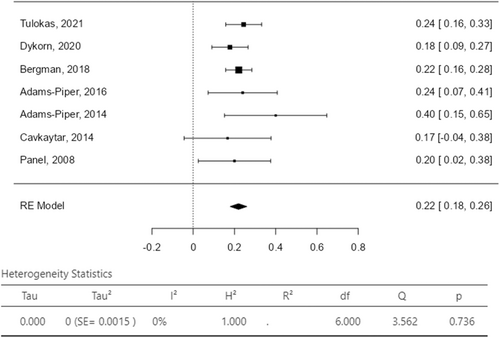
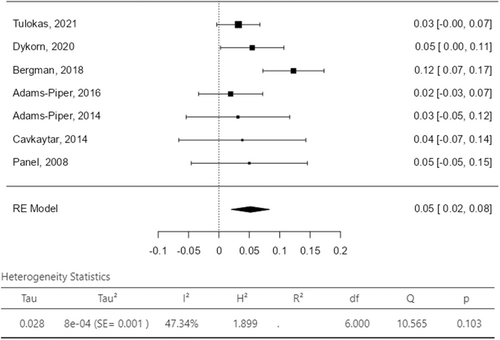
The assessment of failure rate after delivery was reported subjectively as a dichotomous variable (yes/no) in all the studies. Two studies adopted validated questionnaires for the assessment of SUI recurrence: the Urogenital Distress Inventory (UDI) in one study18 and the Norwegian validated short-form urinary disease specific questionnaire in the other.20 SUI recurrence was evaluated by Tulokas et al. by counting the number of visits for incontinence after delivery,21 while Cavkaytar et al. assessed SUI recurrence trough a cough stress test.16 The overall SUI recurrence rate was reported between the 18.0% and 26.0% with a pooled value of 22% in women who delivered after MUS surgery (Figure 3). The related I2 test result was 0% demonstrating statistical homogeneity among studies. In 3 (3/7; 43%) studies women were not submitted to a new incontinence procedure for failure of SUI surgical treatment. SUI reintervention rate was between the 2% and the 8% with a pooled value of 5% (Figure 4); the related I2 test was 47.34%, still showing statistical homogeneity among the included studies. Overall, most reported complications were UTI (range 0%−5.3%), voiding dysfunction (range 0%−5.3%) and persistent/de novo OAB/UUI symptoms (range 0%−54.0%). Vaginal erosion of the tape was not reported in any study. Pelvic or abdominal pain was reported in only one study in the 9% of cases (Table 3).20
| Authors and year | Complications related to MUS, n (%) |
|---|---|
| Tulokas et al.21 | UTI 4 (4.0) during pregnancy; Pain 12 (13.0) during pregnancy |
| Dyrkorn et al.20 | UTI 2 (2.8) during pregnancy; Voiding dysfunction 1 (1.4) during pregnancy; persistent et de novo UUI 39 (54.0) after pregnancy |
| Bergman et al.19 | Voiding dysfunction needing bladder catheterization during the pregnancy 3 (1.8) |
| Adams-Piper, Buono, et al.18 | No complications |
| Adams-Piper, Darbinian, et al.17 | No complications |
| Cavkaytar et al.16 | No complications |
| Panel et al.15 | UTI during pregnancy 1 (5.3); Renal colic during pregnancy 1 (5.3); Voiding dysfunction before and during pregnancy 1 (5.3) |
- Abbreviations: MUS, mid-urethral sling; UTI, urinary tract infection; UUI, urgency urinary incontinence.
According to the metanalysis comparing women who delivered or not after MUS, three studies were included with 329 women who delivered (both vaginally and by caesarian section) after MUS and 860 women who did not deliver after MUS. SUI recurrence was not statistically different in the two groups (RR 1.01, 95% CI 0.73—1.40; p = 0.96), with a related I2—test of 41% (p = 0.18) (Figure 5) and in SUI reintervention (RR 1.45, 95% CI 0.91−2.30; p = 0.12), with a related I2—test of 0% (p = 0.38) demonstrating homogeneity among studies (Figure 6). Moreover, in MUS failure rate comparing delivery mode 6 studies with available data were included in the analysis. When data were available, women with SUI recurrence before pregnancy were excluded from the analysis; 131 women who had vaginal delivery and 163 women who had cesarian section were included. There was no difference between groups (RR 1.24, 95% CI 0.77−2.01; p = 0.37), with a related I2—test of 0% (p = 0.60) (Figure 7). Other risk factors for postpartum UI were a BMI ≥ 30 Kg/m2, UI before and during pregnancy (Table 4). Concerning SUI reintervention, five studies were included in the study analysis with 124 women who delivered vaginally and 159 who were sustained a cesarian section. No differences were found between the two groups (RR 1.61, 95% CI 0.76−3.42; p = 0.22), with a related I2— test of 0% (p = 0.47) (Figure 8).
| Risk factor | No. of studies | Case (n/N) | Control (n/N) | RR (95% CI) | I2 (%) | p Value |
|---|---|---|---|---|---|---|
| BMI ≥ 30 Kg/m2 | 2 | 41/111 | 107/644 | 2.21 (1.6, 3.0) | 0 | <0.0001 |
| Parity ≥ 2 | 6 | 97/456 | 68/375 | 0.87 (0.4, 1.9) | 74 | 0.74 |
| Type of MUS | 3 | TOT 3/20 | TVT 11/32 | 0.55 (0.2, 1.7) | 0 | 0.31 |
| UI before pregnancy | 2 | 8/9 | 4/31 | 5.4 (1.1, 25.7) | 63 | 0.03 |
| UI during pregnancy | 3 | 9/17 | 2/39 | 6.8 (1.8, 25.4) | 0 | 0.004 |
| Mode of delivery | 6 | VD 29/131 | CS 29/163 | 1.2 (0.8, 2.0) | 0 | 0.37 |
- Abbreviations: BMI, body mass index; CS, cesarian section; MUS, midurethral sling; RR, risk ratio; TOT, trans-obturator tape; TVT, Tension-free vaginal tape; UI, urinary incontinence; VD, vaginal delivery.
4 DISCUSSION
This systematic review and metanalysis aimed to evaluate the four main issues concerning the surgical management of SUI in young fertile women: (1) The recurrence rate and reintervention rate after childbirth in women previously submitted to MUS; (2) the risk of relapse of SUI and/or SUI reintervention related to childbirth; (3) the preferred route of delivery to prevent SUI relapse and/or reintervention; (4) safety in women who have pregnancy and childbirth after MUS surgery.
This metanalysis demonstrated a SUI recurrence rate of the 22% (95% CI: 18%−26%) and a SUI reintervention rate of the 5% (95% CI: 2%−8%) after childbirth in women previously submitted to MUS. Moreover, the results of this metanalysis showed that there is no difference in SUI relapse and reintervention between women who had childbirth after MUS procedure and women who did not have childbirth after MUS.
To date, an accepted definition of “young woman” lacks and we use to identify with this term women younger than 40 years-old and specifically those women who have not still completed their reproductive project. For this category of women there is not enough evidence about the consequence of pregnancy and delivery on the efficacy and safety of MUS implantation, and guidelines and recommendations supporting this decision making lack.
Lower urinary tract symptoms are a significant concern for women in all phases of life, including pregnancy and postpartum.22 Urinary disorders may reach the 97.3% of patients during all pregnancy periods, with increased frequency of urination, nocturia and urinary urgency reported as the most prevalent symptoms.23 The prevalence of UI during pregnancy is reported ranging from 20% to 67%, with a tendency to worsen as gestation advances24, 25 and SUI prevalence is reported around the 40%.23 All these considerations led physicians to retain that pregnancy itself and delivery could affect the results of anti-incontinence procedure.6 In addition, performing MUS at a young age may increase the risk of recurrence and the risk of long-term complications. Contrary to this hypothesis, the result of our metanalysis showed that MUS failure (RR 1.01, 95% CI 0.73−1.40; p = 0.96) and reintervention (RR 1.45, 95% CI 0.91−2.30; p = 0.12) for SUI relapse are not dependent from childbirth.
Moreover, in our study, the route of delivery did not affect the efficacy of MUS surgery. Indeed, we did not identify any difference between women who delivered vaginally and women who had cesarian section after MUS implantation both for SUI relapse (RR 1.24, 95% CI 0.77−2.01; p = 0.37) and for anti-incontinence reintervention (RR 1.61, 95% CI 0.76−3.42; p = 0.22). Considering that a successful MUS operation is thought to have a high risk of SUI recurrence if followed by pregnancy,6 many surgeons retain the caesarian section as the route of choice of delivery for pregnant woman after MUS.7 Even if we could not systematically distinguish all the indication for the caesarian delivery, it is likely that MUS has been the indication for several elective caesarian sections. In the included studies we showed a higher rate of caesarian section of the 43% up to the 66.7%; considering that vaginal delivery seems to not affect SUI relapse and reintervention, the mode of delivery should be decided on the base of obstetric indications.
A BMI ≥ 30 Kg/m2, UI before and during pregnancy emerged as factors associated with postpartum UI in women who previously had MUS. Etiopathogenesis of SUI is known to be multifactorial, and in two of the three largest studies included in this systematic review19, 20 a BMI > 30 Kg/m2 was highlighted as a risk factor for SUI relapse. Several risk factors, such as being overweight and obese,26, 27 cigarette smoking,28 and maternal age29 have been proposed for the development of UI23, 30 and should be considered for the management of SUI relapse after MUS procedure. Even if obesity is considered as an established risk factor for SUI,31 some studies presented conflicting results after MUS implantation.32, 33 In this field, the multifactorial etiology of SUI complicates the possibility of preventing SUI relapse. Anyway, patients with SUI submitted to MUS procedure should be educated about the advantages of maintaining a healthy BMI.
Additionally, this metanalysis observed that experiencing incontinence during pregnancy following MUS is correlated with postpartum incontinence. The data concerning the relationship between UI during pregnancy and its persistence or worsening postpartum are conflicting: Solans-Domenech et al.34 found that UI during pregnancy is associated with postpartum incontinence, with vaginal delivery increasing the risk of persistent incontinence. Conversely, Wesnes et al.35 concluded that the link between postpartum incontinence and mode of delivery is not significantly affected by incontinence status during pregnancy, and predicting a high-risk group for incontinence based on delivery mode cannot rely on continence status during pregnancy. According to our results, patients who develop UI during pregnancy after MUS should be counseled about the increased likelihood of this condition persisting after delivery and offered intervention if necessary.
Based on these observations, several studies tested the impact of childbirth on urinary continence in women who previously had MUS.15-21
In the study by Tulokas et al.21 the authors concluded that pregnancy after MUS did not increase the odds for SUI re-procedure or re-visit and that future pregnancy does not need to be viewed as an absolute contraindication for MUS operation. This long-term retrospective case-control study adopted two Finnish national registers: the Care Register for Health Care (Care Register) and the Medical Birth Register. If on one hand these registries offered a substantial population-based sample without any selection bias, on the other hand it was not possible to detect recurrent SUI and other complications unless the women decided to seek help from a doctor, with a possible underestimation of UI relapse. The absence of an objective evaluation of UI at follow-up represent a limitation for this study.
Dyrkorn et al.20 in their retrospective case-control study concluded that no differences in outcomes were seen between women who had childbirth and women who had not, independently of delivery mode. However, having more than one delivery after MUS seems to impact the continence status. Two Danish national registers, the National Norwegian Female Incontinence Registry and the Medical Birth Registry of Norway have been adopted and a validated short-form urinary disease-specific questionnaire for subjective data has been telephonically administered. As in the previous study, register offer a comprehensive long-term evaluation of the study population. However, UI have been only subjectively evaluated through the validated questionnaire, without an objective assessment such as urodynamics test.
The largest retrospective case-control study conducted by Bergman et al.19 concluded that childbirth subsequent to a MUS procedure does not carry an elevated risk of patient-reported SUI, and the continence status remains unaffected by the mode of delivery in the subsequent pregnancy. The study utilized nationwide Swedish health care registers to identify both cases and controls, while the UDI questionnaire was administered during follow-up. However, there was no objective assessment of SUI at follow-up. Additionally, the study did not provide information on continence status between MUS surgery and pregnancy, making it difficult to differentiate between pre-existing incontinence and that arising due to childbirth.
In 2016 and 2014, Adams-Piper and colleagues17, 18 concluded from their case-series that the safety and durability of MUS remained intact even after subsequent pregnancy, that vaginal delivery is not contraindicated and sling-related complications during pregnancy are not prevalent. However, the limited sample size and the absence of an objective assessment of SUI relapse during follow-up, including the lack of a clear definition for diagnosing SUI at follow-up, constrain the generalizability of the findings.
In their case series, Cavkaytar et al.16 concluded that the risk of postpartum SUI recurrence among women who have undergone MUS application appears to be consistent regardless of the mode of delivery, and data suggested that incontinence during pregnancy could potentially be a risk factor for postpartum incontinence. Although the small sample size may have hindered the reproducibility of these findings, the study conducted by Cavkaytar et al. stands out as the sole inclusion in this meta-analysis to utilize the stress test, providing an objective evaluation of SUI as per the study's objectives.
At last, the study by Panel et al.15 affirmed that pregnancy following surgical treatment for SUI with sub-urethral tape appears not to pose significant urinary or obstetric complications for patients. Pregnancy itself constitutes a risk factor for recurrent incontinence, and vaginal delivery did not elevate the risk of recurrence in comparison to cesarean section. However, the study design, the small sample size and the lack of a clear definition for diagnosing SUI at follow-up limit the possibility to spread recommendation related MUS in young women and route of delivery.
Presently, there is a lack of evidence-based data concerning the impact of subsequent pregnancy and delivery on the risk of SUI recurrence following a MUS procedure. In a survey conducted among members of the American Urogynecologic Society (AUGS), nearly 15% of respondents indicated that they would refrain from performing anti-incontinence surgery on patients planning future pregnancies. Additionally, 40% of respondents expressed a preference for cesarean delivery over vaginal delivery for patients who had undergone surgery for SUI.36 AUGS reported that the available data are inadequate to determine whether recurrent rates of SUI vary between vaginal and cesarean deliveries.37 According to the results of this metanalysis, childbirth does not increase the risk of SUI relapse after MUS surgery independently from the route of delivery.
Concerns about synthetic non resorbable materials vaginally implanted raised after several patients' class actions.38 Specific tape complications, such as vaginal sling erosion, infection and chronic pelvic pain emerged, leading several national and international authorities to emanate recommendation for the use of synthetic non resorbable transvaginal tape and particularly MUS.38 Consequently, some authors are persuaded that performing MUS procedures in the young may be associated with good initial outcomes, but with risk of long-term morbidity.39 Moreover, MUS have been accused to lead to dyspareunia and sexual impairment. Indeed, when vaginal exposure of the polypropylene occurs in women submitted to MUS procedures, this may cause sexual dysfunction.40 Moreover, vaginal MUS implantation itself may cause neurovascular tissue damages with sensory loss, pelvic pain, and dyspareunia.40-42 For these several reasons, alternative less-invasive treatment as the urethral bulking agents (UBAs) have been proposed for young women affected by SUI.39 Even if less effective than MUS on SUI,43, 44 UBAs showed a more important safety profile44 and a good efficacy also in improving sexual function,45-47 leading clinicians to consider the possibility of postponing MUS in favor of UBAs during reproductive age.39 In the studies included in this systematic review, MUS did not show to increase complications both during pregnancy and at delivery. Indeed, the rate of complications possibly related to MUS such as UTIs and voiding dysfunction in pregnant women previously submitted to MUS is in line with that of pregnant women not previously submitted to MUS23, 48 at a long follow-up up to 129.6 months.
This systematic review and metanalysis has several limitations. All the included studies were retrospective reports with all the implicit defects of such design; however, MUS in young fertile women can be considered as a “rare condition.” Randomized controlled trials are not ethically possible for the evaluation of the optimal mode of delivery. Several studies presented a low sample size. Moreover, several missing data such as the assessment of SUI, birth weight, obstetric anal sphincter injuries, instrumental delivery, the obstetrician choice for the delivery route limited the possibility to perform metanalysis for these important sub-groups of patients. The number of studies ultimately included and analyzed in this meta-analysis was limited, primarily due to the majority of screened studies being of low quality. Moreover, the analyses were based on only three studies per comparison. Furthermore, the three population-based studies incorporated in this meta-analysis originate from a specific population in Scandinavia, which may restrict the generalizability of the findings to other regions. Therefore, adjustments of the presented data to suit the characteristics of the local population should be considered. However, we retain that this metanalysis presents several strengths too. We utilized prominent clinical literature databases from which data were extracted and analyzed employing rigorous statistical methodologies. The included population is a small population but of main clinical importance for several health care providers (urogynecologist, urologist, obstetrician etc.) that could confront this condition. This metanalysis has the largest overall sample size reported in the literature for this specific clinical issue, with all the included studies that showed homogeneous results. We deeply analyzed several potential risk factors related to postpartum relapse of UI. However, due to the inherent confounding effect in observational studies, these conclusions should be interpreted cautiously. Even if better design studies are needed, RCTs in this field are difficult and not ethically sustainable, making systematic reviews and metanalysis the main typology of research available to propose clinical recommendations.
5 CONCLUSION
The results of this metanalysis show that childbirth do not affect SUI relapse or reintervention in women previously submitted to MUS. Moreover, in the same population of patients, no difference where highlighted concerning the mode of delivery for the outcome SUI relapse or reintervention, and previous MUS surgery may not be an appropriate indication for caesarian birth in subsequent pregnancy. MUS does not increase complications in women who had childbirth after the anti-incontinence procedure at long term follow-up. Even if the results of our metanalysis does not highlight pregnancy, childbirth and mode of delivery as factors impacting MUS efficacy and safety, evidence are insufficient to make widespread recommendations.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.




