A genetic assessment of natural barriers for isolating a proposed Greenback Cutthroat Trout reintroduction area
Abstract
Objective
Native inland trout conservation efforts rely on physical barriers to exclude nonnative salmonids from target habitats. We used genetic techniques to evaluate a series of natural waterfalls for their potential to serve as barriers to prevent nonnative salmonids from entering a proposed reintroduction area for federally threatened Greenback Cutthroat Trout Oncorhynchus virginalis stomias.
Methods
Genetic samples were collected from nonnative Brook Trout Salvelinus fontinalis at 11 sampling reaches above and below natural waterfalls (height: ~1–3 m under base flow conditions) along a 33-km segment of Colorado's upper Cache la Poudre River near the outflow of the proposed reintroduction area. To evaluate whether upstream movement of Brook Trout is restricted by any of these waterfalls, we characterized longitudinal trends in genetic diversity along the river corridor and examined patterns of genetic differentiation and population structure in relation to waterfall locations using a panel of microsatellites.
Result
We found no evidence that the waterfalls served as complete movement barriers for nonnative Brook Trout based on genetic clustering analyses, estimates of population differentiation, and longitudinal genetic patterns. Our multilocus assessment did not identify alleles restricted to downstream reaches, and the river segment was genetically homogenized.
Conclusion
Our evaluation suggests that the existing waterfalls do not fully prevent upstream movement by nonnative Brook Trout, and thus barrier modification would be needed to establish an isolated Greenback Cutthroat Trout population in the proposed wilderness area.
INTRODUCTION
Once widespread throughout the western United States, cutthroat trout species have declined in abundance and distribution following extensive introductions of nonnative salmonids during the past century (Young et al. 1996; Harig et al. 2000; Gresswell 2011; Nordberg et al. 2021). For instance, Brook Trout Salvelinus fontinalis were widely introduced throughout the southern Rocky Mountains beginning in the late 1800s and have become naturalized in habitat that has historically been occupied by cutthroat trout (Fausch 2007; Metcalf et al. 2012). Brook Trout typically outcompete cutthroat trout for food and habitat resources during early life stages, leading to extirpation of cutthroat trout in many cases (De Staso and Rahel 1994; Dunham et al. 2002; Peterson et al. 2004; Roberts et al. 2017). As a result, a majority of remnant native cutthroat trout populations exist in isolated headwater habitats upstream of barriers (Young et al. 1996; Fausch 2007; Nordberg et al. 2021) and successful recovery strategies rely on natural or artificial barriers to prevent encroachment by nonnative fish into reintroduction areas (Harig et al. 2000; Novinger and Rahel 2003; Rahel 2013). Evaluating the effectiveness of natural waterfall features to serve as fish passage barriers is important for identifying suitable reintroduction habitats and determining when artificial barrier construction is necessary.
Under the management-by-isolation paradigm, managers have reintroduced populations of several native inland trout species (Oncorhynchus spp.) including Apache Trout O. apache, Lahontan Cutthroat Trout O. henshawi, Colorado River Cutthroat Trout O. virginalis pleuriticus, and Greenback Cutthroat Trout O. virginalis stomias (Novinger and Rahel 2003; Fausch et al. 2009; Rahel 2013). Headwater habitats that are chosen for reintroductions are often located in remote wilderness areas and other public lands with minimal anthropogenic influence, which has resulted in the preservation of intact aquatic habitats that are suitable for extinction-vulnerable species (Kershner et al. 1997). However, logistic constraints for work in remote areas make locating or constructing barriers challenging, and although native species conservation is a management priority in designated wilderness areas (Association of Fish and Wildlife Agencies 2006), physically modifying habitats in a wilderness setting can be controversial and requires scrutinous evaluation to ensure that proposals align with policy. Therefore, decisions regarding if, where, and how barriers are used or constructed for native trout reintroductions in wilderness areas should be informed by science.
Assessments of fish passage barriers in natural environments often rely on the direct observation of fish movement using techniques such as mark–recapture or passive integrated transponder (PIT) antennas (Thompson and Rahel 1998; Bunt et al. 2012). Although observational studies can confirm fish passage, they may fail to detect movement when it actually occurs because only small fractions of populations are tagged or marked and limited observation windows can miss patterns of episodic or infrequent movement (Kanno et al. 2014; Whiteley et al. 2014). Furthermore, studies relying on mark–recapture of individual fish require multiple visits to each monitored site, which can be labor intensive, costly, and inefficient to implement at many sites. Genetic methods have emerged as an alternative strategy for indirectly detecting barrier effects in stream fish populations (Wofford et al. 2005; Deiner et al. 2007; Whiteley et al. 2014; Timm et al. 2015) and may provide a more efficient way to evaluate fish passage at large numbers of sites. Thus, genetic approaches can help identify and evaluate potential barriers for use in native fish reclamation projects. By blocking gene flow, barriers can produce marked patterns of genetic isolation, reduced genetic diversity, and differentiation between groups (Hudy et al. 2010; Timm et al. 2015; Allendorf et al. 2022). Often, genetic diversity is reduced above barriers because gene flow is restricted in the upstream direction (Wofford et al. 2005; Neville et al. 2006, 2009; Deiner et al. 2007; Torterotot et al. 2014). Barriers have also been widely reported to be a factor that influences spatial genetic structure in salmonids, contributing to increased levels of genetic differentiation between barrier-separated populations in some cases (Costello et al. 2003; Neville et al. 2006; Deiner et al. 2007; Guy et al. 2008; Torterotot et al. 2014; White et al. 2020) but not in others (Wofford et al. 2005; Timm et al. 2015). Furthermore, barriers may produce distinctive longitudinal patterns in allele frequencies along river corridors because alleles that originate from downstream source populations are expected to be absent above barriers that restrict upstream movement (Sundqvist et al. 2016).
In this study, we examined spatial genetic patterns of nonnative Brook Trout in relation to waterfall locations near the downstream boundary of a proposed reintroduction area for federally threatened Greenback Cutthroat Trout. This reintroduction effort, the Poudre Headwaters Project, is an interagency effort to restore a metapopulation of Greenback Cutthroat Trout to 60 km of stream habitat and 106 acres of lake habitat in Colorado's upper Cache la Poudre River basin, the largest cutthroat trout recovery project in the state's history. A permanent barrier at the downstream boundary of the reclamation area is necessary for the project's success, but fish passage observations revealed that the waterfall that was originally selected for this purpose (U.S. Forest Service 2009) does not sufficiently prevent upstream fish movement (M.P. Fairchild, unpublished data). Identifying a suitable natural barrier would best align with management priorities because the project occurs on a federally designated Wild and Scenic River segment located in a wilderness area where the goal of natural resources management is to preserve the free flow of the river and maintain scenic and recreational values (U.S. Forest Service 1990). However, if there is not a natural barrier, modifying an existing waterfall feature or constructing a new artificial barrier would be necessary to establish an isolated Greenback Cutthroat Trout population. The objective of this study was to evaluate a series of bedrock waterfalls for their potential to serve as physical barriers to isolate the proposed reclamation area. We used multilocus microsatellite genotype data that were collected from sampling reaches above and below each waterfall to assess whether genetic patterns indicate restricted upstream movement by nonnative Brook Trout. This evaluation will provide information to assist managers in deciding whether a natural waterfall feature could serve as the downstream boundary for the Poudre Headwaters Project or if waterfall modification will be necessary to ensure the successful isolation of habitat for Greenback Cutthroat Trout recovery.
METHODS
Study area
This study occurred along a 33-km headwater segment of Colorado's Cache la Poudre River within Rocky Mountain National Park and Roosevelt National Forest. The Cache la Poudre River begins at an elevation of 3270 m as a small, snowmelt-driven stream and flows from the Continental Divide through the northwest corner of Rocky Mountain National Park, forming a dendritic network with other tributary streams (Figure 1). The segment of the Cache la Poudre River that is bounded by our sampling reaches is dominated by nonnative trout, primarily Brook Trout, with low abundance of hybridized cutthroat trout (e.g., Colorado River Cutthroat Trout × Yellowstone Cutthroat Trout O. v. bouvieri) originating from stocked reservoirs and tributaries. Brown Trout Salmo trutta also occur in the lower portion of the study area. Historical records show that Brook Trout were stocked in some of the streams and lakes that are hydrologically connected to our study area as early as 1892, with no records of Brook Trout stocking after 1955 (Colorado Parks and Wildlife, unpublished data; U.S. Fish and Wildlife Service, unpublished data). The entire river corridor and most of the tributary streams of the upper Cache la Poudre River that were addressed in this study lie within the bounds of congressionally designated wilderness areas: Commanche Peak Wilderness designated in 1980 and Rocky Mountain National Park designated in 2009. Additionally, the river corridor was designated as a Wild and Scenic River in 1986, which provides water resource protections that preclude impoundments or disturbances to the hydrology or riparian condition along the corridor.
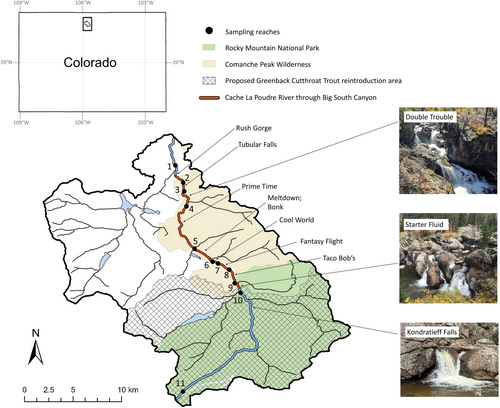
Approximately 20 km downstream from its source, the Cache la Poudre River enters Big South Canyon, where a series of natural bedrock waterfall features have been recognized as potential candidates to serve as the downstream boundary of the Poudre Headwaters Project (Figure 1). These waterfalls are associated with named class IV–V whitewater rapids, with vertical drop heights ranging roughly 1–3 m at base-flow levels. Some rapids consist of multiple vertical cascades that are separated by step pools, which could serve as resting areas for trout. Additionally, there are side channels beside some rapids, which could provide temporary alternate movement paths for trout at high-river stages. Based on the waterfall locations, we selected 11 Brook Trout sampling reaches (length ranging 73–997 m) that were positioned longitudinally along a 33-km segment of the Cache la Poudre River, extending upstream from the mouth of Big South Canyon to the river's origin at the Continental Divide. The sampling locations were chosen so that each adjacent reach was separated by a waterfall feature, with one reach positioned above and below each waterfall. This was not always possible due to practical constraints during fieldwork, and one reach pair is separated by two waterfall features (Figure 1, reaches 5–6).
Sample collection and laboratory genotyping
In September and October of 2022, Brook Trout tissue samples were collected by hook-and-line sampling (reaches 3, 4, 6, 7), backpack electrofishing (reaches 2, 9, 10, 11), or both methods (reaches 1, 5, 8). All the fish were measured for total length, and anal fin clips were collected before releasing the fish alive to the site of capture. Fin clips were dried on chromatography paper and stored in individual coin envelopes. To avoid genetic sampling of closely related individuals, length-frequency distributions were constructed for each reach prior to laboratory work and individuals presumed to be age-0 were excluded from genotyping (Whiteley et al. 2012). The Brook Trout were genotyped at 12 previously described microsatellite loci: SfoC113, SfoC115, SfoC129, SfoC38, SfoC88, SfoD91, SfoB52, SfoC24, SfoC28, SfoC79, SfoC86, and SfoD75 (King et al. 2012). The number of genotyped fish per reach ranged from 20 to 25 (Table S3). Genomic DNA was extracted using Qiagen Dneasy Blood and Tissue Kits following the manufacturer's protocol. The microsatellite markers were amplified using polymerase chain reaction (PCR) in two 10-μL multiplexes, each containing 2 μL genomic DNA, 5 μL Qiagen Multiplex PCR Mastermix, 0.04–0.1 μL each of forward and reverse PCR primer (Table S4), and 2.18–2.2 μL nuclease free water. The thermal profile for PCR amplification consisted of denaturing at 95°C for 15 min, 35 cycles of denaturation at 95°C for 45 s, annealing at 56°C for 45 s, and extension at 72°C for 2 min, followed by a final extension of 60°C for 30 min. Following amplification, the PCR products were treated with a solution of formamide and GeneScan 600 LIZ size standard (Thermofisher Scientific) and visualized using an Applied Biosystems 3500 genetic analyzer. The alleles were scored using GeneMapper version 6.
Population genetic analysis
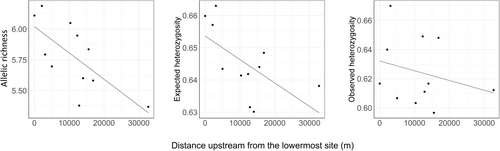
We implemented a suite of analyses to examine whether genetic patterns among sampling reaches that are separated by waterfalls indicate restricted upstream movement. Prior to analysis, the microsatellite genotypes were assessed for potential scoring errors caused by stutter products or large allelic dropout using Micro-Checker version 2.2.3 (Van Oosterhout et al. 2004). We tested for deviations from Hardy–Weinberg equilibrium with 1000 Monte Carlo permutations using the R package pegas (Paradis 2010) and applied a Bonferroni correction for multiple testing comparisons across 132 tests. At each reach, mean rarefied allelic richness (RS) and allele frequencies were calculated using the R package PopGenReport (Adamack and Gruber 2014) and observed (HO) and expected (He) heterozygosity were calculated using the R package hierfstat (Goudet 2004). To test for reduced genetic diversity at farther upstream reaches, linear regression was applied in R (R Core Team 2023) to assess the relationships between reach-specific RS, HO, and He and channel distance upstream from the lower-most sampling reach. Longitudinal variations in allele frequencies along the river corridor were examined in relation to the waterfall locations to assess whether certain alleles were exclusive to downstream reaches and absent above potential barriers, a pattern that would suggest restricted upstream movement. We conducted additional evaluations of each waterfall by aggregating the data across all sampling reaches and calculating the expected number of distinct alleles present in random subsamples of equal size (i.e., total rarefied allelic richness) downstream and upstream of each waterfall using the rarefaction procedure of Hurlbert (1971) as implemented in PopGenReport. The number of alleles sampled for rarefaction was 40, reflecting the minimum pooled sample size among downstream and upstream sample aggregates.
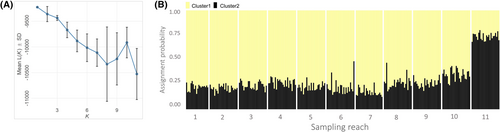
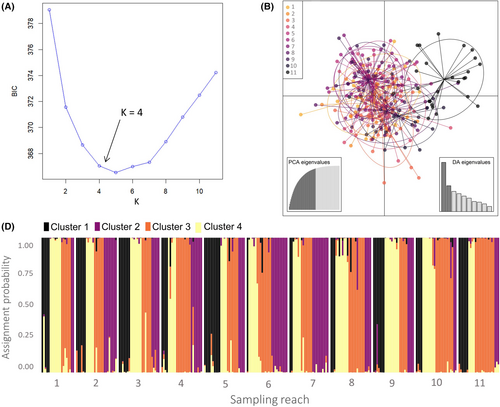
We quantified genetic differentiation between all pairs of sampling reaches using pairwise Fst (Weir and Cockerham 1984) and D (Jost 2008). Differentiation estimates and their corresponding 95% confidence intervals were calculated using the R packages diveRsity (Keenan et al. 2013) for D and hierfstat for Fst. Estimates for Fst and D were considered significant if their 95% confidence intervals did not overlap zero. Two genetic clustering algorithms were applied to further examine the population structure in the study area. The first method, STRUCTURE version 2.3.4 (Pritchard et al. 2000), uses a Bayesian approach to assign individuals to likely genetic clusters based on shared patterns of multilocus genetic variation. We evaluated the likelihood of 1–11 genetic clusters (K), with five independent STRUCTURE runs performed for each K. All runs consisted of a burn-in period of 50,000 iterations followed by 200,000 Markov chain–Monte Carlo iterations, specifying the admixture model, correlated allele frequencies, and sampling locations as priors. Individual cluster assignment plots were assessed visually, and the most plausible number of genetic clusters was determined by assessing the mean log likelihood of K (L[K]; Pritchard et al. 2000) and ΔK (Evanno et al. 2005). The second clustering method, discriminant analysis of principle components (DAPC; Jombart et al. 2010), defines clusters of genetically similar individuals using a multivariate approach. To determine the number of principal components to retain for DAPC, we used the optim.a.score function from the R package adegenet (Jombart 2008). We identified de novo genetic clusters using the find.clusters function from adegenet to evaluate K = 1–11 genetic clusters. Following Jombart and Collins (2015), we chose the optimal number of genetic clusters as the value of K at which the rate of decrease in the Bayesian information criterion began to plateau. In addition to evaluating the de novo genetic clusters, we constructed a DAPC ordination plot to visualize the genetic segregation of individuals that were grouped a priori by sampling location.
RESULTS
A total of 269 Brook Trout were genotyped at 12 microsatellite loci (Table S3), and all loci were polymorphic. After Bonferroni correction, we detected no deviations from the Hardy–Weinberg equilibrium across all loci and reaches. Micro-Checker revealed no evidence of stutter products or large allelic dropout, suggesting a lack of allele-scoring errors. Across all reaches, expected heterozygosity (He) ranged from 0.63 to 0.66 (mean 0.65), observed heterozygosity (HO) ranged from 0.60 to 0.67 (mean 0.62), and rarefied allelic richness (RS) ranged from 5.37 to 6.19 (mean 5.78). Generally, genetic diversity was lower at farther upstream reaches (Figure 2), with linear regression showing significant negative relationships between distance upstream (m) and RS (p = 0.017) and He (p = 0.045) but not HO (p = 0.44). The pairwise Fst and D values indicated minimal genetic differentiation among sampling reaches. Estimates of pairwise Fst ranged from −0.010 to 0.025, with a mean of 0.005, and the estimates of D ranged from −0.012 to 0.020, with a mean of 0.003 (Table S1). Only 9 of the 55 pairwise Fst comparisons between sampling reaches yielded significantly positive estimates, and no pairwise D values were significantly positive. Of the nine significantly positive Fst estimates, seven were associated with the uppermost reach (reach 11), indicating modest genetic isolation of this reach relative to the rest of the study area. Longitudinal variations in the frequencies of specific alleles along the river corridor exhibited no clear patterns to suggest that upstream movement is restricted by any of the waterfalls (Figure S1). Using aggregated data across all sampling reaches, most waterfalls showed slightly fewer distinct alleles present upstream than downstream (mean 2.08 fewer alleles upstream) and Kondratieff Falls showed the greatest discrepancy, with an estimated 9.45 fewer alleles upstream than downstream (Table S2).
Genetic clustering analyses provided minimal evidence of distinct genetic groups in the study area. From our STRUCTURE results, the mean log probability of the data decreased as the number of genetic clusters increased from K = 1 to 11, providing the most support for K = 1 (Figure 3A). Because the ΔK method (Evanno et al. 2005) for identifying the optimal K is unable to verify K = 1 (Evanno et al. 2005; Gilbert 2016; Janes et al. 2017), this method was not used to determine genetic clusters (Figure S2). Although our assessment of the mean log probability of K supports K = 1, a visual examination of the STUCTURE individual assignment plot assuming K = 2 indicated some genetic isolation of reach 11 (Figure 3B). The optimal number of principal components to retain for DAPC was determined to be 48, and de novo DAPC cluster identification showed a steep decline in the values for the Bayesian information criterion until K = 4, indicating 4 as the optimal number of genetic clusters (Figure 4A). However, visualization of individual membership probabilities to each cluster as they relate to the sampling locations revealed no spatial segregation of genetic clusters that were identified by DAPC (Figure 4C). Separately, an ordination plot of DAPC clusters defined a priori as sampling locations showed substantial overlap in ordination space among all reaches, with reach 11 showing moderate isolation relative to the rest of the study area (Figure 4B). Overall, we found no evidence that any of the existing waterfalls served as complete movement barriers.
DISCUSSION
Our evaluation revealed a lack of barrier-driven genetic differentiation among stream reaches that are separated by waterfalls. Furthermore, longitudinal patterns in allele frequencies along the river corridor showed no discernable patterns to suggest that upstream movement is substantially restricted. These results support the possibility of at least minimal upstream movement by Brook Trout along this river segment, with none of the natural waterfalls under evaluation showing genetic evidence of being impassable barriers. We observed a general trend of increasing genetic diversity at farther downstream reaches (Figure 2), which aligns with the results of previous studies on stream fishes (Wofford et al. 2005; Hanfling and Weetman 2006; Neville et al. 2006; Deiner et al. 2007; Lamphere and Blum 2011). In dendritic stream networks, this pattern may arise from biased fish dispersal in the downstream direction and the accumulation of unique genotypes from the convergence of different tributaries in the network (Thomaz et al. 2016). Barriers can contribute to directional asymmetries in fish movement, but downstream-biased dispersal is common in many stream fishes even in the absence of barriers (Hanfling and Weetman 2006; Lamphere and Blum 2011), which can result from passive displacement of fish during high-flow events (Morrissey and Ferguson 2011) or density-dependent dispersal (Fraser et al. 2004). This downstream bias is typical for many fishes, but there is evidence that Brook Trout exhibit a unique tendency for upstream movement; several studies have reported upstream-biased dispersal by Brook Trout (Peterson and Fausch 2003; Hansbarger et al. 2010; Gutowsky et al. 2023) even in steep headwater streams (Adams et al. 2000). Importantly, Brook Trout typically use uppermost headwater tributaries for spawning (Witzel and MacCrimmon 1983) and the higher genetic diversity farther downstream in our study area likely resulted in part from colonization of downstream sites from different spawning locations in the dendritic network, including tributaries that converge with the Cache la Poudre River downstream from the study area. In this case, impassable barriers along our study segment would be expected to produce discernible longitudinal genetic patterns because downstream-sourced genetic variation would be present at lower sites but absent above barriers. Therefore, it is likely that our suite of analyses would have detected some genetic evidence of restricted upstream movement if any of the evaluated waterfalls provides a complete barrier.
The lack of genetic evidence for barriers is consistent with previous observations of fish passage in the study area; two of the waterfalls in our evaluation, Starter Fluid and Kondratieff Falls (Figure 1), have previously been examined in mark–recapture fish passage studies. From 2019 through 2022, U.S. Forest Service personnel administered PIT tags to 1284 Brook Trout and released them below Starter Fluid, the waterfall that was originally selected as the downstream barrier for the Poudre Headwaters Project. A stationary PIT antenna that was positioned directly above the waterfall detected upstream passage of tagged fish, and tagged fish were recaptured upstream of the falls (Fairchild, unpublished data). Movement past this waterfall varied by season, so it is possible that seasonal variations in flow provide temporary opportunities for upstream passage by reducing the drop height, increasing the plunge pool depth, or filling an intermittent side channel. Details of this fish passage study at Starter Fluid will be reported in a separate article. Upstream movement of Brook Trout past Kondratieff Falls was also demonstrated by Myrick and Kondratieff (2004), who released 626 marked Brook Trout below the waterfall and subsequently recaptured three of these marked fish above the falls. Interestingly, our genetic results suggest that Kondratieff Falls is the most likely waterfall in the study area to impede upstream fish movement. This waterfall showed the greatest reduction in total allelic richness from downstream to upstream (Table S2), and sampling reach 11, which is separated from the rest of the study area by Kondratieff Falls (Figure 1), was the most genetically distinct reach based on the Fst and cluster analyses (Table S1; Figures 3B and 4B). Nevertheless, even small numbers of migrants (<10 individuals per generation) have been shown to significantly reduce the genetic differentiation between Brook Trout groups (Nathan et al. 2017), and low pairwise Fst estimates (a maximum value of 0.02 between reaches 11 and 7), along with a lack of quantitative support for distinct genetic clusters, highlight that reach 11's genetic isolation is weak. This reach is also the most spatially isolated sampling location in our study, and isolation by distance may contribute to its slight genetic distinction aside from partial barrier effects.
The tendency of specific waterfall features to restrict fish passage is influenced by many factors, including the physical characteristics of waterfalls and the size and swimming ability of fish. In general, larger drop heights, shallower plunge pool depths, and smaller individual fish sizes reduce the probability of upstream fish passage across barriers (Stuart 1962; Kondratieff and Myrick 2006). A limited number of studies have attempted to quantify the jumping ability of Brook Trout and identify barrier-height thresholds to restrict fish passage. In a laboratory experiment, Kondratieff and Myrick (2006) reported a 0.74-m maximum jump height for Brook Trout <34 cm in total length. However, Adams et al. (2001) documented a 21-cm Brook Trout ascending a 1.5-m waterfall based on mark–recapture observations, highlighting that the maximum observed jumping performances in laboratory studies do not always reflect the behavior of fish in the wild. Timm et al. (2015) applied population genetic analyses to estimate a barrier-height threshold for influencing Brook Trout gene flow, reporting that genetic diversity and Fst were not significantly affected by barriers with drop heights of less than 4 m. Our results are consistent with this 4-m threshold, as none of our evaluated waterfalls exceed this height.
Our work demonstrates the utility of genetic techniques for evaluating fish barriers in an applied management scenario. Genetic patterns are informative about migration trends over long timescales (Allendorf et al. 2022) and account for a wider range of individual movement abilities and past flow conditions compared to studies that rely on direct observation of individual fish. Although traditional fish passage studies are useful for site-specific understanding of seasonal or hydrologic limits to fish passage, a primary advantage of our genetic approach is that it allowed for a rapid assessment of many potential barrier sites simultaneously, without incurring the time and personnel costs of mark–recapture methods, which require multiple visits to sites over time. However, it is important to note that inferences of fish dispersal based on genetic methods can be confounded by factors such as stocking history, unauthorized fish movement, and limited sampling. Additionally, the detection of restricted upstream movement based on longitudinal variations in allele frequencies relies on the existence of alleles that are exclusive to downstream sites and absence upstream of barriers (Sundqvist et al. 2016). In certain cases, such as when most of a region's genetic diversity originates from upstream sources, barriers may be present but undetectable due to a deficiency of unique downstream alleles. However, in our study the observed increase in genetic diversity farther downstream suggests an accumulation of downstream-sourced genetic variation from tributary confluences, which would increase the likelihood of detecting existing barriers along the study segment.
Overall, this genetic assessment provided no evidence that any of the evaluated waterfalls are complete upstream barriers to Brook Trout. Our results, along with observed upstream passage of fish over some of the waterfalls (Myrick and Kondratieff 2004; Fairchild, unpublished data), lead us to conclude that none of these waterfalls in their unaltered states should be considered adequate to isolate habitat in the Poudre Headwaters Project reintroduction area. To successfully reintroduce and establish a Greenback Cutthroat Trout population, it is likely that Starter Fluid, the waterfall that was originally selected to bound the project area (U.S. Forest Service 2009), would need to be modified to serve as a complete barrier to nonnative trout invasion. However, the decision to modify natural habitat features in a designated wilderness area must be carefully weighed against the benefits of restoring native trout populations. We believe that this trade-off will continue to be common because current and future efforts to conserve native inland trout often occur in wilderness headwaters and require physical barriers. Our research serves as a case study on the use of molecular tools as a rapid assessment technique to inform fisheries and other natural resources management decisions based on the best available science.
ACKNOWLEDGMENTS
Funding for this study was provided by Guadalupe River Trout Unlimited, U.S. Forest Service, and National Park Service. We thank volunteers from the Rocky Mountain Flycasters chapter of Trout Unlimited as well as U.S. Forest Service, U.S. Fish and Wildlife Service, and National Park Service personnel for assisting with field collections. We also thank Noël Clark for reviewing the accuracy of biologic names, along with Audrey Harris and two anonymous reviewers for constructive feedback that improved this manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Fieldwork was conducted in accordance with a protocol approved by the Colorado State University Institutional Animal Care and Use Committee (IACUC Protocol Number 1505).
Open Research
DATA AVAILABILITY STATEMENT
The data presented are available online (https://doi.org/doi:10.5061/dryad.3n5tb2rsd).




