Don't count your eggs before they resorb: Early collection of ovarian follicles influences estimates of Lake Trout fecundity in Yellowstone Lake
Abstract
Objective
Understanding recruitment dynamics is necessary to predict population-level responses to exploitation, management actions, or anthropogenic influences. Fecundity is commonly used as a metric of recruitment dynamics and can guide successful management of fisheries. However, an individual female's fecundity is not constant over time; females resorb ovarian follicles to regulate fecundity as they approach spawning. This suggests that sampling for fecundity too early may produce inaccurate estimates of relative fecundity. In Yellowstone National Park, suppression of invasive Lake Trout Salvelinus namaycush reduced the abundance of mature fish by 92% between 2012 and 2022. The continued efficacy of this suppression effort requires accurate assessments of reproductive potential of the population that remains.
Methods
We sought to determine whether the timing of ovarian follicle collection affected estimates of mean relative fecundity. We collected ovarian follicles from female Lake Trout, which are autumn spawners, between mid-August and early October in 2021 and 2022. The number of ovarian follicles per sample was counted to obtain estimates of relative fecundity for each female.
Result
We observed a 13% decline in estimated mean relative fecundity between individuals that were sampled before mid-September and those that were sampled after mid-September.
Conclusion
Our data support strategic timing of fecundity sampling to best capture the true reproductive capability of a population, which is a key metric used in models that guide adaptive management of fishes.
INTRODUCTION
An accurate understanding of recruitment dynamics is needed to predict a population-level response to exploitation or other factors causing a loss of reproductive capacity. Fecundity, defined as the total number of mature ovarian follicles that are oviposited (Pavlov and Emel'yanova 2009), is an important reproductive metric that is commonly used in understanding recruitment dynamics and in the successful management of fishes. Specifically, fecundity data are necessary for calculations derived from life history tables, determining spawning biomass, and recruitment analyses (Kjesbu 2009). Estimates of relative fecundity—the number of developing ovarian follicles divided by fish body weight (Nikolsky 1963; Bagenal 1978; Liermann et al. 2004; Kjesbu 2009)—over time can provide fisheries managers with insight into how exploited populations are changing and whether demographic shifts are occurring (De Roos et al. 2006). Fecundity sampling is typically conducted in the weeks and months immediately prior to spawning in order to avoid sampling females that have already spawned or that are undergoing atresia (resorption of ovarian follicles); however, an individual female's estimated relative fecundity may not be constant across her reproductive cycle. As females approach spawning, they may respond to the energetic demands of environmental stressors by downregulating (or “intentionally” reducing) their fecundity through the process of ovarian follicular atresia, in which the number of oocytes produced at the beginning of the reproductive cycle is reduced to the actual number of follicles that the female can support and spawn (Miranda et al. 1999; Grier et al. 2009; Kjesbu 2009).
As a result of fecundity downregulation through follicular atresia, estimates of relative fecundity have been found to be affected by the timing of sample collection in marine fishes. For example, relative fecundity estimates for female Greenland Halibut Reinhardtius hippoglossoides were shown to be higher when individuals were sampled earlier in vitellogenesis—the process of yolk formation and deposition in the ovarian follicle—compared to later in vitellogenesis as females approached spawning (Kennedy et al. 2009). Kennedy et al. (2009) described a 43% reduction in ovarian follicles through natural follicular atresia as female Greenland Halibut transitioned from potential fecundity to realized fecundity (realized fecundity = potential fecundity – subsequent atresia; Kjesbu 2009) over the course of vitellogenesis. Similarly, a study of Atlantic Herring Clupea harengus, a winter spawner (February–April), reported that females sampled across the prespawning season (July–January) exhibited a 59% reduction in ovarian follicles as a result of follicular atresia (Kurita et al. 2003).
Additional examples of fecundity downregulation through follicular atresia can be found in marine fishes (see Thorsen et al. 2006; Van Damme et al. 2009); however, knowledge gaps appear to exist across freshwater species. Vladykov (1956) observed reductions in fecundity in Brook Trout Salvelinus fontinalis through changes in oocyte size. Similarly, research on Rainbow Trout Oncorhynchus mykiss suggested that 10–30% of ovarian follicles could be resorbed throughout vitellogenesis (Bromage and Cumaranatunga 1988). Because of the impact that fecundity downregulation can have on the accuracy of fecundity estimates and the understanding of long-term population dynamics, it is important to examine this phenomenon further in freshwater fish populations.
Invasive Lake Trout S. namaycush were discovered in Yellowstone Lake, Yellowstone National Park, Wyoming, in 1994 (Kaeding et al. 1996), and the negative effects of their introduction on the native Yellowstone Cutthroat Trout O. virginalis bouvieri population (Ruzycki et al. 2003; Koel et al. 2005) and lake ecosystem (Koel et al. 2019) have been extensively documented. To mitigate the impacts of Lake Trout, the U.S. National Park Service (NPS) implemented gill netting removal to suppress population growth (Koel et al. 2020) in 1995. The suppression program is informed by a statistical catch-at-age (SCAA) model that was created in 2009 (Syslo et al. 2011) and is refined annually (Koel et al. 2020; Syslo et al. 2020). The SCAA model is used to estimate the abundance and biomass of age-2, age-3–5, and age-6 and older (age-6+) Lake Trout and to set benchmarks for the gill netting effort to be applied each year. Gill netting effort increased annually, and in 2012 the effort intensified enough to drive the Lake Trout population into decline (population growth rate λ < 1.0; Koel et al. 2020; Syslo et al. 2020). In 2015, 3 years after the peak Lake Trout abundance was observed in Yellowstone Lake, estimated relative fecundity was not statistically different than that in earlier years when the population was undergoing exponential growth (1996, 2006, and 2007; Ruzycki et al. 2003; Syslo et al. 2011; Heredia et al. 2021). The suppression program emphasized the removal of adults; between 2012 and 2022, the estimated abundance of age-6+ (reproductively mature) Lake Trout declined by 92% (Koel et al. 2024). Despite this, the abundance of age-2 recruits varied but did not decline, suggesting that the population was compensating for the loss of adults (Koel et al. 2024). One compensatory mechanism that is currently being investigated is a population-level shift to a younger age at first maturity.
Contemporary and accurate evaluations of relative fecundity are required to further inform SCAA modeling and maintain the efficacy of Lake Trout suppression in Yellowstone Lake. In the late 1990s, approximately 5 million eggs were produced annually. Lake Trout abundance peaked in 2012, and egg production at that time surpassed 50 million annually. Since then, intensified gillnetting suppression efforts have greatly reduced the adult segment of the population; however, nearly 10 million eggs—two times the management goal of 5 million eggs—continue to be produced annually (Koel et al. 2020). Previous fecundity sampling occurred in late summer and early autumn as part of removal activities that were already in place, conducted several weeks prior to the peak spawning period in Yellowstone Lake (late September and early October; Ruzycki et al. 2003; Syslo et al. 2011; Heredia et al. 2021). In this instance, if the sampling occurred too early in the reproductive cycle (i.e., too early in vitellogenesis), the downregulation of fecundity through follicular atresia may have produced estimates of relative fecundity that reflected the potential fecundity of sampled fish rather than the realized fecundity.
Our goal was to develop fecundity sampling guidelines for Yellowstone National Park that can be used to generate accurate annual fecundity estimates, thereby improving outputs from the statistical population models that guide fishery management actions in Yellowstone Lake. Our specific questions were (1) “Do female Lake Trout have lower relative fecundity closer to the peak of spawning than during the prespawning stages of reproductive development?”; and (2) “Can we identify a time when female Lake Trout in Yellowstone Lake transition from potential fecundity to realized fecundity?” Results of the present study will ensure greater accuracy of individual fecundity made in the field, thus leading to better estimates of total population fecundity produced by the SCAA model that underpins the multi-million-dollar Lake Trout suppression program.
METHODS
Lake Trout ovary collection
Ovaries were collected from mature female Lake Trout (n = 118; 486–866 mm total length) in Yellowstone Lake during September 13–October 6, 2021, and August 18–September 22, 2022. Lake Trout were captured by trap-netting and gill netting methods (Koel et al. 2020) implemented for ongoing Lake Trout suppression efforts. Regardless of size, Lake Trout that were believed to be mature females based on external visual inspection (enlarged abdomens and swollen oviducts) and macroscopic observation of ovarian follicle diameter were removed from the nets and stored on ice in coolers for up to 10 h. In the NPS laboratory at Yellowstone Lake, Lake Trout were measured to the nearest millimeter (total length) and weighed to the nearest gram (fish ≤2200 g) or to the nearest 28 g (fish >2200 g). Ovaries were removed from mature female Lake Trout and weighed to the nearest 0.01 g using an SPX2202 precision scale (Ohaus Corporation). We obtained three ovarian tissue samples (anterior, middle, and posterior) from each ovary, resulting in a total of six ovarian tissue subsamples per female. Each of the six subsamples was weighed to the nearest 0.01 g and preserved in 70% ethyl alcohol.
Ovarian follicle collection and counting
Ovarian follicles were counted at the U.S. Fish and Wildlife Service Bozeman Fish Technology Center in Bozeman, Montana. Each subsample was separated with forceps, and individual follicles were counted in each subsample. We did not have enough fish that were sampled for both histological analysis and fecundity estimates, so we were unable to identify the exact timing of fecundity downregulation with reference to the stage of maturity (i.e., primary, secondary, or tertiary vitellogenesis or spermatogonial proliferation as per Crossman et al. 2022). We recognize the importance of those data and are including them in future work.
Statistical analyses
We determined post hoc whether follicle collection time impacted mean relative fecundity estimates by using follicle count data to visually identify the collection time at which fecundity downregulation likely ended. Based on this, each fish was assigned to one of two groups depending on the date of sampling: (1) mid-August to early September or (2) mid-September to early October. We used Welch's two-sample t-test to identify differences in mean relative fecundity estimates between these two groups as well as differences in the mean relative fecundity estimates obtained if all fish were included in the estimate versus only including fish that were sampled after the end of fecundity downregulation. We also used analysis of variance to determine whether there were differences in ovarian follicle count among sampled regions in an ovary and between ovaries and whether there were differences in fish weight and total length based on when fish were sampled (i.e., prior to or after mid-September).
RESULTS
Data met the assumptions of normality and did not require transformation. Individual fish weight ranged from 1407 to 8278 g, and total length ranged from 486 to 866 mm. Mean individual fish weight ± SE was 3443 ± 123 g, and mean total length ± SE was 661 ± 7 mm. There was no evidence of statistically significant differences in ovarian follicle count among sampled regions in an ovary (F2,705 = 0.2, p = 0.30) or between ovaries (F2,705 = 1.01, p = 0.30). Additionally, there was no evidence of statistically significant differences in individual fish weight (F1,116 = 1.96, p = 0.30) and total length (F1,116 = 1.06, p = 0.30) based on when fish were collected (i.e., prior to or after fecundity downregulation). Four females were statistically identified as biologically extreme outliers based on fecundity data and were excluded from the analyses. Mid-September was the collection time at which fecundity downregulation ended (i.e., there was no further decline in follicle count per gram of fish after this point) and females transitioned from potential fecundity to realized fecundity (Figure 1).
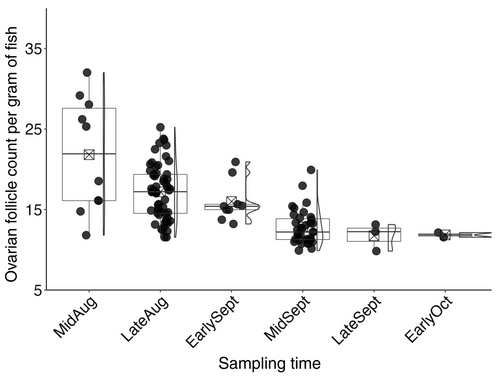
The timing of sampling impacted mean relative fecundity estimates. Mean estimated relative fecundity determined for females that were sampled between early August and early September was 13% higher than for females that were sampled between mid-September and early October (t85 = 2.3, p = 0.02; Table 1), which, based on gill netting catches over the past three decades, is the peak period of spawning in Yellowstone Lake. Additionally, the mean estimated relative fecundity obtained when all females across sampling times were included was 9% higher than the mean estimated relative fecundity determined for females that were sampled between mid-September and early October (t148 = 3.1, p = 0.00; Table 1).
| Collection period | N | Mean estimated relative fecundity (follicles/kg) |
|---|---|---|
| Mid-Aug–early Oct | 118 | 1485 ± 42 y |
| Mid-Aug–early Sep | 78 | 1551 ± 54 y |
| Mid-Sep–early Oct | 40 | 1357 ± 65 z |
DISCUSSION
During our study period, we found evidence of fecundity downregulation in female Lake Trout that were sampled from Yellowstone Lake. Visual inspection of follicle count data demonstrated that fewer follicles were produced in the sampling period after mid-September than in the sampling period before mid-September. This suggests that fecundity downregulation ended at some point in mid-September and that female Lake Trout in this population were likely transitioning from potential fecundity to realized fecundity at that time. Specifically, the mean estimated relative fecundity for fish collected after mid-September was 13% lower than the mean estimated relative fecundity for females collected before mid-September.
Our data suggest that collecting samples for fecundity estimates after the end of fecundity downregulation can benefit fisheries managers. These calculated estimates of realized relative fecundity can then be used in conjunction with other population metrics, such as mean weight, to obtain an improved understanding of the reproductive potential of the population. During this study period, the use of ovarian follicles collected across the entire study (mid-August–early October) misestimated the egg production of an average age-6+ Lake Trout female by 9% (Table 2). In the context of invasive Lake Trout management in Yellowstone Lake, this misestimation could affect output from the SCAA model used to guide the multi-million-dollar management program, budgeting, technician allocation, and the type and scale of removal efforts. Estimates that reflect the realized relative fecundity of a population will also better inform fisheries managers about demographic shifts in the reproductive structure of this population. We compared fecundity estimates from our study with those from Heredia et al. (2021) and found that sampling time affected both the statistical and biological significance of the difference in estimated relative fecundity between the studies (Table 3). When we compared the realized relative fecundity estimates between the two studies, it was evident that Lake Trout reproductive potential in Yellowstone Lake was 16% lower in 2022 than in 2015. A difference of this magnitude in estimated realized relative fecundity may be an indicator of demographic shifts in the reproductive structure of the Lake Trout population, which could impact the management approach used for this population. Because each population of fish is unique, fisheries managers should consider their individual management needs when assessing the relevance of adjusting the timing of ovarian follicle collection in their study population.
| Collection period | Estimated number of eggs produced by an age-6+ individual | Estimated number of eggs produced by all age-6+ females |
|---|---|---|
| Mid-Aug–early Oct | 5113 | 14,219,253 z |
| Mid-Sep–early Oct | 4672 | 12,992,832 y |
| Study year | N | Collection period | Mean relative fecundity (follicles/kg) |
|---|---|---|---|
| 2015 | 40 | Mid-Sep–early Oct | 1535 y |
| 2021–2022 | 118 | Mid-Aug–early Oct | 1485 y |
| 2021–2022 | 40 | Mid-Sep–early Oct | 1357 z |
Although our data suggest that relative fecundity estimates declined between mid-August and early October due to fecundity downregulation, our analyses were limited by not collecting paired samples for both fecundity and histological analyses of each female. Doing so would have enabled us to correlate relative fecundity estimates with a specific phase and stage of maturity, similar to the work of Kennedy et al. (2009). Future sampling in Yellowstone Lake should be designed such that the additional histological analyses can be paired with fecundity estimates to describe the precise stage of vitellogenesis at which fecundity downregulation ends. Similarly, future sample collection could include obtaining ovarian follicle diameters across the spawning season as another indicator of actual spawning time. This is possible because maximum ovarian follicle diameter is typically achieved within 2–3 days of reaching final maturity (Thorsen et al. 2006). Finally, future fecundity sampling in Yellowstone Lake could be designed to account for the impact of potential interannual variation resulting from changes in abiotic factors, such as water quality metrics and food availability.
Across species, effective fisheries management relies on accurate estimates of key life history metrics. We have shown that the timing of collection of fecundity samples in a freshwater fish species can alter estimates of reproductive potential, and we encourage fisheries managers to take this into account when assessing relative fecundity in their study populations. We believe that this study provides evidence that collecting ovarian follicle samples too early can result in misestimation of relative fecundity and, consequently, may be masking changes in the Lake Trout population in Yellowstone Lake. As a result of these findings, fisheries managers in Yellowstone National Park intend to conduct future sample collection after mid-September for Lake Trout fecundity monitoring in Yellowstone Lake. This will enable fisheries managers in Yellowstone National Park to use our findings to revise the current SCAA model and, as needed, focus even greater efforts on the gill-net suppression of adults and the removal of embryos from spawning sites.
ACKNOWLEDGMENTS
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service or the NPS. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This research was funded by Yellowstone Forever (Grant Number G-022) and NPS Yellowstone National Park. We thank Nicholas Heredia for providing access to data from Lake Trout collected for fecundity estimates in 2015. We are grateful to Jeffrey Powell (Bozeman Fish Technology Center) for valuable support and assistance. Special thanks are extended to Pat Bigelow, Drew MacDonald, Cody Vender, and Hickey Brothers Research, LLC, for assistance with Lake Trout collection. Finally, we greatly appreciate the feedback received from the reviewers.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
ETHICS STATEMENT
Sampling methodology was not subject to external ethics review.
Open Research
DATA AVAILABILITY STATEMENT
The authors will make data available.




