Coupling Gear Decontamination Trials and Angler Surveys to Minimize Spread of Invasive New Zealand Mud Snails Potamopyrgus antipodarum
Abstract
The New Zealand mud snail Potamopyrgus antipodarum (NZMS) is a global invader that is readily spread through attachment to recreational fishing gear. Preventing the spread of NZMSs by decontaminating fishing gear such as waders is a key step toward limiting invasive NZMSs and their ecological impacts; however, the effectiveness of decontamination protocols depends on both the efficacy of the protocol and the willingness of anglers to implement it. We tested the efficacy of three decontaminants (Virkon Aquatic, Formula 409, and bleach) at killing NZMSs on waders using two application techniques (spray versus soak) and two exposure durations (10 versus 20 min). We coupled the results of these tests with responses to a self-administered online survey that gauged the willingness of anglers to implement several decontamination strategies. Mortality of NZMSs differed widely among decontaminants, with the greatest mortality caused by Formula 409 (mean ± SE = 100 ± 0%), regardless of application type or duration. Bleach produced a mean mortality of 68.75 ± 11.97%, and Virkon Aquatic resulted in a mean mortality of 56.25 ± 11.97%. Neither exposure duration nor application method significantly influenced the degree of NZMS mortality, and their interaction was not significant. Anglers who responded to the survey (n = 339) revealed that Formula 409 was the decontaminant they would be most willing to use. Further, spraying was highly preferred over soaking for all decontaminants. Based on our experimental trials and the angler survey, we developed an angler decontamination metric (ADM) that helps to determine the decontamination strategy that optimizes NZMS mortality on fishing gear. Our ADM indicates that spraying gear with Formula 409 is the most effective NZMS decontamination strategy that anglers are willing to use. Our study is the first to combine the efficacy of NZMS decontaminants and angler willingness to adopt a decontamination strategy. By doing so, we hope to encourage the widespread use of NZMS decontamination of fishing gear to limit the spread and impacts of this increasingly relevant invasive species.
Introductions of invasive species into new habitats, particularly freshwaters, have accelerated during the past century due to human activities, such as commerce, transport, and recreation (Mack et al. 2000; Sala et al. 2000; Kolar and Lodge 2001; Ricciardi 2006). In turn, research has documented widespread impacts on the biodiversity, ecosystem processes, and ecosystem goods and services of invaded systems (Wilcove et al. 1998; Cox and Rutherford 2000; Kolar and Lodge 2001; Lodge and Shrader-Frechette 2003). Once established, invasive species can be extremely difficult to remove from an area, and resource managers are now increasingly emphasizing the prevention of new introductions as a key facet of effective management (Leung et al. 2002).
New Zealand mud snails Potamopyrgus antipodarum (NZMSs) are prolific invaders and have become established across the globe. New Zealand mud snails are native to the streams and lakes of New Zealand but are now found in 40 countries across six continents (Taybi et al. 2021; Geist et al. 2022). These snails are successful invaders due, in part, to their high reproductive capacity and competitive aptitude while largely being released from predation in their nonnative range. The reproductive strategy of invasive NZMS populations is solely parthenogenic, and invasive populations are predominantly made up of females—a trait that enables a single individual to establish a new population (Zaranko et al. 1997; Dybdahl and Kane 2005). In addition, the NZMS has a solid operculum, giving the snail the ability to enclose itself within its elongated shell to resist environmental stressors (e.g., desiccation) and to persist across a broad range of conditions (Geist et al. 2022). The advantageous life history traits of NZMSs, coupled with increasing globalization, facilitates further spread, posing threats to aquatic systems worldwide.
Impacts of invasive NZMSs on resident communities and ecosystem function are complex and vary widely. New Zealand mud snails can cause major shifts in algal assemblages (Krist and Charles 2012; Bennett et al. 2015) and native invertebrate communities (Kerans et al. 2005; Rakauskas et al. 2017) and can impact fish diets and physiological condition (Vinson and Baker 2008; J. A. Geist and S. D. Tiegs, unpublished data). In other instances, NZMSs have weak or negligible effects on invaded systems (Múrria et al. 2008; Brenneis et al. 2010), and in the case of an Australian stream, NZMSs had positive effects on native invertebrate densities (Schreiber et al. 2002). The negative effects of NZMSs on invaded systems stem in part from their extremely high densities, which in some instances can reach over 500,000 individuals/m2 (Dorgelo 1987; Hall et al. 2006). When elevated densities are achieved, NZMSs can consume as much as 75% of gross primary production and alter nitrogen and carbon cycling (Hall et al. 2003; Moore et al. 2012). Overall, the impacts associated with NZMSs can be severe and their ongoing spread poses threats to the structure and functioning of aquatic systems.
As NZMSs continue to expand their range, identifying pathways of spread and minimizing their effectiveness are key steps toward preventing further invasion into uninfected water bodies (U.S. Fish and Wildlife Service 2015). In the United States, NZMS populations usually occur at popular angling destinations, suggesting a relationship between recreational fishing and NZMS spread (Geist et al. 2022). Recreational angling often involves gear (e.g., waders, wading boots, and other equipment) to which NZMSs can attach, facilitating their spread within and among watersheds (Hosea and Finlayson 2005; Stockton 2011; State of Michigan 2018).
Preventing NZMS spread through individual efforts (e.g., gear decontamination by recreationists) can be effective at reducing introductions into uninvaded water bodies at local and regional scales (Proctor et al. 2007; State of Michigan 2018). Several chemical reagents have been found to be effective at decontaminating equipment (e.g., boats, fishing equipment) and infrastructure (e.g., fish hatcheries). These include copper sulfate, benzethonium chloride, Pine-Sol, Formula 409, Virkon Aquatic, Sparquat 256, Quat 4, Green Solutions High Dilutions Disinfectant 256, and Super HDQ (Hosea and Finlayson 2005; Schisler et al. 2008; Stockton and Moffitt 2013; Stout et al. 2016; Stockton-Fiti and Moffitt 2017; De Stasio et al. 2019). These reagents vary in their effectiveness, availability, and ease of use. Given this variation, it is important to understand the willingness of anglers to use a particular decontamination strategy. Additionally, various strategies for NZMS decontamination are currently recommended by different state and federal agencies, organizations, and recreational user groups, resulting in lack of clarity and consistency about best practices.
Coupling data on the effectiveness of different chemical reagents with data on anglers' willingness to use a treatment should lead to optimized decontamination and reduced NZMS spread into new areas. However, combining data on the willingness to implement a particular protocol with its effectiveness has only rarely been achieved. Angler willingness to adopt a spread prevention strategy—or, more broadly, the likelihood of the public to adopt such a strategy—is often an undervalued component of the overall effort to reduce invasive species spread and minimize new introductions. Here, we evaluate the efficacy of different chemical reagents on NZMS mortality along with the willingness of anglers to implement specific decontamination strategies. We experimentally tested three different chemical reagents for their ability to kill NZMSs: Virkon Aquatic, Formula 409 Multi-Purpose Cleaner (hereafter, “Formula 409”), and bleach. The selected chemicals are currently being advocated by various state and federal agencies as effective NZMS decontaminants for recreational fishing gear. We also developed and distributed an online survey that was completed by 339 members of the regional angling community to gauge their general understanding of the NZMS invasion and their willingness to use specific recommended decontamination strategies. By coupling the results from the experimental trials and the survey, we provide a recommendation for a practical and optimized strategy that resource managers and the public can use for decontaminating recreational angling gear to minimize new introductions of NZMSs.
METHODS
Test organisms
We collected approximately 500 live NZMSs by handpicking sediments from the East Branch Au Sable River (Michigan, USA). We placed NZMSs into a 23-L container filled with stream water and transported them to the laboratory, where we transferred them to a 38-L holding tank filled with ~20°C dechlorinated tap water. We also collected rocks, algae, and organic matter (e.g., leaves) from the Au Sable River and placed them in the holding tank to provide food and habitat. We selected 64 adult snails (mean length ± SD = 4.59 ± 0.05 mm) from the holding tank for experimental trials. Prior to the trials, we assessed snails for viability (via volitional movement) and determined shell length to the nearest millimeter.
Evaluation of three disinfectants for New Zealand mud snail decontamination on waders
We used a fully crossed 4 × 2 × 2 factorial design (3 chemical treatments and 1 control × 2 exposure durations × 2 application types) to test the effectiveness of chemical disinfectants at killing NZMSs and the different means of applying them. The three chemical reagents were Virkon Aquatic, Formula 409, and a bleach solution, with deionized water serving as a control (Figure 1). Chemical disinfectants were prepared according to manufacturer recommendations. We diluted 21 mL of Virkon Aquatic concentrate in 30 L of deionized water to reach a 2% concentration (equivalent to label recommendation). We then used Virkon dilution test strips (Syndel Company) to validate the premeasured and diluted solution to ensure the appropriate concentration. To obtain a 10% household bleach solution, we diluted 5.25% sodium hypochlorite (Clorox Bleach) in 30 L of deionized water (equivalent to the recommended concentration for household bleach; CDC 2016). Formula 409 was not altered from its manufactured concentration.
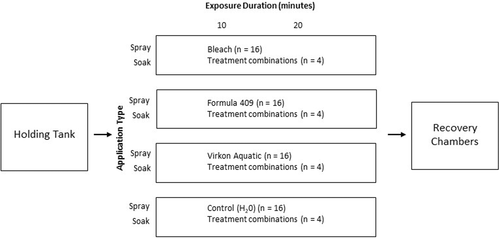
To evaluate the effectiveness of NZMS decontaminants on the surfaces of angling equipment believed to be important for transporting NZMSs, we applied chemical treatments to snails attached to fabric that is commonly used in waders. Nylon/polyester wader fabric from Cabela's Breathable Waders was cut to the size of the experimental chambers (60- × 15-mm polystyrene Petri dishes). We sprayed the cut wader material with deionized water immediately before the start of the experiment to simulate the wet conditions that NZMSs experience when exiting a water body while attached to angling gear.
We tested two exposure durations (10 and 20 min) and two application types: (1) spraying the chemical onto NZMSs and (2) fully submerging NZMSs in the chemical (hereafter, “soaking”). We designated 16 individual NZMSs for each chemical treatment, with four individual NZMSs for each experimental treatment combination (1 of the 3 chemicals with either spray or soak application for either the 10- or 20-min exposure duration). We assigned NZMSs to a designated treatment and then placed the snails directly onto the wader material in each experimental chamber for treatment exposure.
For the spray application, we transferred each chemical solution and water as a control to identical spray bottles from the same manufacturer (Lowe's 946-mL [32-oz] All Purpose Sprayer) to standardize spray volume and velocity when applying chemicals to NZMSs on wader surfaces. We primed each standardized spray bottle by discharging two test sprays away from NZMSs to ensure a full spray (~1 mL) at the time of exposure. With the spray bottle head positioned approximately 10 cm away, we sprayed NZMSs with the chemical and control to ensure full coverage and exposure. For the soak application, we poured enough chemical solution into the experimental chamber with the wader material to fully submerge the NZMSs (~5 mL), and then we placed the snails into the chamber. We interspersed and randomly arranged the experimental chambers and treatment combinations in the testing area. After the designated duration of the chemical exposure (and control) had ended, snails were removed from the experimental chambers, rinsed with clean tap water for 5 s, and placed into a recovery chamber (100- × 15-mm polystyrene Petri dish) with tap water (~5 mL). To assess mortality, we marked the initial placement of NZMSs in the recovery chambers and observed snail movement immediately and at 1, 6, 24, 48, 72, and 96 h posttreatment.
Angler survey
We developed and distributed an online survey to anglers. The survey consisted of 36 questions regarding angler behaviors, invasive species, and NZMS decontamination strategies. We solicited participation in the survey through e-mail to a targeted sample demographic consisting of members of two non-governmental freshwater conservation and recreational fishing organizations: Trout Unlimited and Fly Fishers International. This sample population is a likely transport vector for NZMSs since the spread of this species is speculated to be associated with recreational angling (Bruce and Moffitt 2010; Alonso and Castro-Díez 2012; Stockton and Moffitt 2013). We administered the survey via Google Forms and collected survey data from November 2018 through March 2019.
The survey consisted of three discrete sections (Appendix) and included questions about (1) the respondent's angling behaviors; (2) the respondent's general awareness of regional invasive species—specifically, the NZMS; and (3) the respondent's willingness to implement a specific NZMS decontamination strategy after fishing. Decontamination strategies (i.e., chemical, application technique, and duration) presented in the survey were the same as those used in the experimental trials of this study, previously published studies, and available state and federal management documents (Hosea and Finlayson 2005; Proctor et al. 2007; Stockton and Moffitt 2013; De Stasio et al. 2019). In brief, we asked participants how willing they would be to apply a specific chemical decontaminant (i.e., Virkon Aquatic, bleach, or Formula 409) to their wading gear to help prevent the spread of NZMSs. Each question included a decontamination strategy with a combination of one of the three chemicals, one of the two application methods (i.e., spraying the chemical on wading gear or soaking the wading gear in the chemical), and one of the two exposure durations (10 or 20 min).
Statistical analysis
For the online survey, questions regarding a respondent's willingness to implement various decontamination strategies were developed using a 7-point Likert-type scale (1 = not likely; 7 = very likely) to provide categorical ordinal data. Measures of central tendency (i.e., mean, median, and mode) were calculated for each question. We used the median answer to characterize the sample population's degree of willingness to use a particular decontamination strategy. Additional questions regarding angler behaviors and invasive species and NZMS awareness included various response options that were specific to the question. We summarized these answers by calculating the percentage of each response option selected.
Angler decontamination metric
RESULTS
Evaluation of Three Disinfectants for New Zealand Mud Snail Decontamination on Waders
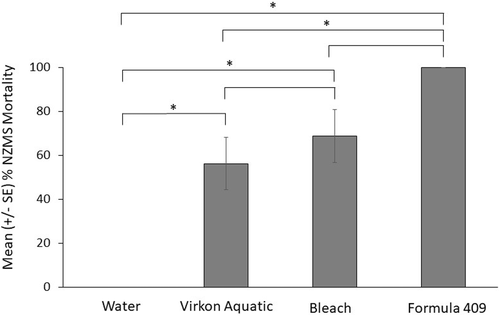
New Zealand mud snail mortality differed widely among decontaminants (ANOVA: P < 0.001; Figure 2; Table 1). The greatest mean NZMS mortality at 1 h after exposure to the decontaminants across all applications and durations was caused by Formula 409, with lower mortality from bleach and even lower mortality from Virkon Aquatic. Neither exposure duration (10 versus 20 min; P = 0.48) nor application method (spraying versus soaking; P = 0.16) affected NZMS mortality (Figures S.1 and S.2 available in the Supplement in the online version of this article); there were no significant interactions in the fully crossed ANOVA model (Table 2).
| Chemical | Application type | Exposure duration (min) | Percent mortality after 1 h | Mean time (h) to mortality |
|---|---|---|---|---|
| Water (control) | Spray | 10 | 0 (0) | >96 (0) |
| Soak | 10 | 0 (0) | >96 (0) | |
| Spray | 20 | 0 (0) | >96 (0) | |
| Soak | 20 | 0 (0) | >96 (0) | |
| Bleach | Spray | 10 | 50 (57) | >96 (0) |
| Soak | 10 | 100 (0) | 60 (45.96) | |
| Spray | 20 | 75 (50) | 66 (45.43) | |
| Soak | 20 | 50 (57) | 60 (45.96) | |
| Virkon Aquatic | Spray | 10 | 50 (57) | 25.5 (47.09) |
| Soak | 10 | 25 (50) | 48 (43.82) | |
| Spray | 20 | 75 (50) | 2 (2.71) | |
| Soak | 20 | 75 (50) | 0.25 (0.5) | |
| Formula 409 | Spray | 10 | 100 (0) | 0 (0) |
| Soak | 10 | 100 (0) | 6 (12) | |
| Spray | 20 | 100 (0) | 0 (0) | |
| Soak | 20 | 100 (0) | 0 (0) |
| Main effect or interaction | df | F-value | P-value |
|---|---|---|---|
| Chemical | 3 | 22.333 | <0.001 |
| Duration | 1 | 0.500 | 0.483 |
| Application | 1 | 2.000 | 0.164 |
| Chemical × duration | 3 | 0.167 | 0.918 |
| Chemical × application | 3 | 1.000 | 0.401 |
| Duration × application | 1 | 0.500 | 0.483 |
| Chemical × duration × application | 3 | 1.500 | 0.226 |
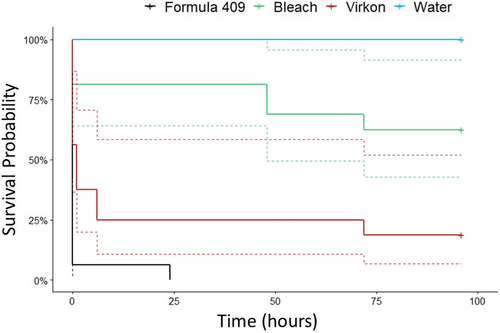
Kaplan–Meier analysis showed a 6.3% survival probability after 1 h for NZMSs treated with Formula 409 across all applications and exposure durations (Figure 3). After 24 h, survival probability was 0% (Figure 3). New Zealand mud snails treated with bleach had an 81.2% probability of survival after 1 h and a 62.5% survival probability after the final 96-h viability assessment (Figure 3). Snails treated with Virkon Aquatic had a 37.5% survival probability after 1 h and an 18.8% survival probability after 96 h (Figure 3). New Zealand mud snails in the control treatment (i.e., water) had a 100% survival probability throughout the entire 96-h duration (Figure 3). Significant differences in survivorship curves were observed between bleach and Formula 409 (P < 0.001), bleach and Virkon Aquatic (P = 0.034), bleach and water (P = 0.044), Virkon Aquatic and water (P < 0.001), and Formula 409 and water (P < 0.001; Table 3).
| Chemical | Formula 409 | Bleach | Virkon Aquatic |
|---|---|---|---|
| Bleach | <0.001 z | – | – |
| Virkon Aquatic | 0.142 | 0.034 z | – |
| Water | <0.001 z | 0.044 z | <0.001 z |
Angler Survey
In total, 339 individuals responded to the online questionnaire, with most respondents answering most questions (Table S.1 available in the Supplement in the online version of this article). Most of the sample population preferred to fish in rivers versus lakes (91% and 9%, respectively), fished multiple rivers in a year (median = 4.5 rivers), fished mostly during summer months (June–August; 58%), and preferred wading versus accessing rivers in a boat (81% and 19%, respectively; Table S.1). Wading gear and material preferences of the sample population were for rubber-soled wading boots versus felt (67% and 29%, respectively) and for Gore-Tex or other breathable material versus neoprene/nylon/rubber (82% and 18%, respectively; Table S.1). Awareness and knowledge of invasive species, specifically the NZMS, varied: 62% of the respondents claimed knowledge of NZMS-invaded rivers in the Great Lakes region, and 43% were able to identify NZMSs (Table S.1). When asked to gauge their own knowledge of NZMSs in the Great Lakes region on a scale of 1–7 (1 = no knowledge; 7 = very knowledgeable), the responses fell within the intermediate range (median = 4; Table S.1).
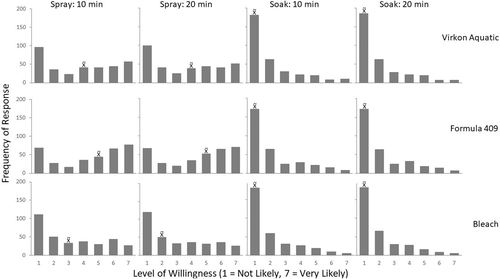
The decontamination strategy that anglers were most likely to use (based on a 7-point Likert-type scale: 1 = not likely; 7 = very likely) was Formula 409 by spray, regardless of the exposure duration (median = 5, mode = 7; Table S.2; Figure 4). Participants were less likely to use Virkon Aquatic by spray application with either exposure duration (median = 4, mode = 1), and they were even less likely to use bleach as a decontaminant by spray application with either exposure duration (10 min: median = 3, mode = 1; 20 min: median = 2, mode = 1; Table S.2; Figure 4). The willingness of the sample population to use any of the chemicals in a soak application, regardless of exposure duration, was low (all combinations: median = 1, mode = 1; Table S.2; Figure 4). When survey participants were asked if they knew where to purchase the chemicals, 99% responded yes for bleach, 88% responded yes for Formula 409, and 12% responded yes for Virkon Aquatic.
Angler Decontamination Metric
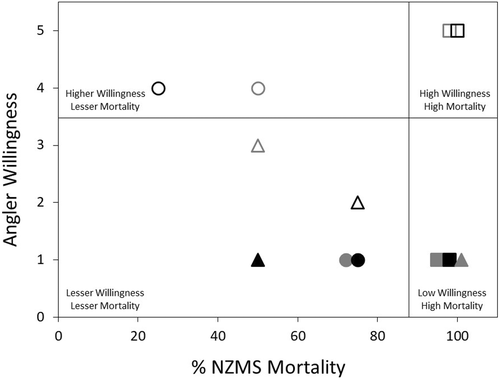
Values of the ADM (higher values indicate greater angler willingness and greater chemical effectiveness) for specific treatment combinations/decontamination strategies ranged from 0.5 (bleach, soak, 20 min) to 5.0 (Formula 409, spray, 20 min; Table 4). The mean ADM value ± SD per chemical reagent was 3.00 ± 2.31 for Formula 409, 1.13 ± 0.60 for Virkon Aquatic, and 1.13 ± 0.49 for bleach across all application types and exposure durations. The mean ADM was 2.80 ± 1.85 for spray application and 0.83 ± 0.20 for soak application across all chemical reagents and exposure durations. For chemical exposure durations, the mean ADM value was 1.85 ± 1.59 for a 10-min exposure and 1.63 ± 1.69 for a 20-min exposure across all chemical reagents and application types (Table 4).
| Decontamination strategy | Mean NZMS mortality (%M [decimal]) | Survey median response (SMR) | ADM (=%M × SMR) |
|---|---|---|---|
| Formula 409, spray, 10 min | 1.00 | 5 | 5.00 |
| Formula 409, spray, 20 min | 1.00 | 5 | 5.00 |
| Virkon Aquatic, spray, 10 min | 0.50 | 4 | 2.00 |
| Bleach, spray, 10 min | 0.50 | 3 | 1.50 |
| Bleach, spray, 20 min | 0.75 | 2 | 1.50 |
| Formula 409, soak, 10 min | 1.00 | 1 | 1.00 |
| Formula 409, soak, 20 min | 1.00 | 1 | 1.00 |
| Bleach, soak, 10 min | 1.00 | 1 | 1.00 |
| Virkon Aquatic, spray, 20 min | 0.25 | 4 | 1.00 |
| Virkon Aquatic, soak, 10 min | 0.75 | 1 | 0.75 |
| Virkon Aquatic, soak, 20 min | 0.75 | 1 | 0.75 |
| Bleach, soak, 20 min | 0.50 | 1 | 0.50 |
| Mean ADM values | |||
| Formula 409 | 3.00 (2.31) | ||
| Bleach | 1.125 (0.49) | ||
| Virkon Aquatic | 1.125 (0.60) | ||
| Spray application | 2.80 (1.85) | ||
| Soak application | 0.83 (0.20) | ||
| 10-min duration | 1.85 (1.59) | ||
| 20-min duration | 1.625 (1.69) | ||
DISCUSSION
- Visually inspect wading and fishing gear and remove NZMSs.
- Brush (using a stiff-bristled brush) and/or wipe off organisms, debris, and organic material from the wading and fishing gear.
- With the angler positioned out of and away from surface waters, spray the wading and fishing gear liberally with Formula 409 (as prepared by the manufacturer), completely covering all material that was in contact with the water body.
- Let Formula 409 remain on the fishing and wading gear for at least 10 min.
- With the angler positioned out of and away from surface waters, rinse the fishing and wading gear with clean water to remove residual Formula 409.
Our results indicate that Formula 409 is the most effective chemical reagent for killing NZMSs using either spray or soak application, with either a 10- or 20-min exposure duration. Formula 409 contains quaternary ammonium compounds, which are toxic to many invertebrates and are commonly found in molluscicides (Vallejo-Freire et al. 1954). Regardless of whether Formula 409 was sprayed or applied via soaking and regardless of whether it was left on angling gear for 10 or 20 min, this reagent killed 100% of NZMSs.
Our findings on the efficacy of Formula 409 and NZMS mortality are consistent with results reported by Schisler et al. (2008), who also observed 100% mortality of NZMSs that were exposed to undiluted Formula 409 for 10 min. Additionally, De Stasio et al. (2019) found Formula 409 to be highly effective, although less so when mud was present. This suggests that NZMSs need to be in direct contact with the chemical reagent for it to have maximum effectiveness. Furthermore, NZMSs can become attached to gear in crevices, under fabric flaps, and/or stuck in Velcro, which may provide conditions inhibiting full exposure to Formula 409. As such, care should be taken to remove mud and organic material, fully examine wading gear for the presence of NZMSs, and treat all suspected areas with the chemical decontaminant. Directly after exiting an infested water body, waders and boots are also likely still damp and exhibit moist conditions, which may dilute Formula 409 and reduce its efficacy. Although we did not test a diluted solution of Formula 409 or an exposure duration <10 min for this chemical, Hosea and Finlayson (2005) found Formula 409 at full strength and 50% dilution to be effective at killing most or all NZMSs with a 5-min exposure duration. This contradicts the study by Schisler et al. (2008), who observed 50% survival of NZMSs that were exposed to 50% diluted Formula 409 for 5 min. As such, we recommend using full-strength Formula 409 for an exposure duration of no <10 min to achieve maximum NZMS mortality.
Virkon Aquatic is commonly used in fish hatcheries and other aquaculture facilities and is currently recommended by many agencies for application to NZMSs (Hosea and Finlayson 2005; Stockton and Moffitt 2013). We found that Virkon Aquatic killed fewer NZMSs in all applications and durations than Formula 409 and usually underperformed relative to bleach (Figure 2; Table 1) in 1-h trials. However, survival analysis indicated that Virkon Aquatic produced the second-lowest survival probability (with Formula 409 generating the lowest) after the full 96-h assessment duration (Figure 3). Furthermore, other studies have shown Virkon Aquatic to be effective at killing NZMSs. Stockton and Moffitt (2013) found that fully submerging wading gear in Virkon Aquatic for 30 min was 100% effective at killing NZMSs at the same concentration as our treatments (i.e., 20 g/L) and that Virkon Aquatic was 99% effective when sprayed and left on wading gear for 30 min. If used for NZMS decontamination, concentrations of Virkon Aquatic should be at least 20 g/L and this chemical should be applied via soaking (i.e., full submersion) for at least 30 min.
Bleach is commonly used for microbial decontamination and has also become an option for anglers as a NZMS decontaminant (Michigan Department of Natural Resources, personal communication). However, there have been limited studies evaluating the efficacy of bleach for NZMS mortality. Our study indicates that bleach can be effective if NZMSs are soaked in a 10% concentration solution, but a spray application of bleach did not result in 100% NZMS mortality (Table 1). Some studies have found that soaking gear in a 5% bleach solution was effective at killing NZMSs (Medhurst and Herbst 2003, cited by Hosea and Finlayson 2005), but another study reported that this procedure was ineffective (Hosea and Finlayson 2005). However, NZMS exposure to higher concentrations of bleach (17% and undiluted) was more effective (Hosea and Finlayson 2005). Overall, the concentrations of bleach that are effective at achieving high levels of NZMS mortality on fishing gear remain unclear.
Responses from the survey of angler behavior and NZMS awareness support the speculation that recreational anglers like those in our survey are probably unintentional vectors of NZMS spread (Table S.1). Ninety-one percent of the respondents indicated that they fish mostly in rivers—aquatic systems where NZMSs have been prominently documented throughout their invasive range (Geist et al. 2022). Furthermore, 99% of the respondents indicated that they fish more than one river in a typical year, suggesting the potential for NZMS spread among watersheds via attachment and transport on fishing gear. Most survey respondents (81%) fished while wading, which entails direct contact with benthic habitats, including NZMSs in invaded systems. Additionally, most respondents preferred to fish during summer (i.e., June–August), when NZMS densities are typically greatest (Vinson 2004; Kerans et al. 2005; Hall et al. 2006; Bennett et al. 2015), increasing the likelihood of anglers contacting NZMSs. Although anglers like those in our sample population have the potential to spread NZMSs, they also have some level of awareness of the invasive NZMS issue (Table S.1). Widespread use of an effective and convenient decontamination strategy will help to limit the spread of NZMSs through this vector.
Responses from survey questions regarding angler willingness to implement various decontamination strategies strongly indicated that Formula 409 is the preferred chemical, with a spray application preferred over soaking (Table S.2; Figure 4). The preference for Formula 409 may be a result of the familiarity and accessibility of the product (i.e., it is available at most convenience stores and markets and requires no preparation prior to use), whereas Virkon Aquatic requires purchase from an online manufacturer and a multi-step process for effective use (i.e., mixing a specific amount of powder with water to achieve the desired concentration). Bleach, while it is a widely available chemical reagent, was not preferred for use by the sample population, perhaps because of its widespread reputation for damaging fabrics, such as those used in waders.
Several studies have shown that chemical decontaminants can damage waders. Bleach can affect wader fabric and boots, resulting in leaks, cracking, and tears (Hosea and Finlayson 2005). Repeated use of Virkon Aquatic can cause leaking along the seams, legs, crotch, and knees of waders (Stockton and Moffitt 2013), and soaking of the wading gear in 50% diluted Formula 409 can cause surficial cracking in the rubber toes of wading boots (Hosea and Finlayson 2005). Damage probably increases with continuing use, but rinsing gear with clean water after the required exposure duration can reduce potential damage (Stockton and Moffitt 2013). Further studies evaluating the long-term effects of chemical reagents used for decontamination of fishing gear are needed.
Preventing the spread of NZMSs and other aquatic invasive species through attachment and transport on water-related recreational gear will help to limit new introductions into uninvaded aquatic systems. While the goal of our study was specific to the invasive NZMS, this approach (i.e., coupling experimental findings with gauged public willingness) could be used to develop spread prevention techniques and decontamination protocols for other invasive species. As such, further research is needed to investigate the efficacy of Formula 409 as a decontaminant for other aquatic invasive species.
We recognize the limitations and drawbacks in our approach combining the experimental results with the results of our angler survey data (i.e., the ADM). Because we used ordinal data as a multiplier for the index, the magnitude of differences among ADM values cannot be assessed. We believe that this technique—combining survey data on the willingness to use a particular decontamination protocol with data on that protocol's effectiveness for causing mortality of an invasive species—can be built upon and improved in future research to maximize the mortality of invasive organisms. Furthermore, as a companion to our ADM, we graphically displayed the coupling of our experimental results with our survey (Figure 5); we hope that together these can provide insight for researchers, resource managers, and the public on the most effective NZMS decontamination strategy that anglers are most willing to use. In all, future research should be directed toward the development of robust and wide-ranging spread prevention strategies that consider public willingness and that adapt to a broad range of recreational styles (fly-fishing, spin fishing, boating, etc.) and invasive species. In this article, we have presented a general approach to quantifying these very different types of data.
Our survey was limited to participants that were willing to respond; as such, the survey respondents may be more likely to implement a decontamination strategy relative to individuals who did not respond. The inherent bias associated with the targeted sample population should be considered when interpreting our results. Nevertheless, recommendations of an effective and convenient decontamination strategy for anglers and other water-related recreationists should increase adoption of the strategy.
As invasive species continue to spread and impact aquatic ecosystems, tools are needed that are not only effective in preventing spread, but also readily adopted and implemented by groups responsible for the transport of the invader. Here, we combine these very different types of data and approaches to develop a protocol that optimizes mortality of the NZMS, a global invader that is continuing to expand its range. Through this effort, we hope to assist in more effective management and conservation of freshwaters where these invaders are increasingly found.
ACKNOWLEDGMENTS
This research was supported by a U.S. Environmental Protection Agency Great Lakes Restoration Initiative award to J.A.G. and an award to S.D.T. through the State of Michigan Invasive Species Grant Program. We thank Keith Berven, Mark Luttenton, and David Strayer for their careful review of the manuscript; Jasmine Mancuso for reviewing and editing; and George Sanders (Department of Sociology and Anthropology, Oakland University) for consultation during survey development. The angler survey portion of the study was conducted using protocols approved by Oakland University's Institutional Review Board. We thank three anonymous peer reviewers and the associate editor for their review of the manuscript. We thank Bryan Burroughs (Michigan Trout Unlimited) for initial discussions of project development and Kennedy Bommarito and Emily Bovee for their assistance during the experimental study. Lastly, we thank Cathy Starnes and Jan Bills (Oakland University) and Danielle Typinski, Kenisha Houston, and Nichol DeMol (Trout Unlimited) for administrative support. There is no conflict of interest declared in this article.
Appendix
Angler Survey for New Zealand Mud Snail Decontamination
- What TU Chapter are you affiliated with?
- What river/stream do you consider ‘home waters’?
- What river/stream do you fish the most?
-
When do you fish most?
- Spring (March–May)
- Summer (June–August)
- Fall (September–November)
- Winter (December–February)
-
What type of freshwater body do you fish in most?
- Rivers
- Lakes
-
Please give your best estimate of the number of times you fish during the spring season (March–May)?
- 0–5 times
- 6–10 times
- 11–15 times
- 16–20 times
- Over 21 times
-
Please give your best estimate of the number of times you fish during the summer season (June–August)?
- 0–5 times
- 6–10 times
- 11–15 times
- 16–20 times
- Over 21 times
-
Please give your best estimate of the number of times you fish during the fall season (September–November)?
- 0–5 times
- 6–10 times
- 11–15 times
- 16–20 times
- Over 21 times
-
Please give your best estimate of the number of times you fish during the winter season (December–February)?
- 0–5 times
- 6–10 times
- 11–15 times
- 16–20 times
- Over 21 times
-
Do you travel to other states/countries to fish?
- Yes
- No
-
If answered yes to the previous question, where do you commonly travel to fish? (check all that apply).
- Western United States
- Around the Great Lakes region
- Eastern United States
- Southern United States
- Other countries
-
Which type of wader boot surface do you primarily use?
- Felt
- Rubber
-
What type of material are your primary waders made from?
- Breathable nylon
- Neoprene
- PVC/Rubber
-
Do you practice any type of wader/gear decontamination after a fishing trip?
- Yes
- No
- If so, describe.
- On a scale of 0–7 (0 = no knowledge, 7 = very knowledgeable), how well would you rate your knowledge on invasive species issues in the Great Lakes Region?
-
How familiar are you on the topic of the New Zealand mud snail?
- Not familiar
- Somewhat familiar
- Very familiar
-
Do you know which rivers currently have existing populations of New Zealand mud snail in Michigan?
- Yes
- No
-
Do you know how to identify a New Zealand mud snail?
- Yes
- No
-
Do you know who to contact if you find a New Zealand mud snail?
- Yes
- No
- To help prevent spread of NZMS, how likely are you to spray your wading gear with Virkon Aquatic decontamination solution (concentrated disinfectant powder available via online purchase) and let sit for 10 min between water bodies?
- Not likely 0–7 Very likely
- To help prevent spread of NZMS, how likely are you to spray your wading gear with Virkon Aquatic decontamination solution and let sit for 20 min between water bodies?
- Not likely 0–7 Very likely
- To help prevent spread of NZMS, how likely are you to spray your wading gear with Formula 409 (home and industrial cleaning product available at most convenience stores) and let sit for 10 min between water bodies?
- Not likely 0–7 Very likely
- To help prevent spread of NZMS, how likely are you to spray your wading gear with Formula 409 and let sit for 20 min between water bodies?
- Not likely 0–7 Very likely
- To help prevent spread of NZMS, how likely are you to spray your wading gear with a bleach solution and let sit for 10 min between water bodies?
- Not likely 0–7 Very likely
- To help prevent spread of NZMS, how likely are you to spray your wading gear with a bleach solution and let sit for 20 min between water bodies?
- Not likely 0–7 Very likely
- To help prevent spread of NZMS, how likely are you to soak your wading gear in a tub of Virkon Aquatic decontamination solution for 10 min between water bodies?
- Not likely 0–7 Very likely
- To help prevent spread of NZMS, how likely are you to soak your wading gear in a tub of Virkon Aquatic decontamination solution for 20 min between water bodies?
- Not likely 0–7 Very likely
- To help prevent spread of NZMS, how likely are you to soak your wading gear in a tub with Formula 409 for 10 min between water bodies?
- Not likely 0–7 Very likely
- To help prevent spread of NZMS, how likely are you to soak your wading gear in a tub with Formula 409 for 20 min between water bodies?
- Not likely 0–7 Very likely
- To help prevent spread of NZMS, how likely are you to soak your wading gear in a tub with a bleach solution for 10 min between water bodies?
- Not likely 0–7 Very likely
- To help prevent spread of NZMS, how likely are you to soak your wading gear in a tub with a bleach solution for 20 min between water bodies?
- Not likely 0–7 Very likely
-
Do you know where to purchase Bleach?
- Yes
- No
-
Do you know where to purchase Formula 409?
- Yes
- No
-
Do you know where to purchase Virkon Aquatic?
- Yes
- No
-
Would you like to become more involved in a current NZMS project?
- Yes
- No




