Function of the Normal Neuromuscular Junction in Young Children
A portion of this study material was presented at the scientific meeting of the Hungarian Society for Clinical Neurophysiology, February 2023, Debrecen, Hungary.
ABSTRACT
Introduction/Aims
The repetitive nerve stimulation (RNS) test is fundamental to test the functional integrity of the neuromuscular junction. RNS data are scarce for newborns and young children, limiting early diagnostic efforts to identify children with neuromuscular diseases. Here, we performed RNS in young children.
Methods
RNS of the axillary nerve was performed in 34 otherwise healthy young children (0 to 48 months of age) undergoing procedural sedation or analgesia. Compound muscle action potentials (CMAP) were recorded over the deltoid muscle. CMAP amplitude and area decrement between the 1st and 4th potential were determined at 3 Hz stimulation. Post-tetanic facilitation, twitch-to-tetanus ratio, and heart rates were recorded.
Results
The largest CMAP amplitude decrement value was 7% at rest, having a median of 1%, and an interquartile range of 1%–3%. There was no correlation between the CMAP amplitude decrement and age. In contrast, a moderate positive correlation was found between the CMAP area decrement and age, occasionally exceeding 10% in infants younger than 6 months. Both post-tetanic facilitation and twitch-to-tetanus ratio exhibited a moderate positive correlation with age.
Discussion
While the CMAP amplitude decrement in young children was within the adult normal range, the area decrement may be higher. Studies of RNS on other nerves in unsedated children are needed to investigate the findings from this study further.
Abbreviations
-
- AChR
-
- acetylcholine Receptor
-
- CMAP
-
- compound muscle action potential
-
- CMS
-
- congenital myasthenic syndrome
-
- EMG
-
- electromyography
-
- HRF
-
- high-rate frequency
-
- MUSK
-
- muscle-specific tyrosine kinase
-
- NMJ
-
- neuromuscular junction
-
- RNS
-
- repetitive nerve stimulation
1 Introduction
In children with suspected congenital myasthenic syndromes (CMS), clinical neurophysiological tests can confirm the functional defect at the neuromuscular junction (NMJ). As a first step, a repetitive nerve stimulation (RNS) test is recommended, which is valuable in distinguishing between pre- and postsynaptic origins of NMJ dysfunction and guiding subsequent genetic analysis in suspected CMS patients [1, 2].
A major challenge in applying these techniques in children is that the structure of the NMJ continues to develop during newborn and childhood. Morphometric analysis of human NMJ showed that the postsynaptic membrane length, postsynaptic area, and membrane length ratio (ratio of postsynaptic to presynaptic membrane length) are decreased in 2-month to 3-year-old children compared to adults [3]. In addition, the fetal form of the acetylcholine receptor, containing a γ subunit (composition α2βγδ) is gradually replaced by an ε subunit-containing adult form (α2βεδ) postnatally. It is unknown to what extent these structural changes are reflected in the functional characteristics of the junction. It was shown that the train-of-four value, post-tetanic facilitation, and twitch-to-tetanus ratio change significantly within the first months and years of life [4]. In addition, pharmacological data revealed that the infant neuromuscular junction exhibits resistance to depolarizing blockers such as succinylcholine compared to non-depolarizing blockade with tubocurarine [5].
Age-specific normal values for RNS have not been agreed upon. Many neurophysiology textbooks and articles suggest using adult cut-off values (10% decrement) to indicate abnormality [2, 6-8]. There have been few RNS studies of healthy children [2, 9]. Some used techniques that are no longer performed in clinical practice.
The primary aims of the study were to collect CMAP amplitude and area decrement values from young children and to analyze their correlation with age. We also aimed to check if these values fall within the established normal cut-off range for adults. The secondary aims were to confirm correlations between age and post-tetanic facilitation and the twitch-to-tetanus ratio. Finally, we tested the safety and feasibility of the RNS test of the axillary nerve.
2 Methods
2.1 Participants
Young children with normal developmental milestones and with no known disorders of the central or peripheral nervous system, undergoing elective surgical procedures, participated in this study. Parents of all study participants provided informed consent. Study procedures were approved by the Ethics Committee at the University of Debrecen and the representative governmental body (OGYÉI/38595/2017). The study conforms with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All examinations were undertaken with each subject's parents' understanding and written consent.
2.2 RNS Test
Recordings were made before the surgical procedure. Anesthesia was initiated by a pediatric anesthesiologist with an intravenous injection of 3 mg/kg propofol, 0.1 mg/kg midazolam, and 1.9 μg/kg fentanyl to reach the necessary level of procedural sedation and analgesia required for the RNS test (after completing the RNS test anesthesia was deepened to the level necessary for the surgical procedure). Non-invasive blood pressure, ECG, and SpO2 were monitored continuously.
Compound muscle action potentials (CMAPs) were recorded using surface stick-on electrodes during repetitive 3 Hz supramaximal stimulation of the axillary nerve just above the clavicle. The stimulating electrode had an inter-electrode (cathode to anode) distance of 1.5 cm. The active electrode was placed over the belly of the deltoid muscle, the reference electrode on the shoulder tip, and the ground electrode over the lateral epicondyle (Supplementary Figure 1A). A sharp initial negative deflection confirmed the correct positioning over the motor point. The stimulus consisted of regulated current pulses with a duration of 0.2 ms. Supramaximal stimulus was determined by increasing the intensity of the stimulating pulse until the CMAP was of maximal size, then increasing the intensity further by 25% (usually around 20 mA). During the studies, the participants lay supine, the upper extremity was immobilized, and the skin temperature was maintained at or above 32°C. Tests were performed using a DANTEC Keypoint Portable EMG machine (Middleton, WI, USA). 3-Hz supramaximal stimulation was given to record CMAP at rest, immediately (within 2 s) following a high-rate stimulation (HRS, 30 Hz for 1 s) and then 20 s and 1 min after the tetanic stimulation. A train of ten stimuli was delivered to the axillary nerve and the resultant CMAPs were sequentially recorded (Supplementary Figure 1B,C). The amplitude and the area of the negative peak of the CMAP were measured, and the decrement between the 1st and 4th potential was determined, as was suggested in previous studies [10]. The percent change in the first post-tetanic CMAP after the HRS relative to the first pre-tetanic CMAP was determined. When the CMAP was elevated after tetanic stimuli, it was defined as post-tetanic facilitation. On the contrary, when the first post-tetanic CMAP was smaller, it was defined as post-tetanic exhaustion. The twitch-to-tetanus ratio (ratio of the height of HRS to the first pre-tetanic CMAP) was also calculated.
2.3 Statistical Analysis
We analyzed data from a convenience sample, which was not based on an a priori sample size calculation. Normal distribution was tested by the Shapiro–Wilk normality test. The correlation of age with CMAP amplitude and area decrement, post-tetanic facilitation, twitch-to-tetanus, and procedural changes in heart rate were tested with the Spearman test. The ranges of values were defined as median ± IQR (interquartile range), as many of the groups did not show normal distribution. The CMAP area decrement values determined at rest, immediately, 20, and 60 s after HRS were evaluated by the Kruskal–Wallis test followed by Dunn's multiple comparisons test. The periprocedural heart rate values before and during stimulation with 3 and 30 Hz were evaluated by the Friedman test, followed by Dunn's multiple comparisons test. The values were considered significant when p < 0.05. The levels of correlations were defined according to the determined correlation coefficients (moderate correlations when rho = 0.40–0.69) [11].
3 Results
54 children undergoing elective surgical procedures (e.g., urethroplasty, pyeloplasty, palatoplasty, inguinal hernioplasty, orchidopexy, ureteral reimplantation, or ureterocele) participated in this study. In 20 patients, it was not possible to complete the RNS test because of time constraints (surgery had priority limiting the time for electrophysiological measurements). Data from 34 young children (23 boys and 11 girls, 0 to 48 months of age) were included in the study evaluations (Table 1. and Supplementary Table 1). One participant was 5 weeks old. Two other participants were immature upon birth. One subject, born at 24 weeks' gestation, was 5 months old at the time of testing (corrected age: 28 days). Another participant was born on the 33rd gestational week, and at the time of the examination, was 1 month and 12 days old (estimated gestational age 40 weeks). Based on their age from conception, all three participants were in the age range corresponding to a term newborn. All the other participants were 2 to 48 months of age (9 subjects aged 2–5 months, 6 aged 6–11 months, 9 aged 12–23 months, and 7 aged 24–48 months). The test was performed on the right side in 4 children and on the left side in 30.
| Characteristic | 0–5 months | 6–48 months | Total |
|---|---|---|---|
| Number of Patients (N) | 9 | 25 | 34 |
| Male (%) | 6 (17.6%) | 17 (50.0%) | 23 (67.6%) |
| Female (%) | 3 (8.8%) | 8 (23.5%) | 11 (32.4%) |
| Age (months) Median (IQR) | 3.0 [1.0–4.0] | 17.0 [11.0–27.0] | 12.5 [5.25–21.0] |
| Height (cm) Median (IQR) | 56.0 [55.0–63.0] | 77.0 [74.0–86.0] | 74.0 [65.0–84.75] |
| Weight (kg) Median (IQR) | 5.0 [4.42–5.2] | 10.0 [8.0–12.0] | 8.07 [6.49–12.0] |
Statistical analysis showed no significant correlation between the age of the young children and the CMAP amplitude decrement values at rest, immediately, 20 s, and 1 min after HRS (Figure 1A–D). Based on this, we decided not to create different age groups for further calculations [4, 12]. RNS CMAP amplitude decrement demonstrated a median of 1% (interquartile range 1% to 3%, full range 0 to 7%).

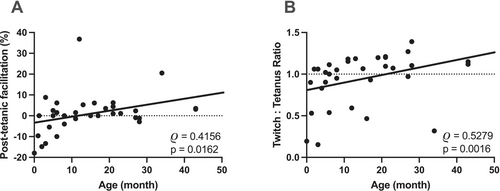
There was a moderate positive correlation between the age of the young children and post-tetanic facilitation (Figure 2A). No facilitation was present below 4 months of age; instead, in most of the cases, exhaustion was detected in this age group. Facilitation was observed in older subjects. Twitch-to-tetanus ratio and the young children's age also showed a moderate positive correlation (Figure 2B), with lower ratios in the younger persons.
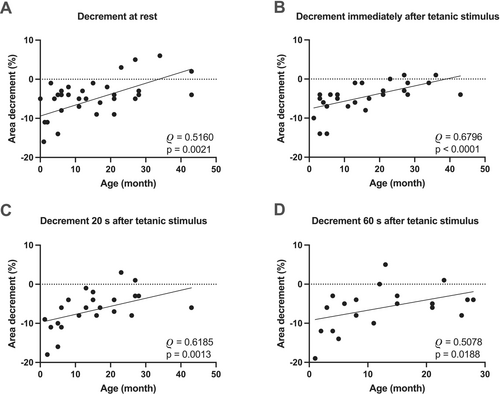
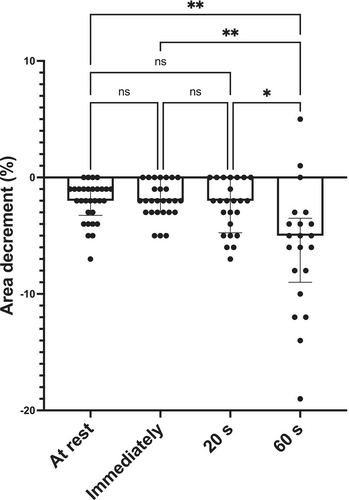
Statistical analysis showed a moderate positive correlation between the CMAP area decrement values and age (Figure 3A). RNS CMAP area decrement demonstrated a median of 11% (interquartile range 5% to 15%) in subjects between 0–5 months of age. Five out of nine infants in this group showed CMAP area decrements exceeding 10%. In contrast, none of the patients between 6–48 months had an area decrement greater than 10% (median of 4%, interquartile range 2% to 5%). A moderate positive correlation was also present immediately, 20 s, and 1 min after HRF stimulation (Figure 3B–D). Statistical analysis showed significant differences among CMAP area decrement values recorded at different time points (Figure 4, p < 0.0005). Multiple comparison showed significantly larger CMAP area decrement values 1 min after HFS compared to rest, immediately, and 20 s after HRS (Figure 4, p = 0.0015, 0.0011, and 0.0174, respectively).
The change in the heart rate did not correlate with the age of children during 3 Hz or 30 Hz stimulations (Supplementary Figure 2A). Moreover, there was no significant difference in the heart rate values before the measurements or during the 3 Hz or 30 Hz stimulations (Supplementary Figure 2B). No clinically significant bradycardia was observed in any child [13].
No safety concerns were raised during or after the investigation. Upon performing RNS, no stimulation artifacts were recorded, and the tracings were usually reproducible. However, in some patients, obtaining and maintaining a steady baseline was difficult, especially when sedation was not sufficiently effective.
4 Discussion
We demonstrated that in young children the median CMAP amplitude decrement was 1%, with a range of 0 to 7% at rest. Although there was no correlation between the CMAP amplitude decrement and age, a moderate positive correlation was found between the CMAP area decrement, post-tetanic facilitation, twitch-to-tetanus ratio, and age.
To date, few studies have investigated the neuromuscular junction in healthy children using RNS. Churchill-Davidson and Wise used needle electrodes to stimulate the ulnar nerve and record from the abductor digiti minimi muscle in five infants, reporting an 11% amplitude decrement at 2.5 Hz in a premature infant. No amplitude decrement was observed at this frequency postnatally (up to 26 weeks), although high-frequency stimulation (50 Hz for 20 s) elicited notable decrements (28%–75%) [14]. Wise and McQuillen found no significant CMAP amplitude reduction in three normal infants aged 1–3 days at 3, 10, or 50 Hz stimulation of the ulnar nerve, although delayed recovery was noted [9]. Koenigsberger et al. analyzed 17 newborns and found no CMAP amplitude decrement at low frequency stimulation of the median nerve (1–2 Hz) but significant decrements at higher frequencies (20–50 Hz) [15]. Crumrine and Yodlowski studied 25 patients aged 1 day to 5 years by EMG with intramuscular motor nerve stimulation and recording in the tibialis anterior muscle. They found no CMAP amplitude decrement at low frequency stimulations (1–3 Hz), whereas infants under age 12 weeks exhibited neuromuscular failure at high frequencies (50–100 Hz) [12]. Maselli et al. reported no CMAP amplitude decrement upon ulnar nerve stimulation in three healthy controls with 2 Hz stimulation, though their ages were not specified [16].
Epidemiological studies indicate that while symptoms of congenital myasthenic syndromes (CMS) can appear at birth or shortly thereafter, diagnosis may be delayed for years [17]. When a myasthenic condition is suspected, RNS should be performed first, because this test is widely available and is a sensitive and specific functional test of the NMJ. In limb-girdle forms of CMS, abnormalities may be limited to distal muscles, making RNS preferable for testing limb muscles in floppy infants [18, 19]. While stimulating the accessory nerve is technically straightforward in adults, it poses challenges in small young children due to access difficulties and the risk of vagal nerve stimulation. In contrast, the supraclavicular region is more accessible, and the deltoid muscle's size allows for robust motor potential recording. Therefore, we chose to stimulate the axillary nerve and record from the deltoid muscle.
Textbooks and neurophysiology literature indicate that a decrement greater than 10% between the fourth and first potentials signifies an abnormal response [10, 18, 20]. Moreover, CMAP amplitude and area decrements are considered to be interchangeable in adults [21]. Some laboratories consider decrements over 7% (e.g., facial muscles [22]) or even 5% as abnormal if artifacts are excluded [20]. All studies establishing these cut-off values were conducted in adults. In our study, we found no CMAP amplitude decrement values higher than 7%, suggesting that young children's CMAP amplitude decrement values are in the adult range. In contrast, more than half of those 0–5 months of age demonstrated CMAP area decrements larger than 10%. In contrast, all older subjects had a CMAP area decrement of less than 10%. These findings may have implications for quantitative neuromuscular monitoring during surgery in infants. Although data from adults suggest that quantitative assessment of the depth of neuromuscular blockade is clinically interchangeable when calculated using CMAP amplitude or area [21], this may not be true for young children.
The larger CMAP area decrements in young children under 6 months indicate different NMJ functioning and skeletal muscle physiology. The CMAP amplitude reflects the peak of summed potentials, which may remain stable if most muscle fibers are activated, whereas the area measurement is sensitive to muscle fiber synchronization. Increased temporal dispersion can lead to phase cancellation, reducing the total area under the curve. In younger children, immature nerve and muscle fiber conduction velocities may result in larger area decrements, even when peak amplitudes appear stable. This can be attributed to small variations in resting intracellular calcium concentrations, affecting muscle fiber response synchronization.
In healthy adults, post-tetanic facilitation is absent, and any observed increase in CMAP amplitude is attributed to pseudofacilitation [20]. In our study, we found post-tetanic exhaustion immediately following high-rate stimulation (HRS) in infants under four months. These results align with previous findings that both premature and term infants exhibit decrements rather than increments with high-frequency stimulation [12, 14, 15] indicating altered presynaptic NMJ function in younger children.
Our study has several limitations: it is a single-center investigation with a relatively small sample size from a Caucasian pediatric population in Central Europe, which may limit the generalizability of the results. Additionally, all children were sedated, which could influence their responses. We focused exclusively on the axillary nerve, a less commonly studied nerve for RNS, complicating generalizations to unsedated conditions and other nerves. We recognize the possibility of type II statistical error in interpreting the Spearman's rank correlation test, where insufficient sample size may lead to failure of rejecting the null hypothesis of no correlation.
In conclusion, this study provides novel insights into the functioning of the neuromuscular junction (NMJ) in pediatric populations, particularly in young children. Our findings indicate that while CMAP amplitude decrements align with adult norms, CMAP area decrements in infants under 6 months are often higher than the cut-off values for adults, emphasizing the need for age-specific reference values in RNS testing. In general, our results suggest the preference for CMAP amplitude decrement over CMAP area decrement in young children. Future studies should explore NMJ function across a broader range of nerves and in diverse pediatric populations, ideally in unsedated conditions, to validate and expand upon our findings.
Author Contributions
Márk Kozák: investigation, data curation, writing – original draft. Katalin Szatmári: investigation, data curation. Erzsébet Németh: investigation, data curation. Béla Fülesdi: writing – review and editing. Attila Nagy: formal analysis. Attila Tóth: formal analysis, writing – review and editing, writing – original draft. Judit Boczán: conceptualization, data curation, investigation, formal analysis, writing – review and editing, writing – original draft.
Acknowledgments
The authors thank the anesthesiology and surgery team of the pediatric surgery operating room for their assistance in carrying out the repetitive nerve stimulation test of the subjects.
Ethics Statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Consent
All examinations were undertaken with each subject's parents' understanding and written consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




