Switching Enzyme Replacement Therapy for Late-Onset Pompe Disease From Alglucosidase Alfa to Cipaglucosidase Alfa Plus Miglustat: Post Hoc Effect Size Analysis of PROPEL
Funding: This work was supported by Amicus Therapeutics, Inc.
ABSTRACT
Introduction/Aims
The randomized, double-blind PROPEL study (NCT03729362) suggested benefits for cipaglucosidase alfa plus miglustat (cipa+mig) versus alglucosidase alfa plus placebo (alg+pbo) in enzyme replacement therapy (ERT)-experienced adults with late-onset Pompe disease (LOPD). To further assess treatment response and the effect of switching treatment from alg to cipa+mig, we conducted a within-group effect size analysis in ERT-experienced patients.
Methods
In this post hoc analysis, standardized within-group effect sizes (Cohen's d for correlated measurements from baseline to week 52) were calculated by dividing the mean change from baseline by the corresponding standard deviation for motor function, lung function, and muscle strength outcomes; patient-reported outcomes/quality of life; and biomarker levels (creatine kinase and hexose tetrasaccharide).
Results
In PROPEL, 77% of patients received ERT with alg before study entry (median ERT duration 7.4 years). ERT-experienced patients remaining on alg+pbo (n = 30) generally showed within-group worsening (d ≤ −0.2) or stability (−0.2 < d < 0.2) across most outcomes, while those switched to cipa+mig (n = 65) mostly showed improvement (d ≥ 0.2) or stability. Patients remaining on alg+pbo demonstrated statistically significant worsening for several lung function outcomes, biomarker levels, and significant improvement for Patient-Reported Outcomes Measurement Information System (PROMIS)-Dyspnea. Patients switched to cipa+mig did not demonstrate significant worsening for any outcomes and showed significant improvements for 6-min walk distance (absolute and % predicted); upper, lower, and overall manual muscle testing; PROMIS-Fatigue; Physician (overall score) and Subject Global Impression of Change (5/8 subdomains); and biomarker levels.
Discussion
ERT-experienced patients with LOPD who switched from alg to cipa+mig treatment achieved improvements or stability in most outcomes.
Trial Registration: ClinicalTrials.gov identifier: NCT03729362
Graphical Abstract
A total of 95 ERT-experienced adults with LOPD were randomized to switch to cipaglucosidase alfa + miglustat or remain on alglucosidase alfa treatment. After 52 weeks, patients remaining on alglucosidase alfa showed worsening or stability for most outcomes, whereas patients who switched to cipaglucosidase alfa + miglustat generally showed stability or improvement.
Abbreviations
-
- 6MWD
-
- 6-min walk distance
-
- Alg
-
- alglucosidase alfa
-
- CFBL
-
- change from baseline
-
- CI
-
- confidence interval
-
- Cipa
-
- cipaglucosidase alfa
-
- CK
-
- creatine kinase
-
- EQ-5D-5L
-
- EQ-5D 5 Response Levels
-
- ERT
-
- enzyme replacement therapy
-
- FVC
-
- forced vital capacity
-
- GAA
-
- acid α-glucosidase
-
- GSGC
-
- Gait, Stairs, Gowers' maneuver, Chair
-
- Hex4
-
- hexose tetrasaccharide
-
- LOCF
-
- last observation carried forward
-
- LOPD
-
- late-onset Pompe disease
-
- MCID
-
- minimal clinical important difference
-
- MEP
-
- maximal expiratory pressure
-
- Mig
-
- miglustat
-
- MIP
-
- maximal inspiratory pressure
-
- MMT
-
- manual muscle test
-
- Pbo
-
- placebo
-
- PGIC
-
- Physician Global Impression of Change
-
- PROMIS
-
- Patient-Reported Outcomes Measurement Information System
-
- Q
-
- quartile
-
- QoL
-
- quality of life
-
- QMT
-
- quantitative muscle test
-
- rhGAA
-
- recombinant human GAA
-
- R-PAct
-
- Rasch-built Pompe-specific Activity
-
- SAS
-
- Statistical Analysis System
-
- SD
-
- standard deviation
-
- SGIC
-
- Subject Global Impression of Change
-
- SNIP
-
- sniff nasal inspiratory pressure
-
- SVC
-
- slow vital capacity
-
- TEAE
-
- treatment-emergent adverse event
-
- TUG
-
- timed up and go
-
- VAS
-
- visual analog scale
1 Introduction
Pompe disease is a rare, inherited, multisystemic, heterogeneous, and progressive disease caused by biallelic pathogenic variants in the acid α-glucosidase (GAA) gene leading to deficiency of the GAA enzyme, acid α-glucosidase [1-3]. Pompe disease is broadly classified into two clinical subtypes: the severe, infantile-onset form associated with rapidly progressive disease and cardiomyopathy in the first year of life, which is lethal without treatment, and the late-onset form [2]. Late-onset Pompe disease (LOPD) can present any time after the first year of life through late adulthood and is characterized by progressive loss of muscle and respiratory function, which often leads to wheelchair use and the need for assisted ventilation. Cardiac involvement is often absent in LOPD [1, 2, 4].
Enzyme replacement therapy (ERT) with alglucosidase alfa (alg), a recombinant human GAA (rhGAA), was the first approved Pompe disease-specific treatment [5, 6]. Cipaglucosidase alfa (cipa), a next-generation rhGAA enriched with naturally (Chinese hamster ovary cell) derived bis-phosphorylated mannose-6-phosphate N-glycans, was designed to improve the delivery of recombinant GAA to skeletal muscle lysosomes when administered in combination with the oral enzyme stabilizer miglustat (mig) [7-10].
The randomized, double-blind PROPEL study (ATB200-03; NCT03729362) compared the efficacy and safety of cipa+mig with the standard of care, alg, plus placebo (pbo) for the treatment of adults with LOPD [11]. Patients previously treated with alg were eligible to participate in the PROPEL study, and most enrolled patients were ERT experienced (95/123; 77.2%) [11], receiving prior ERT for a median of 7.4 years. PROPEL did not achieve statistical superiority for cipa+mig over alg+pbo for the primary endpoint of change from baseline to week 52 in 6-min walk distance (6MWD) in the overall population (between-group difference 13.6 m [95% confidence interval (CI): −2.8, 29.9]). However, the totality of primary and secondary efficacy endpoint data suggested clinically meaningful improvements with cipa+mig versus alg+pbo in various measures of motor functions, lung functions, and biomarkers in the overall and ERT-experienced patient populations [11, 12].
We performed a comprehensive post hoc analysis of the magnitude of within-group treatment effects across all primary and secondary efficacy and pharmacodynamic (biomarker) outcomes in ERT-experienced patients in PROPEL to further assess treatment response and the effect of switching treatment from alg to cipa+mig across motor function, lung function, muscle strength, biomarkers, and patient- and physician-reported quality of life (QoL) measures [11].
2 Methods
2.1 Study Design
The PROPEL study design (Supplementary Figure S1) has been previously reported [11]. Briefly, PROPEL was an international, randomized, double-blind, parallel-group, phase 3 study conducted in 24 countries. The study was performed in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice. The study protocol was approved by the appropriate independent ethics committee or institutional review board at each participating study site, and all participants provided written informed consent. Interventions and outcomes and assessments are detailed in the supplementary Information (S1.1, S1.2).
2.2 Patients
Full eligibility criteria have been described [11]. Patients were classified as either ERT experienced (defined as currently receiving standard-of-care ERT with alg, 20 mg/kg every 2 weeks, for ≥ 24 months) or ERT naïve (defined as no prior ERT).
2.3 Statistical Methods
We performed a post hoc analysis to assess the magnitude of within-group treatment effects across efficacy outcomes. The analyses presented here focus on the subgroup of patients who were ERT experienced prior to entering the study.
Standardized effect size analysis describes the absolute effect size relative to the variability of the data. It transforms the effect size into a scale with no units of measurement (Cohen's d) [13-16]. We calculated standardized within-group effect sizes (Cohen's d for within-group comparisons) and corresponding 95% CIs for the change from baseline to week 52 for the primary, secondary, and pharmacodynamic endpoints of the PROPEL study.
Standardized within-group effect sizes and corresponding 95% CIs were calculated by dividing the mean change from baseline values and CIs at week 52 by the standard deviation (SD) of the difference scores. Values were reversed for endpoints, for which a negative change from baseline indicated an improvement (Gait, Stairs, Gowers' maneuver, Chair [GSGC] total score, time to complete GSGC component tests [10-m walk, four-stair climb, Gowers' maneuver, rising from chair], timed up and go [TUG], Patient-Reported Outcomes Measurement Information System [PROMIS]-Fatigue and -Dyspnea, EQ-5D 5 Response Levels [EQ-5D-5L] scores for Mobility, Self-care, Usual activities, Pain/discomfort, Anxiety/depression, and creatine kinase [CK] and hexose tetrasaccharide [Hex4] levels). Consistent with medical literature [13-16], effect sizes were defined as stable (−0.2 < d < 0.2), small (0.2 ≤ d < 0.5), medium (0.5 ≤ d < 0.8), or large improvement (d ≥ 0.8), or as small (−0.5 < d ≤ −0.2), medium (−0.8 < d ≤ −0.5), or large worsening (d ≤ −0.8), and were considered statistically significant if the standardized 95% CIs did not cross zero.
Analyses were carried out using Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary, NC).
3 Results
3.1 Patients
In total, 125 patients were enrolled and randomly assigned to cipa+mig (n = 85) or alg+pbo (n = 40) in PROPEL. Two patients in the alg+pbo group were not dosed because of the absence of genotype confirmation. Most patients (95/123; 77.2%) were ERT experienced (n = 65 in the cipa+mig group and n = 30 in the alg+pbo group), with an overall median duration of prior treatment with alg of 7.4 years (range, 2.0–13.7 years; quartile 1–3, 4.0–10.4 years). Baseline characteristics of ERT-experienced patients were largely similar between groups, with some differences in four-stair climb, Gowers' maneuver, rising from chair, maximal inspiratory pressure (MIP), and maximal expiratory pressure (MEP) (Table 1 and Supplementary Table S1).
| Cipa+mig (n = 65) | Alg+pbo (n = 30) | |
|---|---|---|
| Median (range) age, years | 48.0 (21–74) | 46.5 (24–66) |
| Median (range) age at diagnosis, years | 39.0 (1–63) | 39.0 (7–62) |
| Males, n (%) | 28 (43.1) | 14 (46.7) |
| Race, n (%) | ||
| Asian | 3 (4.6) | 1 (3.3) |
| Japanese | 2 (3.1) | 4 (13.3) |
| American Indian or Alaska Native | 0 | 1 (3.3) |
| Black or African American | 0 | 1 (3.3) |
| White | 55 (84.6) | 22 (73.3) |
| Other | 5 (7.7) | 1 (3.3) |
| Median (Q1–Q3) ERT duration, years | 7.6 (4.3–10.2) | 7.1 (3.8–10.4) |
| Range | 2.0–13.7 | 2.1–13.2 |
| Motor function, mean (SD) | ||
| 6MWD, a meters | 346.9 (110.21) | 334.6 (114.02) |
| GSGC | 15.6 (4.07) | 15.5 (4.35) |
| 6MWD, a % predicted | 56.6 (15.85) | 54.8 (17.38) |
| Muscle strength, mean (SD) | ||
| Lower extremity MMT score | 26.4 (5.14) | 26.1 (5.81) |
| Lung function, mean (SD) | ||
| Sitting FVC, % predicted | 67.9 (19.05) | 67.5 (20.99) |
| Patient-reported outcomes, mean (SD) | ||
| PROMIS-Physical function score | 64.4 (11.38) | 66.9 (12.29) |
| PROMIS-Fatigue score | 22.0 (7.92) | 20.4 (5.38) |
- Note: Table adapted from Kishnani PS et al. 2024 [17], used under CC BY 4.0.
- Abbreviations: 6MWD, 6-min walk distance; alg+pbo, alglucosidase alfa plus placebo; cipa+mig, cipaglucosidase alfa plus miglustat; ERT, enzyme replacement therapy; FVC, forced vital capacity; GSGC, Gait, Stairs, Gowers' maneuver, Chair; LOPD, late-onset Pompe disease; MMT, manual muscle test; PROMIS, Patient-Reported Outcomes Measurement Information System; Q, quartile; SD, standard deviation.
- a Mean of two screening values.
3.2 Standardized Within-Group Effect Size Analysis
For measures of motor function, significant improvements were seen for % predicted 6MWD (d = 0.50) and for 6MWD in meters (d = 0.42) with cipa+mig. In patients who remained on alg+pbo, no significant improvements or worsening were seen (Figure 1).
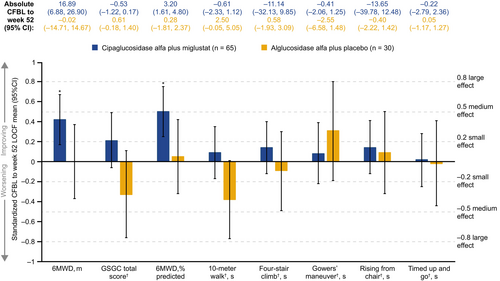
For measures of muscle strength, significant small to medium improvements were seen for lower (d = 0.39), upper (d = 0.50), and total (d = 0.49) manual muscle test (MMT) scores with cipa+mig. Effects on MMT scores with alg+pbo were generally smaller and did not reach significance. In both groups, the effect size on the total quantitative muscle test (QMT) score was 0 < d < 0.2, indicating stability (Figure 2).
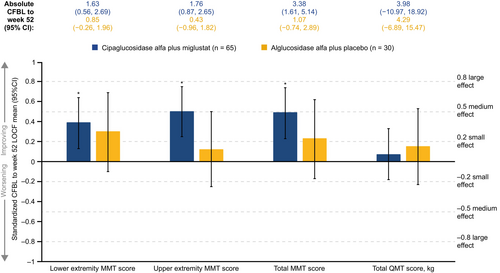
For measures of lung function, effect sizes indicated stability (−0.2 < d < 0.2) in five of the six outcomes assessed in the cipa+mig group, with a small non-significant worsening in sitting slow vital capacity (SVC). In the alg+pbo group, four of the six lung function assessments showed a significant small to large worsening, with Cohen's d ranging from −0.80 to −0.39 (Figure 3).
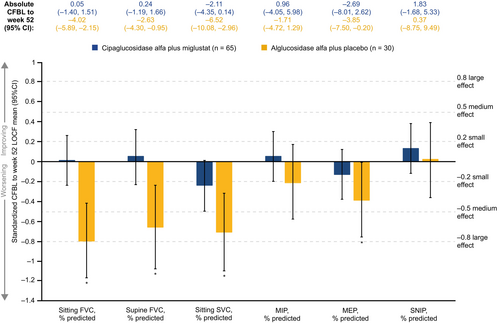
For patient-reported outcomes, significant improvements were seen for several of the Subject Global Impression of Change (SGIC) domains with cipa+mig, with Cohen's d ranging from 0.27 to 0.49, namely muscle function, ability to move around, activities of daily living, overall physical wellbeing, and energy level. Effect sizes for SGIC domains were in the range of −0.2 < d < 0.2 in the alg+pbo group, indicating stability (Figure 4). These effects reflected a higher percentage of patients who experienced improvements and lower percentages who experienced worsening in SGIC domains with cipa+mig than with alg+pbo, as shown for SGIC overall physical wellbeing (Figure 5) and other domains (Supplementary Figure S2). Significant improvements were also observed for PROMIS-Fatigue (d = 0.32) with cipa+mig and for PROMIS-Dyspnea (d = 0.38) with alg+pbo (Figure 4).
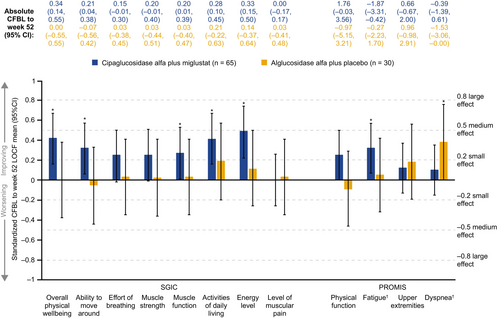
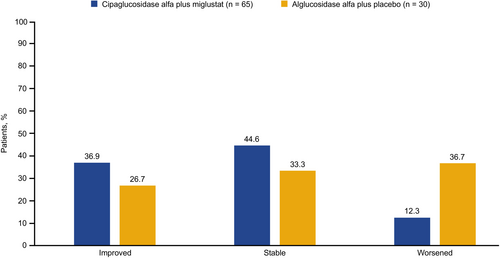
Effect size analysis for the EQ-5D-5L domains, index score, and visual analog scale (VAS), and Rasch-built Pompe-specific Activity (R-PAct) showed mainly stability for both treatment groups (Supplementary Figure S3A,B). Physician Global Impression of Change (PGIC) suggested significant improvements with cipa+mig (d = 0.36) and stability with alg+pbo (d = 0.00; Supplementary Figure S3B). Analysis of biomarker data showed significant medium improvements with cipa+mig for both CK and Hex4 and significant worsening with alg+pbo (Supplementary Figure S4).
Effect size analyses for all endpoints are summarized in Supplementary Table S2.
3.3 Safety
Cipa+mig was generally well tolerated in ERT-experienced patients, with a similar safety profile to that in the alg+pbo group (Table 2). One serious treatment-emergent adverse event (TEAE) occurred in the cipa+mig group, which was considered potentially related to study treatment (anaphylaxis), and three TEAEs resulted in withdrawal from the study (anaphylaxis and chills each in one patient in the cipa+mig group, and stroke in one patient in the alg+pbo group unrelated to study drug) (Table 2).
| Cipa+mig (n = 65) | Alg+pbo (n = 30) | |
|---|---|---|
| TEAEs, n (%) | 62 (95.4) | 29 (96.7) |
| TEAEs potentially related to treatment | 19 (29.2) | 12 (40.0) |
| Serious TEAEs | 6 (9.2) | 1 (3.3) |
| Serious TEAEs potentially related to treatment a | 1 (1.5) | 0 |
| TEAEs leading to study drug discontinuation | 2 (3.1) b | 1 (3.3) c |
| TEAEs leading to death | 0 | 0 |
| Infusion-associated reactions, n (%) | 16 (24.6) | 8 (26.7) |
- Abbreviations: alg+pbo, alglucosidase alfa plus placebo; cipa+mig, cipaglucosidase alfa plus miglustat; ERT, enzyme replacement therapy; LOPD, late-onset Pompe disease; TEAE, treatment-emergent adverse event.
- a Relatedness to treatment was determined by the investigator.
- b Anaphylactic reaction and chills.
- c Cerebrovascular accident (stroke) unrelated to study drug.
4 Discussion
Overall, this effect size analysis of the PROPEL study showed that patients with LOPD who were receiving ERT with alg and switched to cipa+mig mostly showed within-group improvement (d ≥ 0.2) or stability (−0.2 < d < 0.2) in standardized mean change from baseline to week 52. The largest effect sizes and significant improvements were seen for various assessments of motor function, muscle strength, global impression of change scales, and biomarkers; significant worsening was not observed for any assessments. Patients who remained on alg treatment mainly showed worsening (d ≤ −0.2) or stability (−0.2 < d < 0.2) in standardized mean change from baseline to week 52 across most outcomes. In this group, the largest effect sizes were significant worsening for various lung function assessments and biomarkers; in contrast, a significant improvement in the standardized change from baseline was seen for PROMIS-Dyspnea scores. Generally, these results reflect those of the primary analysis for ERT-experienced patients [11]. It is important to note that patients who remained on alg treatment had received prior treatment with alg for 2.1–13.2 years (median 7.1 years) prior to entering PROPEL. As these patients continued their previous treatment regimen during the study, it is possible that outcomes showing a worsening in the first 52 weeks of PROPEL, including lung function assessments and biomarkers, were already worsening prior to the start of PROPEL. A decline in outcomes after several years of ERT with alg has been reported previously [18]. The reason why ERT treatment with alg may become less effective over time is not known but could include a decline in effective cation-independent mannose 6-phosphate receptor-mediated uptake of ERT and trafficking to the lysosome due to autophagic buildup [19-21], challenges that ERT with cipa+mig aims to overcome [8, 10]. Recent European Pompe Consortium recommendations suggest that switching ERT may be considered if there is no stabilization or improvement in skeletal muscle or respiratory function within 12 months of standard ERT, or in cases of severe infusion-related reactions. Decisions to switch should be made on a shared and patient-by-patient basis as there is currently insufficient evidence regarding switching to next-generation ERTs [22].
A standardized effect size analysis provides a way to assess treatment responses across multiple study endpoints and is informative for conditions with a range of symptoms, such as LOPD. However, it is essential to emphasize that the classifications of ‘small’, ‘medium’, or ‘large’ in this effect size analysis are not indicative of clinical meaningfulness, that is, there is no defined Cohen's d that indicates a minimal clinical important difference (MCID). PROPEL assessed a large number of efficacy outcomes; however, MCIDs for patients with LOPD have not been established for most of these outcomes. A recent analysis using data from PROPEL estimated that, for the overall population of patients with LOPD, MCIDs for 6MWD ranged from 2.27% to 8.11% predicted (or 23.7 to 57.2 m for absolute values) [23]. Another analysis using data from two prospective follow-up studies established an MCID of 0.35% to 7.47% predicted (corresponding to 2.2 to 46.6 m or 2.0 to 42.1 m, respectively, for a male or female of 50 years of age with a height of 1.75 m and a weight of 80 kg) [24]. Based on these MCIDs, the mean change from baseline to week 52 in % predicted 6MWD for ERT-experienced patients in PROPEL who switched from alg to cipa+mig of 3.20% (Figure 1) may be considered clinically meaningful; the corresponding standardized effect size for this improvement was 0.50 (i.e., just meeting the threshold for a ‘medium’ improvement). The clinical meaningfulness of the observed effect sizes across the various outcome measures is also supported by the SGIC assessments, which are often used as anchors to estimate MCIDs. These SGIC assessments, including overall physical wellbeing, generally showed more patients reporting improvement or stability and fewer patients reporting worsening in the cipa+mig arm versus the alg+pbo arm.
Safety assessments showed that cipa+mig was generally well tolerated in ERT-experienced patients. The safety profile of cipa+mig in ERT-experienced patients was similar to that of alg+pbo, and safety findings in ERT-experienced patients were similar to those seen in the overall population [11].
A limitation of the study is the relatively small sample size. As a post hoc analysis, this effect size analysis was not powered for detecting between-group differences in the ERT-experienced cohort, nor was it intended to, and was not adjusted for multiplicity. The ERT-naïve cohort was not included in this analysis to focus on patients switching treatments. Differences in outcomes between the ERT-naïve and ERT-experienced cohorts in the PROPEL primary analysis [11] may have been due to the low number of ERT-naïve patients, differences in baseline characteristics between ERT-naïve and ERT-experienced patients [11], or the potential of prior ERT as a treatment effect modifier [25]. Another limitation is the 52-week study duration. LOPD is a chronic and progressive disease; therefore, treatment must remain effective over an extended period. The ongoing open-label extension of PROPEL [26] may provide opportunities to conduct further effect size analyses after longer treatment durations. We cannot fully explain some discrepancies seen across individual assessments, for example, the worsening of lung function outcomes alongside improvement in patient-reported dyspnea in the alg+pbo group. We also saw improvement in MMT scores outcomes alongside improvement in patient-reported dyspnea in the alg+pbo group. We also saw improvement in MMT scores alongside stability of QMT scores in the cipa+mig group; however, both MMT and QMT suggested stability or improvement in muscle strength in patients switching to cipa+mig from alg+pbo. Such findings may have been impacted by using generic rather than Pompe disease-specific assessments or by chance, and further studies would be needed to explore this in more detail.
In summary, our analyses suggest a potential benefit of switching to cipa+mig for ERT-experienced patients. ERT-experienced patients who switched from alg to cipa+mig experienced improvement or stability across endpoints; no endpoints showed significant worsening.
Author Contributions
Antonio Toscano: conceptualization, investigation, data curation, supervision, writing – review and editing. Barry J. Byrne: conceptualization, investigation, data curation, supervision, writing – review and editing. Benedikt Schoser: conceptualization, investigation, data curation, supervision, writing – review and editing. Paula R. Clemens: conceptualization, investigation, data curation, supervision, writing – review and editing. Drago Bratkovic: conceptualization, data curation, investigation, supervision, writing – review and editing. Fred Holdbrook: conceptualization, formal analysis, methodology, validation, visualization, writing – review and editing. Hani Kushlaf: conceptualization, data curation, investigation, supervision, writing – review and editing. Jeff Castelli: conceptualization, formal analysis, methodology, validation, visualization, writing – review and editing. Jordi Díaz-Manera: conceptualization, investigation, data curation, supervision, writing – review and editing. Kristl G. Claeys: conceptualization, investigation, data curation, supervision, writing – review and editing. Mark Roberts: conceptualization, investigation, data curation, supervision, writing – review and editing. Mazen M. Dimachkie: conceptualization, investigation, data curation, supervision, writing – review and editing. Mitchell Goldman: conceptualization, formal analysis, methodology, validation, visualization, writing – review and editing. Tahseen Mozaffar: conceptualization, investigation, data curation, supervision, writing – review and editing. Pascal Laforêt: conceptualization, investigation, data curation, supervision, writing – review and editing. Priya S. Kishnani: conceptualization, investigation, data curation, supervision, writing – review and editing. Sheela Sitaraman Das: conceptualization, formal analysis, methodology, validation, visualization, writing – review and editing.
Acknowledgments
The authors thank the patients, their families, the Pompe disease patient organizations, and the PROPEL study investigators. The authors also thank Yasmine Wasfi and Syed Raza, formerly of Amicus Therapeutics Inc., for their valuable contributions to this analysis. Several authors are members of the European Reference Network for rare neuromuscular diseases (ERN EURO-NMD). Editorial assistance was provided by Alyson Bexfield, PhD, and Simone Boldt, PhD, at AMICULUM, and was funded by Amicus Therapeutics Inc. This study was supported by Amicus Therapeutics Inc.
PROPEL/ATB200-07 Study Group Members: Hashiguchi Akihiro, Jorge Alonso-Pérez, Hernan Amartino, Henning Andersen, Shahram Attarian, Jennifer Avelar, Halina Bartosik-Psujek, Julie Berthy, Cynthia Bodkin, Francoise Bouhour, Drago Bratkovic, Thomas Burrow, Ernest Butler, Barry J. Byrne, Jonathan Cauci, Yin-Hsiu Chien, Kristl G. Claeys, Paula R. Clemens, Stephanie DeArmey, Patrick Deegan, Jordi Díaz-Manera, Mazen M. Dimachkie, Aleksandra Dominovic-Kovacevic, Crystal Eldridge, Simona Fecarotta, Miriam Freimer, Ozlem Goker-Alpan, Kristina Gutschmidt, Robert Henderson, Tarekegn Hiwot, Robert Hopkin, Derralynn Hughes, Jozsef Janszky, Aneal Khan, Gee Kim, Priya S. Kishnani, Hiroshi Kobayashi, Blaž Koritnik, Cornelia Kornblum, Hani Kushlaf, Pascal Laforêt, Vescei Laszlo, Heather Lau, Christopher Lindberg, Wolfgang Löscher, Nicola Longo, Maria Judit Molnar, Tahseen Mozaffar, Olimpia Musumeci, George Konstantinos Papadimas, Giancarlo Parenti, Helio Pedro, Alan Pestronk, Colin Quinn, David Reyes-Leiva, Mark Roberts, Richard Roxburgh, Tobias Ruck, Sabrina Sacconi, Emmanuelle Salort-Campana, Tomo Sawada, Benedikt Schoser, Agnes Sebok, Jin-Hong Shin, Hideaki Shiraishi, Ela Stefanescu, Celine Tard, Ivaylo Tarnev, Mark Tarnopolsky, Michel Tchan, Antonio Toscano, Ans T. van der Ploeg, Jaime Vengoechea, Nuria Vidal-Fernandez, Marie Wencel, Stephan Wenninger.
Ethics Statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflicts of Interest
Hani Kushlaf has received grant support (paid to his institution) from the Muscular Dystrophy Association and has served as a consultant on advisory boards for Alexion AstraZeneca Rare Disease, Amgen, Argenx, and Sanofi Genzyme.
Jordi Díaz-Manera has received consulting fees/honoraria from Amicus Therapeutics Inc., Astellas, Sarepta, Sanofi, Argenx, and Lupin and grant support from Sanofi, Spark, Sarepta, and Boehringer Ingelheim. He has received payment for speaking from Sanofi, Sarepta, and Lupin.
Drago Bratkovic declares no competing interests.
Barry J. Byrne has participated as a consultant/advisory board member for Pfizer, Amicus Therapeutics Inc., and Sanofi.
Kristl G. Claeys received research funding from Alnylam, Biogen, Pfizer, Roche, and Sanofi Genzyme; received advisory board member honoraria from Alexion, Alnylam, Amicus Therapeutics Inc., Argenx, Biogen, Ipsen, Janssen Pharmaceutics, Lupin, Pfizer, Roche, Sanofi Genzyme, Vertex and UCB.
Paula R. Clemens has received grant support from Amicus Therapeutics Inc., Sanofi Genzyme, Spark, ReveraGen, NS Pharma, MDA, National Institutes for Health (NIH), FDA, and Foundation to Eradicate Duchenne. She received an educational honorarium to speak on Pompe disease by Catalyst Medical Education and Medical Learning Group. She was a consultant for Epirium Bio Inc. and Catalyst Pharmaceuticals. She is a member of the North American Pompe Registry Advisory Board for Sanofi.
Mazen M. Dimachkie has received grants or contracts from Alexion/AstraZeneca, Alnylam Pharmaceuticals, Amicus Therapeutics Inc., Argenx, Bristol-Myers Squibb, Catalyst, CSL Behring, FDA/OOPD, GlaxoSmithKline, Genentech, Grifols, Mitsubishi Tanabe Pharma, MDA, NIH, Novartis, Octapharma, Orphazyme, Ra Pharma/UCB, Sanofi Genzyme, Sarepta Therapeutics, Shire Takeda, Spark Therapeutics, and the Myositis Association; consultancy fees, payment, or honoraria for lectures, presentations, manuscript writing, or educational events; participated on a Data Safety Monitoring Board or Advisory Board for Abata/Third Rock, Abcuro, Amicus Therapeutics Inc., ArgenX, Astellas, Cabaletta Bio, Catalyst, CNSA, Covance/Labcorp, CSL Behring, Dianthus, Horizon, EMD Serono/Merck, Fortrea, Ig Society Inc., Ipsen, Janssen, Medlink, Nuvig, Octapharma, Priovant, Sanofi Genzyme, Shire Takeda, and TACT/Treat NMD; and received royalty fees or licenses, consultancy fees, and payment or honoraria for manuscript writing or educational events from Wolters Kluwer Health/UpToDate.
Priya S. Kishnani has received research/grant support from Sanofi Genzyme and Amicus Therapeutics Inc., and has received consulting fees and honoraria from Sanofi Genzyme, Amicus Therapeutics Inc., Maze Therapeutics, Bayer, and Asklepios Biopharmaceutical Inc. (AskBio). She is a member of the Pompe and Gaucher Disease Registry Advisory Board for Sanofi Genzyme, Pompe Disease Advisory Board for Amicus Therapeutics Inc., and Advisory Board for Baebies. Priya S. Kishnani has held equity in Asklepios Biopharmaceuticals and may receive milestone payments related to that equity in the future.
Pascal Laforêt is a consultant/advisory board member for Amicus Therapeutics Inc., Sanofi Genzyme, and Spark Therapeutics. He has received travel expenses from Sanofi Genzyme and Spark Therapeutics.
Mark Roberts has received honorarium for educational symposia from Sanofi Genzyme and Amicus Therapeutics Inc. and for participation on advisory boards for Sanofi and Amicus Therapeutics Inc.
Benedikt Schoser has received unrestricted research grants from AMDA Foundation, Amicus Therapeutics Inc., DM-Entry, EU Horizon programs COMPASS, PaLaDIn, Marigold Foundation, Roche Diagnostics, and speaker's honoraria from Alexion, Amicus Therapeutics Inc., Argenx, Dyne, Kedrion, and Sanofi. He has also been a scientific adviser for Amicus Therapeutics Inc., Alexion, Astellas, Avidity, Sanofi, and Taysha. He declares no stocks or shares.
Antonio Toscano received honorarium for educational talks from Sanofi Genzyme and Amicus Therapeutics Inc., and for participation on advisory boards for Sanofi, Amicus Therapeutics Inc., Aro, and Spark. He is a member of the ERN EURO-NMD, Project ID 739543.
Jeff Castelli, Fred Holdbrook, and Sheela Sitaraman Das are employees of, and hold stocks and shares in, Amicus Therapeutics Inc.
Mitchell Goldman is a former employee of Amicus Therapeutics Inc.
Tahseen Mozaffar has served in an advisory capacity for Alexion, Amicus, AnnJi, Argenx, Arvinas, Ask Bio, Audentes, AvroBio, Horizon Therapeutics, Immunovant, Maze Therapeutics, Momenta (now Janssen), Sanofi Genzyme, Spark Therapeutics, UCB, and Zogenix. He serves on the speaker's bureau for Argenx and Sanofi Genzyme, and on the medical advisory board for the Myositis Association, Neuromuscular Disease Foundation, Myasthenia Gravis Foundation of California and Myasthenia Gravis Foundation of America. Dr. Mozaffar receives research funding from the Myositis Association, the Muscular Dystrophy Association, the NIH and from the following sponsors: Alexion, Amicus Therapeutics Inc., AnnJi, Argenx, Audentes/Astellas Gene Therapy, Bristol-Myers-Squib, Cartesian Therapeutics, Grifols, ML-Bio, Momenta, Ra Pharmaceuticals, Sanofi Genzyme, Spark Therapeutics, and Valerion. He serves on the data safety monitoring board for Acceleron, Avexis, Sarepta, and the NIH.
Editorial assistance was funded by Amicus Therapeutics Inc.
Open Research
Data Availability Statement
Data sharing proposals and requests will be reviewed on a case-by-case basis. Requests for data should be addressed to Nicholas A. Rees at [email protected].





