Peripheral Nervous System Involvement of Hereditary Transthyretin Amyloidosis in the United States: A Multi-Center Perspective
Funding: Dr. Hristelina S. Ilieva is grateful for startup funds from the Farber Institute at Thomas Jefferson University.
Initial data was presented virtually at the Peripheral Nerve Society Meeting in 2021.
ABSTRACT
Introduction/Aims
Hereditary transthyretin amyloidosis (ATTRv) is an autosomal dominant multisystem disorder that occurs worldwide. The most common mutation in the United States, V142I, has previously been described as having a primarily cardiac presentation. However, the prevalence of peripheral neuropathy (PN) in V142I ATTRv patients is unclear. We aimed to characterize and compare the peripheral nervous system involvement of the Val142Ile (V142I, previously V122I) ATTRv mutation with other known ATTRv mutations.
Methods
A retrospective, cross-sectional study was carried out on patients with genetically confirmed ATTRv at 3 institutions from 2018 to 2022. Neuropathic, autonomic, and cardiac symptoms and signs, as well as electrodiagnostic study results, were reviewed for each patient.
Results
Fifty-eight V142I and eighteen non-V142I ATTRv patients were evaluated. The majority of V142I patients had signs of PN, with abnormal pinprick sensitivity and temperature loss (74%), weakness (60%), and loss of deep tendon reflexes (59%). The presence of lightheadedness (29%) and gastrointestinal symptoms (14%) suggested autonomic involvement. PN characteristics and the prevalence of median mononeuropathy did not differ significantly between V142I and non-V142I patients. The population of V142I patients was disproportionately African American (86%) as expected.
Discussion
Polyneuropathy is more commonly found in V142I ATTRv patients than previously reported and has a wide range of phenotypes. A low threshold for neurology referral and electrodiagnostic studies in at-risk populations is encouraged.
Abbreviations
-
- ATTRv
-
- hereditary transthyretin amyloidosis
-
- BNP
-
- brain natriuretic peptide
-
- CIDP
-
- chronic inflammatory demyelinating polyneuropathy
-
- CTS
-
- carpal tunnel syndrome
-
- ECG
-
- 12-lead electrocardiography
-
- ECHO
-
- echocardiogram
-
- GI
-
- gastrointestinal
-
- IENFD
-
- intraepidermal nerve fiber density
-
- NCS
-
- nerve conduction study
-
- PN
-
- peripheral neuropathy
-
- PYP
-
- pyrophosphate
-
- TTR
-
- transthyretin
1 Introduction
Hereditary transthyretin amyloidosis (ATTRv) is a rare genetic disorder characterized by the deposition of abnormal amyloid protein aggregates in various tissues and organs throughout the body. These aggregates primarily consist of mutated transthyretin (TTR) protein [1]. In early-onset cases, late-onset cases, and wild-type, TTR can also occasionally deposit as amyloid fibrils. Buildup of amyloid deposits can occur in the heart, peripheral nerves, leptomeninges, eyes, tendons, kidneys, and gastrointestinal (GI) tract [2, 3]. If left untreated, ATTRv can significantly reduce a patient's quality of life and may ultimately lead to organ failure and premature death [2, 3]. Many of the symptoms of ATTRv, particularly those related to neuropathy and autonomic dysfunction, can overlap with other neuropathic conditions like diabetic neuropathy [4, 5]. This similarity can lead to a misdiagnosis as chronic inflammatory demyelinating polyneuropathy (CIDP) [6, 7], or delay in recognizing the underlying genetic cause, resulting in profound consequences for patients by delaying appropriate treatment [4, 5, 8].
ATTRv is inherited in an autosomal dominant manner, and more than 150 point mutations have been described for the TTR gene [5]. These pathogenic variants result in the production of abnormal TTR proteins that have a propensity to misfold and aggregate, leading to amyloid deposition [9]. Many ATTRv variants are associated with peripheral neuropathy (PN) [3, 10, 11]. Common neuropathy-associated variants so far have included V30M and A97S [12, 13].
Some ATTRv variants are more strongly associated with cardiomyopathy [14]. These result in amyloid deposits in the heart, leading to restrictive cardiomyopathy [15]. A variant of TTR with a point mutation that substitutes isoleucine for valine at position 142 (V142I, formerly V122I) is classically known as causing late-onset cardiomyopathy [16]. This is the most common pathogenic variant in the United States and is disproportionately present in individuals of African origin with West African ancestry [17, 18].
Previous reports propose that 30% of V142I ATTRv patients present with neurological signs and symptoms [14]. However, careful observation of V142I ATTRv patients suggests that PN is an underappreciated piece. Establishing genotype–phenotype correlations can aid in diagnosis and prognosis, and is crucial for personalized management and treatment decisions. Additionally, understanding the specific mutation an individual carries is crucial for personalized management and treatment decisions, particularly in populations with a higher risk of carrying that mutation. Thus, in this study, we compared the neurologic, autonomic, and cardiac phenotypes of V142I ATTRv patients with the phenotypes of patients carrying other ATTRv variants. We aimed to increase physician awareness and aid diagnosis, particularly in African American patients.
2 Methods
2.1 Participants
Patients were identified, retrospectively, by their treating physicians from three neuromuscular centers in the USA (Vanderbilt University Medical Center, Nashville, TN; Atrium Health, Charlotte, NC; Thomas Jefferson University, Philadelphia, PA). Vanderbilt (Approval # 21138) and Thomas Jefferson (Approval # 21D.1136) IRB committees at each institution reviewed and, if needed, approved the study, and informed consent was obtained where appropriate (due to the retrospective data collection, Atrium IRB did not require consent). Patients aged 18 years and older who had received a diagnosis of ATTRv between 2018 and 2022 were included in the study irrespective of their initial presentation to neurology or cardiology clinics. While the majority of patients were referred from cardiology clinics, a few were recognized based on an extension of genetic testing in family members, and some were patients presenting to neurology clinics with red-flag symptoms suggestive of amyloidosis. Patients found to have additional variants that could independently cause neuropathy were excluded. Each patient's ATTRv variants were determined by genotyping. Age, race, sex, and use of tafamidis, diflunisal, inotersen, or patisiran were collected. Comorbid status was also documented, including other potential causes of polyneuropathy besides or associated with ATTRv, such as prediabetes, diabetes, and alcohol exposure. Coexistent amyloid pathologies such as renal dysfunction, spinal stenosis, and heart failure were also documented. Patients with additional etiologies for polyneuropathy, such as diabetes, were included when they had significant median mononeuropathy, cardiomyopathy, or neuropathy features atypical for the additional diagnosis to be the sole etiology of the neuropathy. Patients' medical records were also reviewed for descriptions of neurological and autonomic symptoms and neurological examination components. Skin biopsies for intraepidermal nerve fiber density were performed at two locations (calf and distal thigh) when available. Controls were selected from patients seen in the Vanderbilt EMG laboratory between 2020 and 2021 for an evaluation of median mononeuropathy. Medical records of controls were reviewed to rule out patients with possible TTR etiology and other etiologies of PN.
2.2 Nerve Conduction Studies
Where available, motor and sensory nerve conduction studies (NCS) from ATTRv patients were reviewed and recorded. Data included sensory studies for sural, ulnar, and median nerves and motor studies for median, ulnar, peroneal, and tibial nerves. Peak latencies were recorded for sensory nerves. Compound muscle action potentials were measured from baseline to negative peak. Median mononeuropathy was defined as prolonged median sensory and/or motor latency compared to other nerves in the hand (typically ulnar) based on AANEM guidelines and the criteria of each health center's diagnostic laboratory [19].
2.3 Cardiac Assessment
Plasma brain natriuretic peptide (BNP) levels, 12-lead electrocardiography (ECG) and echocardiogram (ECHO) results, and pyrophosphate (PYP) scans were reviewed and recorded where available. As this manuscript's focus was neuropathy features and was retrospective, cardiac test results were determined by the referring cardiologists. We included what was available in order to document the number of patients with coexistent cardiomyopathy and neuropathy.
2.4 Statistical Analysis
SPSS version 29 (IBM, Armonk, NY) was used for statistical analyses. Data was analyzed using non-parametric tests for comparison of means (non-normally distributed) given the small sample size so as to not assume the normality of the data. Chi-square tests were used to compare the categorical data, while Wilcoxon rank-sum and Kruskal–Wallis tests were used to compare continuous variables. p < 0.05 was considered statistically significant.
3 Results
3.1 Patient Demographics and Characteristics
The demographic selected other characteristics of the investigated patient cohort are shown in Table 1. A total of 58 ATTRv patients had a confirmed V142I genotype, while 18 ATTRv patients carried a mutation other than V142I. Of those 18 non-V142I ATTRv patients, 11 had a confirmed T80A (formerly T60A) genotype. The presence of spinal stenosis did not differ significantly across V142I patients, non-V142I patients, and control individuals. Of note, the prevalence of cardiomyopathy was also not significantly different between V142I and non-V142I patients. However, V142I patients were significantly older and had a lower median BMI than non-V142I patients and controls. As anticipated, V142I patients were disproportionately African American compared to non-V142I patients. V142I patients were also more likely to have coexistent diabetes than those with other mutations or controls. Seven patients (of 22) in the V142I group had no cardiomyopathy but had significant neuropathy and median mononeuropathies felt to be out of proportion to their diabetes and were included in this analysis.
| Variable | V142I (n = 58) | Non-V142I (n = 18) | Control (n = 26) | p a |
|---|---|---|---|---|
| T80A mutation, n (%) | 0 (0%) | 11 (61%) | 0 (0%) | N/A |
| Age (years), median (range) | 68 (34–87) | 59 (34–73) | 55 (29–78) | < 0.001 |
| Sex (Female), n (%) | 13 (25%) | 13 (62%) | 13 (50%) | 0.169 |
| Race (African American), n (%) | 50 (86%) | 1 (5%) | 4 (15%) | < 0.001 |
| BMI (kg/m2), median (range) | 29 (18–56) | 31.8 (20.4–55.7) | 32 (23–65) | 0.018 |
| Cardiomyopathy, n (%) | 42 (72%) | 16 (88%) | N/A | 0.532 |
| Spinal stenosis, n (%) | 11 (19%) | 5 (28%) | N/A | 0.191 |
| Coexistent diabetes, n (%) | 23 (40%) | 0 | 4 (15%) | < 0.001 |
- Note: Categorical variables were compared using a chi-square test. Bold values = 0.05 as cutoff.
- Abbreviation: BMI: body mass index.
- a Quantitative variables were compared using a Kruskal–Wallis test.
3.2 Neuropathy in V142I ATTRv
In terms of neuropathy presentation, no significant differences were observed between V142I and non-V142I patients (Table 2). Most patients experienced pin and temperature sensation loss, while slightly more than half displayed weakness and loss of vibration sense. Deep tendon reflexes were diminished or absent in approximately half of the patients. However, pain was only reported in 29% of V142I patients and 28% of non-V142I patients. The most common autonomic symptom was GI disturbance, which presented with either diarrhea, early satiety, or alternating constipation and diarrhea. Again, no difference in the occurrence of autonomic symptoms was identified between V142I and non-V142I patients. Of note, two patients displayed rapidly progressive proximal weakness and were initially misdiagnosed as CIDP but were found upon genetic testing to have an ATTRv mutation, one V142I mutation, one T80A. The various presentations of neuropathy phenotypes in V142I patients (Figure 1) are similar to those described in other mutations. The common findings of pin and temperature disturbance with autonomic symptoms suggest that most patients had small fiber abnormalities, though not all were confirmed with skin biopsy pathologically.
| V142I (n = 58), n (%) | Non-V142I (n = 18), n (%) | p a | |
|---|---|---|---|
| Weakness | 35 (60%) | 10 (50%) | 0.586 |
| Pin/temperature loss | 43 (74%) | 15 (83%) | 0.641 |
| Deep tendon reflexes diminished or absent | 34 (59%) | 8 (44%) | 0.253 |
| Pain | 17 (29%) | 5 (28%) | 0.883 |
| Proprioception loss | 3 (5%) | 3 (16%) | 0.095 |
| Vibration loss | 32 (55%) | 11 (61%) | 0.931 |
| Lightheadedness | 13 (29%) | 6 (33%) | 0.519 |
| Gastrointestinal symptoms | 24 (14%) | 8 (50%) | 0.350 |
- a Quantitative variables were compared using a Wilcoxon rank-sum test. Categorical variables were compared using a chi-square test.
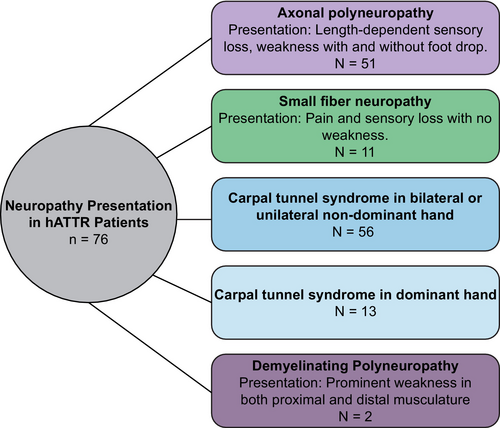
NCS studies were also reviewed and compared across V142I patients, non-V142I patients, and control individuals (Table 3). Overall, NCS results did not differ between V242I and non-V142I patients, with two exceptions. The mean ulnar motor latency was prolonged in non-V142I patients when compared to controls. Additionally, decreased median motor amplitudes, particularly in the non-dominant hand, were observed in non-V142I patients, which was out of proportion to median sensory abnormalities. When assessing V142I patients, 98% displayed nerve conduction abnormalities. This included axonal polyneuropathy, normal lower extremity NCS (small fiber neuropathy confirmed by skin biopsy), or demyelinating polyneuropathy (Table 4). This breakdown in neuropathy presentation is similar to that observed in all ATTRv patients, regardless of mutation status (Figure 1; Table 4). Additionally, 9% of patients had normal NCV parameters, but abnormal intraepidermal nerve fiber density (IENFD) on skin punch biopsy.
| V142I (n = 58), a median (range) | Non-V142I (n = 18), median (range) | Control (n = 26), median (range) | p b | |
|---|---|---|---|---|
| Sural amplitude (μV) | 5.1 (0–15.8) | 3.7 (0–20.6) | N/A | 0.264 |
| Peroneal | ||||
| Motor amplitude ankle (mV) | 3.5 (0.4–9.9) | 2.6 (0–6.4) | N/A | 0.619 |
| Motor conduction velocity (m/s) | 41 (29–50) | 39 (27–45) | N/A | 0.832 |
| Tibial | ||||
| Motor amplitude ankle (mV) | 4.8 (0–15.0) | 4.4 (0–12.8) | N/A | 0.991 |
| Motor conduction velocity (m/s) | 39.0 (25–52) | 36 (27–42) | N/A | 0.682 |
| Ulnar | ||||
| Motor amplitude wrist (mV) | 9.2 (4.2–29.6) | 6.7 (0.9–11.2) | 9.6 (6.3–14.1) | 0.256 |
| Motor latency (ms) | 2.8 (2.0–4.0) | 3.1 (2.1–4.7) | 2.6 (2.1–3.2) | 0.032 |
| Sensory amplitude (μV) | 17.3 (4.7–53.4) | 11.6 (0–46) | 18.1 (1.0–53.5) | 0.307 |
| Sensory latency (ms) | 3.29 (2.1–5.3) | 3.6 (2.8–5.0) | 3.6 (2.5–5.0) | 0.150 |
| Right | ||||
| Median motor amplitude wrist (mV) | 6.8 (0.8–14.3) | 4.3 (0.7–10.1) | 7.0 (1.2–15.4) | 0.179 |
| Median motor latency wrist (ms) | 5.3 (2.6–15.5) | 4.5 (3.0–5.5) | 5.4 (2.9–10.2) | 0.128 |
| Median sensory amplitude (μV) | 14.2 (0–45.0) | 8.2 (0–33) | 14.4 (0–55) | 0.414 |
| Median sensory latency (ms) | 4.7 (2.9–8.4) | 3.9 (3.4–4.9) | 4.5 (3.6–6.0) | 0.317 |
| Left | ||||
| Median motor amplitude wrist (mV) | 7.4 (0.3–17.0) | 3.8 (0.3–8.0) | 7.1 (0.8–17.5) | 0.047 |
| Median motor latency wrist (ms) | 4.5 (2.8–9.5) | 4.9 (3.7–7.3) | 4.8 (3.1–9.0) | 0.573 |
| Median sensory amplitude (μV) | 15.0 (0–85.0) | 11.2 (0–42.0) | 15.5 (0–68.9) | 0.836 |
| Median sensory latency (ms) | 5.4 (2.9–28.0) | 4.0 (2.7–5.0) | 4.6 (3.7–6.9) | 0.853 |
- Note: Bold values = 0.05 as cutoff.
- a Not all patients had nerve conduction studies performed. Latencies were only recorded for recordable wave forms.
- b Quantitative variables were compared using a Kruskal–Wallis test. Categorical variables were compared using a chi-square test. Latencies for sensory nerves are peak latencies.
| No mutation (wild-type) (n = 2), n (%) | V142I (n = 59), n (%) | T60A (n = 8), n (%) | V30M (n = 1), n (%) | Other (n = 6), n (%) | Total a (n = 76), n (%) | |
|---|---|---|---|---|---|---|
| No neuropathy | 0 (0%) | 1 (2%) | 1 (13%) | 0 (0%) | 0 (0%) | 2 (3%) |
| Small fiber neuropathy | 0 (0%) | 9 (15%) | 2 (35%) | 0 (0%) | 0 (0%) | 11 (14%) |
| Axonal polyneuropathy | 2 (100%) | 48 (81%) | 4 (50%) | 1 (100%) | 6 (100%) | 61 (80%) |
| Demyelinating polyneuropathy | 0 (0%) | 1 (2%) | 1 (13%) | 0 (0%) | 0 (0%) | 2 (3%) |
- a Not all patients had sufficient nerve conductions performed to generate a specific neuropathy phenotype. Axonal and small fiber polyneuropathy were defined using ACTTION criteria [21].
3.3 Median Mononeuropathy in V142I ATTRv
Median mononeuropathy was defined as median conduction slowing across the wrist with or without axonal loss. We included controls for median mononeuropathy assessment to determine whether there were electrodiagnostic features in TTR patients that would help in the identification of these patients. Median mononeuropathy was a shared feature between V142I and non-V142I patients (Table 5). The majority of ATTRv patients presented with median mononeuropathy in their dominant hand. Thirty-five V142I patients and 8 non-V142I patients had bilateral median mononeuropathies. The prevalence of right, left, or bilateral median mononeuropathies did not differ between V142I and non-V142I patients. However, V142I patients were more likely to be diagnosed with severe right median mononeuropathy than non-V142I patients (Table 5).
| V142I (n = 58), a n (%) | Non-V142I (n = 18), n (%) | Control (n = 26), n (%) | p b | |
|---|---|---|---|---|
| Bilateral median mononeuropathy | 35 (60%) | 8 (44%) | 19 (73%) | 0.141 |
| Right median | 46 (79%) | 12 (67%) | 24 (92%) | 0.538 |
| Right severe median mononeuropathy | 10 (17%) | 1 (5%) | 4 (15%) | 0.047 |
| Left median mononeuropathy | 38 (65%) | 9 (50%) | 14 (54%) | 0.418 |
| Left CTS severe median mononeuropathy | 12 (21%) | 2 (11%) | 6 (23%) | 0.735 |
- Note: Severe median mononeuropathy was defined as significant motor amplitude loss and/or denervation of appropriate median innervated muscles using guideline: Stevens JC. AAEM minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1997;20 (12):1477–1486. Bold values = 0.05 as cutoff.
- a Numbers may not add up to total as not all patients had both hands, median NCS examined bilaterally.
- b Quantitative variables were compared using a Kruskal–Wallis test. Categorical variables were compared using a chi-square test.
4 Discussion
The retrospective review presented here provides evidence that point mutation-driven predominant phenotype classifications (i.e., cardiac vs. neuropathic) of ATTRv may be inaccurate due to incomplete characterization of disease burden [14]. Specifically, we identified a substantially increased prevalence of polyneuropathy in V142I ATTRv patients compared to previously reported, with nearly all patients having some sign of polyneuropathy by either NCS or abnormal IENFD on skin biopsy.
The prevalence of cardiomyopathy did not vary between V142I and non-V142I patient groups. This may be reflective of the experience in the United States. The lack of difference between the two groups may be due to the presence of 11 individuals with a T80A genotype in the non-V142I cohort, which is also highly associated with cardiac symptoms [23].
The only significant differences between the mutations were differences in race and age. While the V142I genotype is not strictly limited to the African American population and has been detected in small cohorts of Caucasians [24], several studies have identified the V142I mutation as a common genetic variant in the African American population, particularly those of West African Ancestry [17, 18, 25, 26]. V142I patients were also older compared to non-V142I TTR patients, and it is difficult to determine if this is secondary to penetrance or to socioeconomic or other disparity factors affecting age at presentation. While not significantly different, V142I patients were less likely to be female than non-V142I patients, which has been reported in the literature [27]. However, as disease-modifying therapies are now available, it is important to have a low threshold for detection of this underlying etiology in this patient population.
In this analysis of ATTRv patients across three health centers, polyneuropathy presented with a wide phenotype variation, including weakness, autonomic involvement, electrodiagnostic features, and pain [10, 26, 28, 29]. A recent study of V142I patients also reported that a high percentage of asymptomatic patients exhibited impaired pinprick and temperature sensation on neurological examination [30]. This underscores the importance of a comprehensive neurological assessment in asymptomatic genetic carriers and supports the recommendation of annual routine assessments beginning at least 10 years before anticipated amyloidosis onset [31, 32].
When NCSs were systematically assessed for the presence of neuropathy in V142I ATTRv patients, our study illustrates a high percentage of nerve conduction abnormalities in V142I cases, suggesting most patients have mixed small and large fiber polyneuropathy in addition to cardiomyopathy. The concurrent prevalence of both cardiomyopathy and polyneuropathy patients observed in the current and previous studies continues to suggest that ATTRv cannot be easily separated into cardiomyopathy or polyneuropathy presentations [33-37]. This also implies that treatment plans should focus on both cardiomyopathy and polyneuropathy symptoms. Additionally, very few differences in neuropathic presentation were observed between V142I and non-V142I patients. However, decreased median motor amplitudes, particularly in the non-dominant hand, were observed in non-V142I patients. Thus, significantly abnormal median motor amplitudes may help differentiate amyloid PN in some cases.
Variable frequencies of CTS based on genotype have been reported in previous studies [38, 39], with CTS being used as a unique feature to differentiate between ATTRv genotypes. For example, CTS was found to be common in an A97S cohort, but not in a V30M cohort [40]. In the current study, the presence of bilateral, right, and left median mononeuropathy was higher in V142I patients than had been observed in previous reports for the V142I genotype [26, 41]. However, the presence of median mononeuropathy could not be used to distinguish V142I patients from non-V142I individuals.
Approximately 50% of the cases had a co-existing condition, with diabetes (n = 23) and prediabetes (n = 12) being the most frequent, which could also contribute to polyneuropathy and illustrate the etiologic complexity of the disease. In these cases, the burden of demonstrating pathologic evidence, or demonstrating atypical presentations such as proximal weakness that would not typically occur in patients with diabetic neuropathy, is greater. Similarly, as highlighted in this report, the wide phenotypic variation of neuropathic symptoms in these patients makes diagnosing ATTRv a challenge and ATTRv is consistently under-diagnosed [16, 42, 43]. Misdiagnosis also often occurs when ATTRv symptoms are attributed to another, more common disease such as CIDP [7, 43-45]. Therefore, there is a need to enhance our understanding of the initial and multisystem red flag indicators of ATTRv and it is crucial that healthcare professionals who may encounter these patients are knowledgeable regarding these indicators. Given the recent advances in ATTRv therapies, early diagnosis is essential for patients to receive maximal benefit from the available therapies [34, 46, 47]. This is particularly true for the V124I genotype, as carriers display higher mortality rates than patients with other forms of heart disease [48-50].
Our study had several limitations, including its small size and retrospective nature. The small size of the study may limit the statistical power to detect significant associations. Additionally, we did not exclude patients who had received tafamidis, diflunisal, Inotersen, or partisan for treatment of ATTRv; use of these disease-modifying therapies may impact symptom presentation and blur genotype–phenotype relationships.
To conclude, polyneuropathy is more commonly found in V142I patients than previously reported. The findings presented here carry clinical relevance, as they underscore the necessity of increasing physician awareness of the broad spectrum of cardiac, GI, autonomic, and neuropathic symptoms associated with ATTRv amyloidosis, regardless of TTR genotype, to mitigate diagnostic delays of this rapidly progressive disease. Given the recent introduction of effective therapies tailored to ATTRv, early diagnosis and prompt treatment initiation hold promise for enhancing patient outcomes.
Author Contributions
Urvi Desai: conceptualization, investigation, writing – original draft, writing – review and editing, data curation. Hristelina S. Ilieva: conceptualization, investigation, writing – original draft, writing – review and editing, data curation. James E. Eyer: investigation, data curation, writing – original draft. Amanda C. Peltier: conceptualization, investigation, writing – original draft, methodology, validation, writing – review and editing, software, formal analysis, project administration, data curation, supervision.
Acknowledgments
The authors thank Drs. Eva L. Feldman and Emily J. Koubek for their expert editorial assistance. The authors also thank Dr. Rebecca Hsu and Mattison Worthy at Thomas Jefferson University, and Clara Schommer and Lessy Lwea for data collection at Atrium Health, Charlotte for their assistance obtaining informed consent and data submission.
Ethics Statement
We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Consent
The study was approved by the Vanderbilt University IRB Committee (Approval No. 21138). Informed consent was obtained for all living patients in accordance with local and federal regulations and the Declaration of Helsinki. Consent was waived for the deceased or those lost to follow-up.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




