Treatment With Full-Spectrum Cannabidiol Oil Improved the Pathological Findings of Dystrophic Mutant Mice
Laís Leite Ferreira
Department of Anatomy, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Methodology, Investigation, Validation, Formal analysis, Data curation, Writing - original draft
Search for more papers by this authorFabricio Souza Gomes
Department of Anatomy, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Methodology, Investigation, Formal analysis
Search for more papers by this authorBeatriz Godinho Nascimento
Department of Anatomy, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Methodology, Formal analysis
Search for more papers by this authorWagner Corsini
Department of Anatomy, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Methodology
Search for more papers by this authorLuis Felipe Cunha dos Reis
Department of Structural Biology, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Methodology, Formal analysis
Search for more papers by this authorJoão Marcos Oliveira-Silva
Natural Sciences Institute, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Investigation, Methodology, Formal analysis, Data curation
Search for more papers by this authorJosie Resende Torres da Silva
Motor Sciences Institute, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Writing - review & editing, Resources
Search for more papers by this authorMarcelo Lourenço da Silva
Motor Sciences Institute, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Writing - review & editing, Resources
Search for more papers by this authorAngel Maurício Castro Gamero
Natural Sciences Institute, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Funding acquisition, Writing - review & editing, Visualization, Resources, Supervision
Search for more papers by this authorCorresponding Author
Túlio de Almeida Hermes
Department of Anatomy, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Correspondence:
Túlio de Almeida Hermes ([email protected]; [email protected])
Contribution: Conceptualization, Project administration, Resources, Funding acquisition, Writing - original draft, Validation, Data curation, Supervision
Search for more papers by this authorLaís Leite Ferreira
Department of Anatomy, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Methodology, Investigation, Validation, Formal analysis, Data curation, Writing - original draft
Search for more papers by this authorFabricio Souza Gomes
Department of Anatomy, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Methodology, Investigation, Formal analysis
Search for more papers by this authorBeatriz Godinho Nascimento
Department of Anatomy, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Methodology, Formal analysis
Search for more papers by this authorWagner Corsini
Department of Anatomy, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Methodology
Search for more papers by this authorLuis Felipe Cunha dos Reis
Department of Structural Biology, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Methodology, Formal analysis
Search for more papers by this authorJoão Marcos Oliveira-Silva
Natural Sciences Institute, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Investigation, Methodology, Formal analysis, Data curation
Search for more papers by this authorJosie Resende Torres da Silva
Motor Sciences Institute, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Writing - review & editing, Resources
Search for more papers by this authorMarcelo Lourenço da Silva
Motor Sciences Institute, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Writing - review & editing, Resources
Search for more papers by this authorAngel Maurício Castro Gamero
Natural Sciences Institute, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Contribution: Funding acquisition, Writing - review & editing, Visualization, Resources, Supervision
Search for more papers by this authorCorresponding Author
Túlio de Almeida Hermes
Department of Anatomy, Federal University of Alfenas (UNIFAL-MG), Alfenas, Brazil
Correspondence:
Túlio de Almeida Hermes ([email protected]; [email protected])
Contribution: Conceptualization, Project administration, Resources, Funding acquisition, Writing - original draft, Validation, Data curation, Supervision
Search for more papers by this authorFunding: This work was supported by National Council for Scientific and Technological Development (CNPq) grant 402493/2021-4, by Coordination of Superior Level Staff Improvement (CAPES) Financing Code 001; doctoral scholarship: 88887.799640/2022-00;88881.708989/2022-01, and by Research Support Foundation of the State of Minas Gerais (FAPEMIG) APQ-02291-18; Doctoral Scholarship, #12988.
ABSTRACT
Introduction/Aims
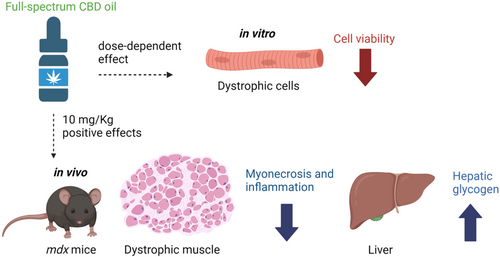
Duchenne muscular dystrophy (DMD) is caused by pathogenic variants in the DMD gene, making muscle fibers susceptible to contraction-induced membrane damage. Given the potential beneficial action of cannabidiol (CBD), we evaluated the in vitro effect of full-spectrum CBD oil on the viability of dystrophic muscle fibers and the in vivo effect on myopathy of the mdx mouse, a DMD model.
Methods
In vitro, dystrophic cells from the mdx mouse were treated with full-spectrum CBD oil and assessed with cell viability and cytotoxic analyses. In vivo, fourteen-day-old mdx mice received 10 mg/kg/day of the full-spectrum CBD oil for 14 days. We analyzed creatine kinase (CK) levels, liver damage markers, and histopathology of the diaphragm (DIA) and quadriceps (QUA [myonecrotic fibers with positive IgG staining, regenerated fibers/central nuclei, the minimum Feret's diameter, the fibrosis area, the inflammatory area, the presence of macrophages, and NF-kappa B content]).
Results
In vitro treatment with full-spectrum CBD oil showed a dose-dependent cytotoxic effect; however, in vivo 10 mg/kg treatment was safe and effectively improved DMD histopathological assessment parameters in DIA and QUA: reduction of central nuclei: 1.7% ± 2.0% versus 22.4% ± 5.3% and 11.1% ± 10.7% versus 32.3% ± 4.6%; reduction of IgG+ myofibers: 0.6% ± 0.7% versus 8.4% ± 1.6% and 0.9% ± 0.3% versus 7.5% ± 1.0%; increase in myofiber size: 85.2 ± 3.2 versus 64.3 ± 4.0 μm and 106.5 ± 8.6 versus 81.2 ± 4.8 μm; decrease in inflammatory area: 6.2% ± 2.7% versus 15.1% ± 2.6% and 5.3 ± 4.1 versus 17.3% ± 2.8%; reduced macrophage area: 0.05% ± 0.1% versus 10.8% ± 4.3% and 1.0% ± 0.7% versus 10.3% ± 4.9%; NF-κB levels: 0.6% ± 0.1% versus 1.7% ± 0.2% and 1.7% ± 0.1% versus 5.2% ± 2.1%; and fibrosis: 5.6% ± 1.8% versus 12.0% ± 3.7% and 1.3% ± 0.5% versus 4.7% ± 1.5%. It also reduced serum CK.
Discussion
Full-spectrum CBD oil may represent a promising new approach to treating DMD, but its potential toxicity must be considered.
Graphical Abstract
In vitro and in vivo full-spectrum CBD effects in the mdx mice model. Created with BioRender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| mus28337-sup-0001-supinfo.docxWord 2007 document , 14.9 KB |
Data S1: Supporting Information. |
| mus28337-sup-0002-FigureS1.tifTIFF image, 10.4 MB |
Figure S1: Western blotting analysis of GAPDH content in the cell cultures. Bands corresponding to proteins (top row) and Ponceau S staining used as a loading control (bottom row) are shown from Ctrl, Vehicle, Ethanol 25%, CBD 18.75 μM (minimum dose) and 300 μM (maximum dose) groups. |
| mus28337-sup-0003-TableS1.docxWord 2007 document , 13.8 KB |
Table S1: List of cannabinoids and terpenes in the full-spectrum cannabidiol oil. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. D. Grounds, H. G. Radley, G. S. Lynch, K. Nagaraju, and A. De Luca, “Towards Developing Standard Operating Procedures for Pre-Clinical Testing in the Mdx Mouse Model of Duchenne Muscular Dystrophy,” Neurobiology of Disease 31, no. 1 (2008): 1–19, https://doi.org/10.1016/j.nbd.2008.03.008.
- 2N. Beastrom, H. Lu, A. Macke, et al., “Mdx(5cv) Mice Manifest More Severe Muscle Dysfunction and Diaphragm Force Deficits Than Do Mdx Mice,” American Journal of Pathology 179, no. 5 (2011): 2464–2474, https://doi.org/10.1016/j.ajpath.2011.07.009.
- 3H. G. Radley and M. D. Grounds, “Cromolyn Administration (To Block Mast Cell Degranulation) Reduces Necrosis of Dystrophic Muscle in Mdx Mice,” Neurobiology of Disease 23, no. 2 (2006): 387–397, https://doi.org/10.1016/j.nbd.2006.03.016.
- 4M. Guglieri, P. R. Clemens, S. J. Perlman, et al., “Efficacy and Safety of Vamorolone vs Placebo and Prednisone Among Boys With Duchenne Muscular Dystrophy: A Randomized Clinical Trial,” JAMA Neurology 79, no. 10 (2022): 1005–1014, https://doi.org/10.1001/jamaneurol.2022.2480.
- 5K. Bushby, R. Finkel, D. J. Birnkrant, et al., “Diagnosis and Management of Duchenne Muscular Dystrophy, Part 1: Diagnosis, and Pharmacological and Psychosocial Management,” Lancet Neurology 9, no. 1 (2010): 77–93, https://doi.org/10.1016/S1474-4422(09)70271-6.
- 6L. Bello, H. Gordish-Dressman, L. P. Morgenroth, et al., “Prednisone/Prednisolone and Deflazacort Regimens in the CINRG Duchenne Natural History Study,” Neurology 85, no. 12 (2015): 1048–1055, https://doi.org/10.1212/WNL.0000000000001950.
- 7A. B. Macedo, D. S. Mizobuti, T. A. Hermes, et al., “Photobiomodulation Therapy for Attenuating the Dystrophic Phenotype of Mdx Mice,” Photochemistry and Photobiology 96, no. 1 (2020): 200–207, https://doi.org/10.1111/php.13179.
- 8G. Patterson, H. Conner, M. Groneman, C. Blavo, and M. S. Parmar, “Duchenne Muscular Dystrophy: Current Treatment and Emerging Exon Skipping and Gene Therapy Approach,” European Journal of Pharmacology 947 (2023): 175675, https://doi.org/10.1016/j.ejphar.2023.175675.
- 9N. Elangkovan and G. Dickson, “Gene Therapy for Duchenne Muscular Dystrophy,” Journal of Neuromuscular Diseases 8, no. s2 (2021): S303–S316, https://doi.org/10.3233/JND-210678.
- 10S. Atalay, I. Jarocka-Karpowicz, and E. Skrzydlewska, “Antioxidative and Anti-Inflammatory Properties of Cannabidiol,” Antioxidants (Basel) 9, no. 1 (2019): 21, https://doi.org/10.3390/antiox9010021.
- 11E. A. Thiele, E. D. Marsh, J. A. French, et al., “Cannabidiol in Patients With Seizures Associated With Lennox-Gastaut Syndrome (GWPCARE4): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial,” Lancet 391, no. 10125 (2018): 1085–1096, https://doi.org/10.1016/s0140-6736(18)30136-3.
- 12O. J. Cassol, Jr., C. M. Comim, B. R. Silva, et al., “Treatment With Cannabidiol Reverses Oxidative Stress Parameters, Cognitive Impairment and Mortality in Rats Submitted to Sepsis by Cecal Ligation and Puncture,” Brain Research 1348 (2010): 128–138, https://doi.org/10.1016/j.brainres.2010.06.023.
- 13S. Giacoppo, F. Pollastro, G. Grassi, P. Bramanti, and E. Mazzon, “Target Regulation of PI3K/Akt/mTOR Pathway by Cannabidiol in Treatment of Experimental Multiple Sclerosis,” Fitoterapia 116 (2017): 77–84, https://doi.org/10.1016/j.fitote.2016.11.010.
- 14A. E. Odieka, G. U. Obuzor, O. O. Oyedeji, M. Gondwe, Y. S. Hosu, and A. O. Oyedeji, “The Medicinal Natural Products of Cannabis sativa Linn.: A Review,” Molecules 27, no. 5 (2022): 1689, https://doi.org/10.3390/molecules27051689.
- 15E. C. Berthold, S. H. Kamble, S. R. R. Kanumuri, et al., “Comparative Pharmacokinetics of Commercially Available Cannabidiol Isolate, Broad-Spectrum, and Full-Spectrum Products,” European Journal of Drug Metabolism and Pharmacokinetics 48, no. 4 (2023): 427–435, https://doi.org/10.1007/s13318-023-00839-3.
- 16C. Patikorn, O. Nerapusee, K. Soontornvipart, et al., “Efficacy and Safety of Cannabidiol for the Treatment of Canine Osteoarthritis: A Systematic Review and Meta-Analysis of Animal Intervention Studies,” Frontiers in Veterinary Science 10 (2023): 1248417, https://doi.org/10.3389/fvets.2023.1248417.
- 17H. Thurgur, M. Lynskey, A. K. Schlag, C. Croser, D. J. Nutt, and E. Iveson, “Feasibility of a Cannabidiol-Dominant Cannabis-Based Medicinal Product for the Treatment of Long COVID Symptoms: A Single-Arm Open-Label Feasibility Trial,” British Journal of Clinical Pharmacology 90 (2023): 1081–1093, https://doi.org/10.1111/bcp.15988.
10.1111/bcp.15988 Google Scholar
- 18F. A. Iannotti, E. Pagano, A. S. Moriello, et al., “Effects of Non-Euphoric Plant Cannabinoids on Muscle Quality and Performance of Dystrophic Mdx Mice,” British Journal of Clinical Pharmacology 176, no. 10 (2019): 1568–1584, https://doi.org/10.1111/bph.14460.
- 19C. A. Collins and J. E. Morgan, “Duchenne's Muscular Dystrophy: Animal Models Used to Investigate Pathogenesis and Develop Therapeutic Strategies,” International Journal of Experimental Pathology 84, no. 4 (2003): 165–172, https://doi.org/10.1046/j.1365-2613.2003.00354.x.
- 20A. C. Campos and F. S. Guimarães, “Involvement of 5HT1A Receptors in the Anxiolytic-Like Effects of Cannabidiol Injected Into the Dorsolateral Periaqueductal Gray of Rats,” Psychopharmacology 199, no. 2 (2008): 223–230, https://doi.org/10.1007/s00213-008-1168-x.
- 21C. Y. Matsumura, A. Pertille, T. C. Albuquerque, H. Santo Neto, and M. J. Marques, “Diltiazem and Verapamil Protect Dystrophin-Deficient Muscle Fibers of MDX Mice From Degeneration: A Potential Role in Calcium Buffering and Sarcolemmal Stability,” Muscle & Nerve 39, no. 2 (2009): 167–176, https://doi.org/10.1002/mus.21188.
- 22R. D. Novaes, R. V. Gonçalves, D. C. Marques, et al., “Effect of Bark Extract of Bathysa Cuspidata on Hepatic Oxidative Damage and Blood Glucose Kinetics in Rats Exposed to Paraquat,” Toxicologic Pathology 40, no. 1 (2012): 62–70, https://doi.org/10.1177/0192623311425059.
- 23D. A. Fu and M. Campbell-Thompson, “Periodic Acid-Schiff Staining With Diastase,” Methods in Molecular Biology 1639 (2017): 145–149, https://doi.org/10.1007/978-1-4939-7163-3_14.
- 24T. A. Hermes, P. Fratini, B. G. Nascimento, et al., “Trilobatin Contributes to the Improvement of Myopathy in a Mouse Model of Duchenne Muscular Dystrophy,” International Journal of Experimental Pathology 105, no. 2 (2024): 75–85, https://doi.org/10.1111/iep.12502.
- 25J. Dubach-Powell, “Quantitative Determination of Muscle Fiber Diameter (Minimal Feret's Diameter) and Percentage of Centralized Nuclei,” TREAT-NMD Neuromuscular Network (2014), https://www.treat-nmd.org/wp-content/uploads/2023/07/MDX-DMD_M.1.2.001.pdf.
- 26S. Pagano, M. Coniglio, C. Valenti, et al., “Biological Effects of Cannabidiol on Normal Human Healthy Cell Populations: Systematic Review of the Literature,” Biomedicine and Pharmacotherapy 132 (2020): 110728, https://doi.org/10.1016/j.biopha.2020.110728.
- 27T. Soundara Rajan, S. Giacoppo, D. Scionti, et al., “Cannabidiol Activates Neuronal Precursor Genes in Human Gingival Mesenchymal Stromal Cells,” Journal of Cellular Biochemistry 118, no. 6 (2017): 1531–1546, https://doi.org/10.1002/jcb.25815.
- 28Y. Urasaki, C. Beaumont, M. Workman, J. N. Talbot, D. K. Hill, and T. T. Le, “Potency Assessment of CBD Oils by Their Effects on Cell Signaling Pathways,” Nutrients 12, no. 2 (2020): 357, https://doi.org/10.3390/nu12020357.
- 29J. M. Adams and S. Cory, “The Bcl-2 Protein Family: Arbiters of Cell Survival,” Science 281, no. 5381 (1998): 1322–1326, https://doi.org/10.1126/science.281.5381.1322.
- 30A. J. Primeau, P. J. Adhihetty, and D. A. Hood, “Apoptosis in Heart and Skeletal Muscle,” Canadian Journal of Applied Physiology 27, no. 4 (2002): 349–395, https://doi.org/10.1139/h02-020.
- 31A. Pena-Blanco and A. J. Garcia-Saez, “Bax, Bak and Beyond – Mitochondrial Performance in Apoptosis,” FEBS Journal 285, no. 3 (2018): 416–431, https://doi.org/10.1111/febs.14186.
- 32U. B. Hendgen-Cotta, S. Esfeld, K. Rudi, I. Miinalainen, J. P. Klare, and T. Rassaf, “Cytosolic BNIP3 Dimer Interacts With Mitochondrial BAX Forming Heterodimers in the Mitochondrial Outer Membrane Under Basal Conditions,” International Journal of Molecular Sciences 18, no. 4 (2017): 1–15, https://doi.org/10.3390/ijms18040687.
- 33J. M. Adams and S. Cory, “The BCL-2 Arbiters of Apoptosis and Their Growing Role as Cancer Targets,” Cell Death and Differentiation 25, no. 1 (2018): 27–36, https://doi.org/10.1038/cdd.2017.161.
- 34P. J. Dlugosz, L. P. Billen, M. G. Annis, et al., “Bcl-2 Changes Conformation to Inhibit Bax Oligomerization,” EMBO Journal 25, no. 11 (2006): 2287–2296, https://doi.org/10.1038/sj.emboj.7601126.
- 35S. Pagano, C. Valenti, P. Negri, et al., “Acute and Chronic Cannabidiol Treatment: In Vitro Toxicological Aspects on Human Oral Cells,” Food and Chemical Toxicology 185 (2024): 114513, https://doi.org/10.1016/j.fct.2024.114513.
- 36A. Colell, D. R. Green, and J. E. Ricci, “Novel Roles for GAPDH in Cell Death and Carcinogenesis,” Cell Death and Differentiation 16, no. 12 (2009): 1573–1581, https://doi.org/10.1038/cdd.2009.137.
- 37M. Brzozowa-Zasada, J. Kurek, A. Piecuch, and K. Steplewska, “Correlation Study of GAPDH, Bcl-2, and Bax Protein Immunoexpression in Patients With Colorectal Adenocarcinoma,” Gastroenterology Review/Przegląd Gastroenterologiczny 13, no. 4 (2018): 322–331, https://doi.org/10.5114/pg.2018.79813.
- 38W. Tatton, R. Chalmers-Redman, and N. Tatton, “Neuroprotection by Deprenyl and Other Propargylamines: Glyceraldehyde-3-Phosphate Dehydrogenase Rather Than Monoamine Oxidase B,” Journal of Neural Transmission (Vienna) 110, no. 5 (2003): 509–515, https://doi.org/10.1007/s00702-002-0827-z.
- 39K. Tsuchiya, H. Tajima, T. Kuwae, et al., “Pro-Apoptotic Protein Glyceraldehyde-3-Phosphate Dehydrogenase Promotes the Formation of Lewy Body-Like Inclusions,” European Journal of Neuroscience 21, no. 2 (2005): 317–326, https://doi.org/10.1111/j.1460-9568.2005.03870.x.
- 40S. Li, H. He, L. J. Parthiban, H. Yin, and A. T. Serajuddin, “IV-IVC Considerations in the Development of Immediate-Release Oral Dosage Form,” Journal of Pharmaceutical Sciences 94, no. 7 (2005): 1396–1417, https://doi.org/10.1002/jps.20378.
- 41M. A. Huestis, R. Solimini, S. Pichini, R. Pacifici, J. Carlier, and F. P. Busardo, “Cannabidiol Adverse Effects and Toxicity,” Current Neuropharmacology 17, no. 10 (2019): 974–989, https://doi.org/10.2174/1570159x17666190603171901.
- 42J. Li, J. W. Zagorski, and N. E. Kaminski, “Establishment of a Point of Departure for CBD Hepatotoxicity Employing Human HepaRG Spheroids,” Toxicology 488 (2023): 153469, https://doi.org/10.1016/j.tox.2023.153469.
- 43D. I. Stapleton, X. Lau, M. Flores, et al., “Dysfunctional Muscle and Liver Glycogen Metabolism in Mdx Dystrophic Mice,” PLoS One 9, no. 3 (2014): e91514, https://doi.org/10.1371/journal.pone.0091514.
- 44C. A. Szigyarto and P. Spitali, “Biomarkers of Duchenne Muscular Dystrophy: Current Findings,” Degenerative Neurological and Neuromuscular Disease 8 (2018): 1–13, https://doi.org/10.2147/DNND.S121099.
- 45P. Dowling, S. Murphy, M. Zweyer, M. Raucamp, D. Swandulla, and K. Ohlendieck, “Emerging Proteomic Biomarkers of X-Linked Muscular Dystrophy,” Expert Review of Molecular Diagnostics 19, no. 8 (2019): 739–755, https://doi.org/10.1080/14737159.2019.1648214.
- 46E. G. de Carvalho, W. Corsini, and T. A. Hermes, “Severe Muscle Damage After a Short Period of Ischemia and Reperfusion in an Animal Model,” Surgery 174, no. 2 (2023): 363–368, https://doi.org/10.1016/j.surg.2023.04.033.
- 47E. Isenmann, S. Veit, L. Starke, U. Flenker, and P. Diel, “Effects of Cannabidiol Supplementation on Skeletal Muscle Regeneration After Intensive Resistance Training,” Nutrients 13, no. 9 (2021): 3028, https://doi.org/10.3390/nu13093028.
- 48A. Briguet, I. Courdier-Fruh, M. Foster, T. Meier, and J. P. Magyar, “Histological Parameters for the Quantitative Assessment of Muscular Dystrophy in the Mdx-Mouse,” Neuromuscular Disorders 14, no. 10 (2004): 675–682, https://doi.org/10.1016/j.nmd.2004.06.008.
- 49M. J. Marques, R. Ferretti, V. U. Vomero, E. Minatel, and H. S. Neto, “Intrinsic Laryngeal Muscles Are Spared From Myonecrosis in the Mdx Mouse Model of Duchenne Muscular Dystrophy,” Muscle & Nerve 35, no. 3 (2007): 349–353, https://doi.org/10.1002/mus.20697.
- 50R. D. Mâncio, T. A. Hermes, A. B. Macedo, D. S. Mizobuti, I. F. Rupcic, and E. Minatel, “Dystrophic Phenotype Improvement in the Diaphragm Muscle of Mdx Mice by Diacerhein,” PLoS One 12, no. 8 (2017): e0182449, https://doi.org/10.1371/journal.pone.0182449.
- 51R. D. Mâncio, T. A. Hermes, A. B. Macedo, et al., “Vitamin E Treatment Decreases Muscle Injury in Mdx Mice,” Nutrition 43-44 (2017): 39–46.
- 52T. A. Hermes, R. D. Mâncio, A. B. Macedo, et al., “Tempol Treatment Shows Phenotype Improvement in Mdx Mice,” PLoS One 14, no. 4 (2019): e0215590, https://doi.org/10.1371/journal.pone.0215590.
- 53T. A. Hermes, A. B. Macedo, A. R. Fogaça, et al., “Beneficial Cilostazol Therapeutic Effects in Mdx Dystrophic Skeletal Muscle,” Clinical and Experimental Pharmacology & Physiology 43, no. 2 (2016): 259–267, https://doi.org/10.1111/1440-1681.12521.
- 54L. M. Apolinário, S. C. De Carvalho, H. Santo Neto, and M. J. Marques, “Long-Term Therapy With Omega-3 Ameliorates Myonecrosis and Benefits Skeletal Muscle Regeneration in Mdx Mice,” Anatomical Record (Hoboken) 298, no. 9 (2015): 1589–1596, https://doi.org/10.1002/ar.23177.
- 55S. Acharyya, S. A. Villalta, N. Bakkar, et al., “Interplay of IKK/NF-kappaB Signaling in Macrophages and Myofibers Promotes Muscle Degeneration in Duchenne Muscular Dystrophy,” Journal of Clinical Investigation 117, no. 4 (2007): 889–901, https://doi.org/10.1172/JCI30556.
- 56S. Messina, A. Bitto, M. Aguennouz, et al., “Nuclear Factor Kappa-B Blockade Reduces Skeletal Muscle Degeneration and Enhances Muscle Function in Mdx Mice,” Experimental Neurology 198, no. 1 (2006): 234–241, https://doi.org/10.1016/j.expneurol.2005.11.021.
- 57J. M. Peterson, W. Kline, B. D. Canan, et al., “Peptide-Based Inhibition of NF-κB Rescues Diaphragm Muscle Contractile Dysfunction in a Murine Model of Duchenne Muscular Dystrophy,” Molecular Medicine 17, no. 5–6 (2011): 508–515, https://doi.org/10.2119/molmed.2010.00263.
- 58M. Giovarelli, F. Arnaboldi, S. Zecchini, et al., “Characterisation of Progressive Skeletal Muscle Fibrosis in the Mdx Mouse Model of Duchenne Muscular Dystrophy: An In Vivo and In Vitro Study,” International Journal of Molecular Sciences 23, no. 15 (2022): 1–15, https://doi.org/10.3390/ijms23158735.