Performance fatigability in adults with spinal muscular atrophy treated long-term with nusinersen
Abstract
Introduction/Aims
Persons with spinal muscular atrophy (pwSMA) report progressive muscle weakness but also reduced endurance when performing repetitive tasks in daily life, referred to as “performance fatigability” (PF). Data regarding the effects of the new disease-modifying drugs on PF are scarce. Thus, our main objective was to examine PF in adult ambulatory pwSMA treated long-term with nusinersen.
Methods
Six-minute walk test (6MWT) data from 14 adult pwSMA treated with nusinersen for up to 70 months were retrospectively analyzed to determine PF. Performance fatigability was defined as the percentage change in the distance covered between the last and first minute of the 6MWT. In addition, relationships between PF and other clinical features were assessed.
Results
Performance fatigability was found in 12/14 pwSMA (85.7%) prior to treatment. The mean distance walked in the sixth minute (71.1 m) was shorter than the distance covered in the first minute (81.8 m), corresponding to a mean PF of 13.1% (95% confidence interval (CI): 6.5–19.6, p = .0007). During treatment with nusinersen, there was a mean reduction in PF of 5.6% (95% CI: −10.0 to −1.3, p = .0148). We found no relationship between PF and fatigue as measured by the Fatigue Severity Scale.
Discussion
This study demonstrates the presence of PF as an independent component of motor impairment and as a potential therapeutic target in our cohort of adult ambulatory pwSMA. Furthermore, the observations in our cohort suggest that nusinersen may have a beneficial effect on PF.
Abbreviations
-
- 6MWD
-
- six-minute walk distance
-
- 6MWT
-
- six-minute walk test
-
- CI
-
- confidence interval
-
- FSS
-
- Fatigue Severity Scale
-
- HFMSE
-
- Hammersmith Functional Motor Scale Expanded
-
- PF
-
- performance fatigability
-
- pwSMA
-
- persons with spinal muscular atrophy
-
- RULM
-
- revised upper limb module
-
- SMA
-
- spinal muscular atrophy
-
- SMN
-
- survival of motor neuron
-
- SMN
-
- survival of motor neuron gene
1 INTRODUCTION
5q-associated spinal muscular atrophy (SMA) is an inherited neuromuscular disorder that results in progressive muscle atrophy and weakness because of degeneration of anterior horn cells.1 Nusinersen is an intrathecally administered antisense oligonucleotide that is capable of increasing SMN protein production.2 In pivotal trials, treatment with nusinersen led to meaningful benefits for survival and motor function in infants and children.3, 4 Moreover, the feasibility, safety, and efficacy of nusinersen therapy have also been demonstrated in adult persons with spinal muscular atrophy (pwSMA).5-13
In addition to progressive muscle weakness, pwSMA often report reduced endurance when performing activities of daily life, such as brushing teeth, chewing, writing, wheelchair use, or walking.14, 15 This lack of endurance is characterized by an objectively measurable decline in physical performance during a repetitive task, and is therefore often referred to as “performance fatigability” (PF).15-18 It is important to clearly distinguish PF from “fatigue.”15-17 Fatigue is a well-known phenomenon in many conditions including SMA that affects quality of life, and is often described as a subjective “overwhelming sense of tiredness, lack of energy, feeling of exhaustion, mental, physical, or both.”17-23 In contrast, PF is increasingly considered an important additional dimension of motor impairment in pwSMA that may be linked to neuromuscular junction dysfunction and could represent an independent therapeutic target.14, 15, 24-27 The six-minute walk test (6MWT) has been shown to be a sensitive tool for determining PF in ambulatory pwSMA and is therefore useful for evaluating the potential efficacy of new SMA treatments in this context.27-33 Systematic data on the effect of disease-modifying drugs on PF are scarce. Hence, our main objective was to investigate the effects of long-term nusinersen therapy on PF in adult ambulatory pwSMA.
2 METHODS
2.1 Participants and data collection
Data from adult (defined as 18 years or older) ambulatory pwSMA type 3 treated with nusinersen in the Department of Neurology at the Essen University Hospital, Germany, were retrospectively analyzed. All participants had genetically confirmed SMA, with homozygous deletion of exons 7, 8, or both, or with compound heterozygous mutations. Only pwSMA who were able to walk a distance of at least 10 m without the need for walking aids or other medical conditions that would prevent proper completion of the 6MWT were included. Intrathecal nusinersen therapy was administered according to the approved dosing schedule, with four loading doses on treatment days 0, 14, 28, and 63, followed by maintenance doses every 4 months. Each dose comprised 12 mg of nusinersen. Demographic data, medical history, and clinical findings were obtained before treatment started. Questionnaires were completed and motor assessments were conducted prior to each injection of nusinersen, including the 6MWT, the revised upper limb module (RULM), the Hammersmith Functional Motor Scale Expanded (HFMSE), and the Fatigue Severity Scale (FSS). The HFMSE allows the assessment of activities of daily living in pwSMA and consists 33 items, each scored on a scale from 0 to 2, with a maximum total score of 66 points. Higher scores indicate better motor function.34 The RULM is designed to assess upper limb function in a wide range of pwSMA types 2 and 3. It consists of 19 items graded on a 3-point system (with the exception of one item), with a maximum total score of 37 points. Higher scores indicate better upper limb function.35 The FSS is a questionnaire designed to objectify the degree of subjective fatigue and consists nine items, with higher scores indicating greater fatigue severity.36 The study was approved by the Ethics Committee of the University Duisburg-Essen, Germany (approval number: 18-8071-BO) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was given by every participant included in this study.
2.2 Six-minute walk test
The 6MWT was conducted under standardized conditions based on the American Thoracic Society guidelines.37 Briefly, a 25-m distance was marked out with two cones in a quiet, flat corridor with a solid surface. Participants were instructed to walk as long a distance as possible over a period of 6 min, without jogging or running. They were asked to walk the 25-m course, turn around the distant cone, walk back, go around the cone at the starting point, and repeat this loop as many times as possible. Participants were allowed to slow down or stop and rest in between, but without sitting down. Throughout the test, participants were encouraged every minute with standardized phrases. The time required for each 25-m section, the distance covered in each full minute, and the total distance walked in 6 min (6MWD) were recorded.
Only participants with available results from the baseline 6MWT (first loading dose) and at least one additional 6MWT after a minimum of 6 months (first maintenance dose) were included in the final analysis; the data from the other three loading doses were not evaluated.
2.3 Evaluation of PF
PF was defined as the percentage change in the distance covered between the last and first minute of the 6MWT. Accordingly, PF was calculated from the raw data of the 6MWT using the following formula: with a positive value indicating the presence and a negative value indicating the absence of PF. To evaluate the change in walking capacity with nusinersen treatment, the changes from baseline for PF (expressed as percentage changes) and 6MWD (expressed as changes in meters) were examined for each injection. Furthermore, possible relationships between PF and other clinical features and outcomes were assessed.
2.4 Statistical analyses
Statistical analyses and visualization of results were performed with GraphPad Prism version 10.1.0 (GraphPad Software, San Diego, California, United States) and SAS version 9.4 (SAS Institute, Cary, North Carolina, United States). Continuous variables were described by the median, mean, standard deviation, quartiles and range. Categorical data were expressed by absolute and relative frequencies. The paired t-test was used to calculate the difference between the distance walked in the last minute and the first minute at baseline. Point estimates with 95% confidence intervals (CIs) were calculated for each time point to evaluate the changes in PF and 6MWD. A linear mixed model was used for the variables “changes in PF and 6MWD” to estimate the mean difference in PF and 6MWD during the observation period. In both cases, the model was set up with global intercept as fixed effect and participant as random effect, so that the correlation between all observations within a participant is identical (compound symmetry), while observations between any two participants are uncorrelated. After graphically checking for normal distribution, correlations were assessed using Pearson correlation analysis. Correlation coefficients <0.3 were considered negligible, 0.3 to <0.5 was interpreted as a low correlation and 0.5 to <0.7 as a moderate correlation.38 A two-sided p-value <.05 was interpreted as significant. Due to the nature of the study, all statistical test results should be interpreted as exploratory.
3 RESULTS
3.1 Participant characteristics
From July 2017 to October 2023, 24 ambulatory pwSMA type 3 aged 18 years or older were treated with nusinersen. Of these, two individuals required a walking aid, so the 6MWT was not performed. In addition, the raw data of the baseline 6MWT were not available in six cases, as the initial treatment took place elsewhere and/or only the total 6MWD was recorded. Finally, two individuals received only the first or second loading dose until the data cut-off. Thus, data from 14 pwSMA were included in the final analysis. In all participants, nusinersen was administered via conventional lumbar punctures, with no missed injections or relevant deviations from the recommended dosing schedule. Raw data for the 6MWT were available for 156 of 189 time points examined (82.5%). The longest observation period was 70 months, achieved by three individuals. Detailed participant characteristics, including motor function and results for the 6MWT at baseline, are provided in Table 1.
| Number of participants, n (%) | 14 |
| Female | 10 (71.4) |
| Spondylodesis | 0 (0) |
| Non-invasive ventilation | 0 (0) |
| Nutritional support | 0 (0) |
| Age, years | |
| Mean ± SD | 33.7 ± 11.7 |
| Median (range) | 28.5 (18–58) |
| Height ± SD, cm | 174.6 ± 8.1 |
| Weight ± SD, kg | 69.9 ± 12.8 |
| SMN2 copy number, n (%) | |
| 3 | 2 (14.3) |
| 4 | 11 (78.6) |
| 5 | 1 (7.1) |
| Age at symptom onseta, years | |
| Mean ± SD | 8.3 ± 5.5 |
| Median (range) | 9.5 (1.5–16.0) |
| RULM, mean ± SD | 35.9 ± 2.1 |
| HFMSE, mean ± SD | 48.9 ± 9.1 |
| FSS, mean ± SD | 5.4 ± 1.1 |
| 6MWD, meters | |
| Mean ± SD | 444.5 ± 94.7 |
| Median (range) | 415.5 (325.0–611.9) |
| 6MWT performance fatigability, % | |
| Mean ± SD | 13.1 ± 11.3 |
| Median (range) | 11.0 (−5.0–35.4) |
- Abbreviations: 6MWD, six-minute walk distance; 6MWT, six-minute walk test; FSS, Fatigue Severity Scale; HFMSE, Hammersmith Functional Motor Scale Expanded; RULM, revised upper limb module; SD, standard deviation; SMN2, survival of motor neuron 2 gene;
- a Age at symptom onset was mostly based on the participants' memories.
3.2 Performance fatigability and 6MWD under treatment with nusinersen
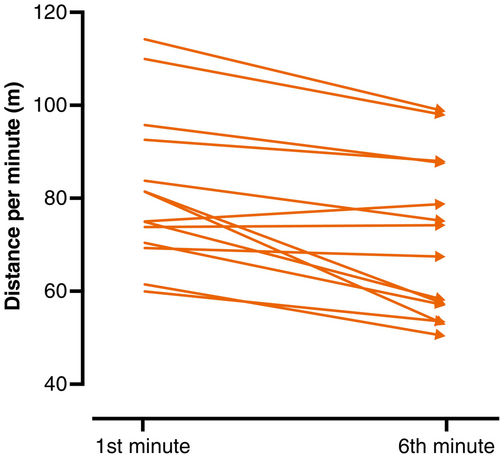
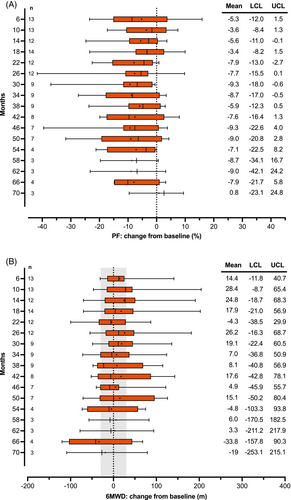
At baseline, PF was detected in 12/14 individuals (85.7%). In the total cohort, the mean distance walked in the sixth minute (71.1 m) was shorter than the distance covered in the first minute (81.8 m), corresponding to a mean PF of 13.1% (95% CI: 6.5–19.6, p = .0007) and a reduction in average speed over 1 min of 0.18 m/s (Table 1, Figure 1). During treatment with nusinersen, there was a mean reduction in PF of 5.6% (95% CI: −10.0 to −1.3, p = .0148) (Figure 2A). Overall, the 6MWD remained stable (mean change 12.4 m; 95% CI: −25.2 to 50.0; p = .4889). In the total cohort, a clinically meaningful change in 6MWD, defined as a change of more than 30 m,33, 39 was only observed for month 66 (Figure 2B). However, this time point was only reached by four individuals. There was a moderate positive correlation between PF and HFMSE score at baseline (Pearson r = 0.537; p = .048), while no other correlation was significant (Table 2).
| Performance fatigability vs. | Pearson r | p-value |
|---|---|---|
| HFMSE | 0.537 | 0.048 |
| 6MWD | −0.343 | 0.230 |
| Fatigue (FSS) | −0.149 | 0.612 |
| SMN2 copy number | −0.407 | 0.149 |
| Female sex | −0.395 | 0.162 |
| Age | −0.391 | 0.167 |
| Body weight | −0.333 | 0.245 |
| Body height | 0.143 | 0.627 |
- Abbreviations: 6MWD, six-minute walk distance; FSS, Fatigue Severity Scale; HFMSE, Hammersmith Functional Motor Scale Expanded; SMN2, survival of motor neuron 2 gene.
4 DISCUSSION
Our study demonstrates the presence of PF in our cohort of adult ambulatory pwSMA. Data on PF in pwSMA are available from other studies, in which participants were younger than in our cohort. Montes et al. observed a PF of 17% in a cohort of 18 ambulatory pwSMA with a mean age of 15.3 years, while Dunaway Young et al. demonstrated a PF of 18.1% in 30 pwSMA with a mean age of 23.7 years.29, 33 These slightly higher PF values may be attributable to differences in disease severity, as the 6MWD and age at symptom onset were lower compared to our cohort, and both studies showed a higher PF in participants with SMA type 3a (age at symptom onset <3 years) than in participants with SMA type 3b (age at onset >3 years). In another study, an average PF of about 13.7% was found in 38 pwSMA with a mean age of 14.1 years, which is also in line with our findings.40 Furthermore, the average speed reduction between the first and last minute in our cohort agrees well with the results of two further studies by Montes et al., in which reductions of 0.11 m/s (11%) and 0.19 m/s (21%) were reported in pwSMA with a mean age of 22 and 31.2 years, respectively.30, 32 Overall, our observations are consistent with studies conducted in younger pwSMA and indicate a high prevalence of PF in older pwSMA as well.
To date, little is known about the effects of new SMA therapies on PF. In a post-hoc analysis of the CS2 and CS12 studies of nusinersen, in which participants received nusinersen at overall lower doses compared to the current approval, Montes et al. investigated changes in PF and 6MWD in 14 children and adolescents with SMA aged 2–15 years.28, 41 At baseline, the total cohort showed a mean 6MWT PF of 38.2%, which is higher compared to our cohort and the studies mentioned above. This deviation is likely due to outliers, as the medians of PF were considerably lower than the mean values and there was a wide range, with a PF of up to 100%. During treatment with nusinersen, Montes et al. observed a median change in PF of −0.1% at day 253 and –3.8% at day 1050. The authors interpreted these findings as modest decreases or stabilization of PF.28 In our cohort, we observed a reduction of PF with a mean change of 5.6%, suggesting a beneficial effect of nusinersen on PF and thus supporting the findings of Montes et al. Although these data should be interpreted with care, the absence of deterioration in our cohort indicates a potential long-term effect of nusinersen therapy on PF, as previous data on the natural course of the disease would suggest a significant worsening of PF even within much shorter periods of time.31
Performance fatigability is increasingly recognized as an important and independent component of motor impairment in pwSMA that has a relevant impact on activities of daily life.14, 15, 24-27 Of note, 6MWT PF has not been observed in healthy controls, treated persons with myasthenia gravis, individuals with Duchenne and Becker muscular dystrophy, or those with glucose transporter protein type 1 deficiency and various mitochondrial disorders, which suggests that PF is a characteristic feature of SMA and not secondary to muscle weakness.14, 16, 30, 31, 42 Remarkably, the presence of PF in pwSMA was demonstrated not only with the 6MWT, but also with other performance tests, such as the Endurance Shuttle Nine Hole Peg Test, the Endurance Shuttle Box and Block Test, and the Endurance Shuttle Walk Test for the upper and lower limbs, respectively.14, 43 Recently, Kant-Smits et al. demonstrated the presence of PF even in the respiratory muscles.44 Van der Heul et al. and others showed that PF also affects mastication in pwSMA, underlining the relevance of PF in various aspects of daily life.45-47 Whether nusinersen therapy also has an effect on these repetitive tasks remains to be investigated.48
Consistent with previous findings, PF and fatigue did not correlate in our cohort, confirming the concept of PF as a distinct and independent dimension of motor dysfunction in pwSMA.17, 21, 42
We found no correlation between PF and 6MWD, which is in line with the observations of Pera et al.,27 who assessed 15 pwSMA type 3 aged 9–66 years, but contrasts with the results of Montes et al.,28 who found an inverse relationship between these parameters in the CS2/CS12 study cohort. During long-term treatment with nusinersen, we observed an overall stable 6MWD. In a previous study on untreated adult pwSMA, a mean annual decline in 6MWD of 9.7 m was observed during the natural course of the disease.49 Thus, our long-term data on 6MWT support results from previous studies in which stabilization, and in some cases even improvement, of 6MWD were observed in adult pwSMA treated with nusinersen — a finding that would not be expected in a progressive disease.5, 6, 50-53
The relatively small number of participants, especially the decrease in sample size over time, is a limitation of our study, as evidenced by a marked increase of confidence intervals particularly during the second half of the observation period. Furthermore, placebo effects cannot be ruled out, since this was a retrospective study without a control group. However, it should be noted that the improvement as well as stabilization of the current clinical state, including fatigue and endurance, is an important therapeutic goal for pwSMA.54, 55
5 CONCLUSION
We have confirmed the presence of PF as an independent component of motor impairment and a potential therapeutic target in adult pwSMA. The observations in our cohort suggest that nusinersen may have a beneficial effect on PF. Prospective studies in larger cohorts are required to confirm this, and PF should be considered as an outcome measure in future studies of SMA therapies.
AUTHOR CONTRIBUTIONS
Benjamin Stolte: Conceptualization; data curation; formal analysis; methodology; investigation; writing – original draft; visualization; project administration. Svenja Neuhoff: Investigation; writing – review and editing. Jaqueline Lipka: Investigation; data curation; writing – review and editing. Melina Schlag: Writing – review and editing; investigation; data curation. Cornelius Deuschl: Investigation; writing – review and editing. Christoph Kleinschnitz: Resources; supervision; writing – review and editing. Tim Hagenacker: Conceptualization; investigation; data curation; supervision; resources; project administration; writing – review and editing; methodology.
ACKNOWLEDGMENT
Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
This research received no specific grants from any funding agency in the public, commercial or non-profit entities.
CONFLICT OF INTEREST STATEMENT
B.S. received advisory honoraria from Alexion and speaker honoraria and travel reimbursement from Biogen. S.N., J.L., M.S., O.V., and C.D. declare no conflicts of interest. T.K. received honoraria for lecturing as well as financial research support from Biogen. C.K. received honoraria for lecturing and consulting as well as financial research support from Ablynx, Almirall, Amgen, Bayer Vital, Bristol-Mayers Squibb, Biotronik, Boehringer Ingelheim, Biogen, CSL Behring, Daiichi-Sankyo, Desitin, Eisai, Ever Pharma, Sanofi Genzyme, Merck Serono, Mylan, Medday, Novartis, Pfizer, Roche, Siemens, Stago, and Teva. T.H. received speaker fees and advisory honoraria from Novartis, Roche and Biogen and research support from Biogen, Roche, and Novartis.
ETHICS STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.