Six-minute walk test as outcome measure of fatigability in adults with spinal muscular atrophy treated with nusinersen
Alessandra Govoni and Giulia Ricci contributed equally to this study.
Abstract
Introduction/Aims
Fatigue (subjective perception) and fatigability (objective motor performance worsening) are relevant aspects of disability in individuals with spinal muscular atrophy (SMA). The effect of nusinersen on fatigability in SMA patients has been investigated with conflicting results. We aimed to evaluate this in adult with SMA3.
Methods
We conducted a multicenter retrospective cohort study, including adult ambulant patients with SMA3, data available on 6-minute walk test (6MWT) and Hammersmith Functional Motor Scale—Expanded (HFMSE) at baseline and at least at 6 months of treatment with nusinersen. We investigated fatigability, estimated as 10% or higher decrease in walked distance between the first and sixth minute of the 6MWT, at baseline and over the 14-month follow-up.
Results
Forty-eight patients (56% females) were included. The 6MWT improved after 6, 10, and 14 months of treatment (p < 0.05). Of the 27 patients who completed the entire follow-up, 37% improved (6MWT distance increase ≥30 m), 48.2% remained stable, and 14.8% worsened (6MWT distance decline ≥30 m). Fatigability was found at baseline in 26/38 (68%) patients and confirmed at subsequent time points (p < 0.05) without any significant change over the treatment period. There was no correlation between fatigability and SMN2 copy number, sex, age at disease onset, age at baseline, nor with 6MWT total distance and baseline HFMSE score.
Discussion
Fatigability was detected at baseline in approximately 2/3 of SMA3 walker patients, without any correlation with clinical features, included motor performance. No effect on fatigability was observed during the 14-month treatment period with nusinersen.
Abbreviations
-
- 6MWT
-
- six-minute walk test
-
- HFMSE
-
- Hammersmith Functional Rating Scale Expanded
-
- NMJ
-
- neuromuscular junction
-
- RULM
-
- revised upper limb module
-
- SMA
-
- spinal muscular atrophy
-
- SMN
-
- survival motor neuron
1 INTRODUCTION
Fatigue, which is the patient's subjective perception of difficulty and exhaustibility in performing motor tasks, is among the most disabling symptoms noted by individuals with spinal muscular atrophy (SMA) with a relevant impact on daily activities, although it is often underestimated in clinical practice.1 It is important to evaluate the impact of SMA therapies on this, and fatigability is the objective parameter to estimate the worsening of motor performance.2 Fatigue has been traditionally classified as peripheral or central fatigue, with the former related to disorders of muscle or neuromuscular junction (NMJ) and the latter associated with diseases affecting the neuronal drive to the muscle. In this study, we will always refer to fatigability as the counterpart of peripheral fatigue.3, 4
To date, there is no consensus on which outcome measure should be used to test fatigability in SMA patients. The 6-minute walk test (6MWT) is among the main outcome measures to test motor performance in SMA patients and has been used also to assess fatigability, most commonly measured as the difference between the distances in meters walked in the first and sixth minute.5, 6
A few studies focused on longitudinal evaluation of fatigability through the 6MWT in untreated SMA3 patients. Among them, Dunaway et al. investigated 30 adult and pediatric SMA patients, detecting similar fatigability at baseline and at the end of the follow-up, although the observation period was limited to 1 month.7 Two studies with mixed pediatric and adult SMA patients included a 12-month follow-up period with contrasting results.5, 8
Treatment with the antisense oligonucleotide nusinersen significantly changed the disease course in infantile and later-onset pediatric forms of SMA,9, 10 leading to drug approval in Europe and the United States. Thereafter, many observational studies reported some benefits from nusinersen, mainly on motor function, even in SMA adult patients.11 In two large cohorts of adult SMA3 patients treated with nusinersen, 6MWT significantly improved across all the time points (6, 10, and 14 months) compared to the baseline,12, 13 but no data on fatigability were included.
Among the studies investigating the effect of nusinersen on fatigability through the 6MWT performance in adult SMA patients,14 conflicting results have been reported, partially due to the different methods used to calculate fatigability and different inclusion criteria. Indeed, Montes et al. showed improvement of fatigability comparing the distances in meters walked in the sixth and first minute of the 6MWT, while Pechman et al. reported no fatigability either at baseline or after 38 months of treatment considering the time needed to walk the first and the last 50 m.14, 15
We aimed here to evaluate fatigability through the 6MWT, before and after treatment with nusinersen in adult SMA3 patients.
2 METHODS
2.1 Patients
The patients included in the study are part of a larger cohort of patients regularly followed through a shared protocol (ID: SMADU; approved by the Ethics Committee of Fondazione IRCCS Istituto Neurologico ‘Carlo Besta’, coordinating center, on July 10, 2019) in 18 Italian secondary or tertiary care centers for SMA. Inclusion criteria were: (1) clinical and molecular diagnosis of SMA3; (2) nusinersen treatment started in adulthood; (3) ability to walk at least a few steps independently or with assistive devices (e.g., cane), but without the assistance of others for the entire follow-up; and (4) clinical data, including 6MWT, available at least at baseline (treatment initiation) and at 6 months. All participants provided written informed consent for the SMADU study, according to the Helsinki declaration.
2.2 Measures of clinical outcomes
The following assessments were evaluated at baseline (T0) before initiating nusinersen therapy, and at 6 (T6), 10 (T10), and 14 (T14) months after starting treatment: 6MWT,5 Hammersmith Functional Motor Scale—Expanded (HFMSE),16 and the revised upper limb module (RULM).17
We investigated fatigability, estimated as 10% or greater decrease in walked distance between the first and sixth minute of the 6MWT, as already reported,18 at baseline and over each time point. Our primary outcome was the evaluation of fatigability at baseline and its changes over the 14-month treatment period with nusinersen.
We also analyzed the total meters walked during the 6MWT at each time point, considering a minimum increase of 30 m as clinically meaningful improvement, a decrease of at least 30 m as meaningful worsening and stability when the difference ranged between −30 and +30 m.7
Due to the great variability in meters covered at baseline, we analyzed the 6MWT trend in two subgroups: longer walker and shorter walker, according to the ability to walk more or less than 300 m at baseline, respectively.7
2.3 Statistical analysis
Shapiro–Wilk's test was applied to check for normality. Distributed Gaussian variables were summarized as mean ± SD. Comparisons between the two subgroups were estimated with the Student's t-test for unpaired data or, if appropriate, with the Mann–Whitney test. Furthermore, an analysis of variance for repeated measures and post hoc analysis were performed to compare data at baseline, and at 6, 10, and 14 months after therapy. We evaluated the associations between HFMSE score and total 6MWT scales, at baseline and T6, T10, and T14, by Spearman's correlation test.
We have defined strong correlations as those with coefficients of 0.70–1.00, moderate correlations as 0.40–0.69, and weak as 0.10–0.39.19 We considered a p-value <0.05 as statistically significant. When necessary, p-level was adjusted for multiple comparisons. Statistical analysis was performed by IBM SPSS® Statistics package for Mac Os X (IBM, Armonk, NY, USA).
3 RESULTS
3.1 6MWT distance analysis
The characteristics of the subjects included in the study combine the diagnosis of SMA3 and the categories of motor milestone according to the World Health Organization (WHO) of “walking with assistance” or “walking alone”.20
The clinical features and performance of HFMSE, RULM, and 6MWT at baseline are summarized in Table 1. Thirteen (27.0%) patients had a treatment period limited to 6 months, 8 (16.7%) to 10 months and 27 (56.3%) to 14 months. None of the patients lost the walking ability during the follow-up period.
| Number of patients (% female) | 48 (56.3%) |
|---|---|
| Age at symptom onset (years) | 8.6 ± 5.4 |
| SMA type 3a/3b | 15/33 |
| Age at baseline (years) | 39.7 ± 13.5 |
| SMN2 copy number | |
| 3 | 10 |
| 4 | 28 |
| Unknown | 10 |
| HFMSE score at baseline | 49 ± 9.7 |
| RULM score at baseline | 35.2 ± 3.1 |
| 6MWT score at baseline | 306.4 ± 140.3 |
- Abbreviations: 6MWT, six-minute walk test; HFMSE, Hammersmith Functional Rating Scale expanded; RULM, revised upper limb module; SMA, spinal muscular atrophy; SMN2, survival motor neuron 2.
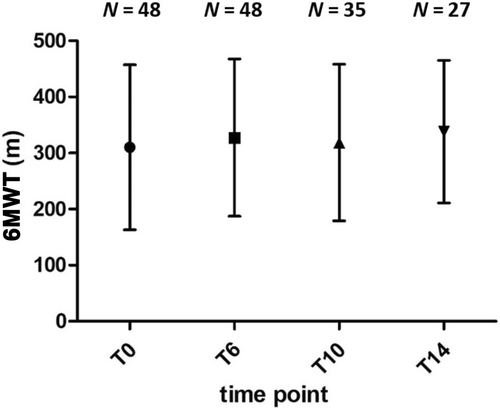
HFMSE score and 6MWT distance were moderately correlated at baseline (r = 0.66; p = 0.02), T6 (r = 0.63; p = 0.15), T10 (r = 0.65; p = 0.02) and strongly correlated at T14 (r = 0.79; p = 0.01). No correlation was found between RULM score and 6MWT distance. Compared to baseline, total meters covered at 6MWT significantly increased at T6 (306.4 ± 140.3 m vs. 321.5.3 ± 134.6 m, p < 0.05), T10 (315.7 ± 136.5 m, p < 0.05) and T14 (333.9. ± 127.3 m, p < 0.05), although with different subgroups size (Figure 1).
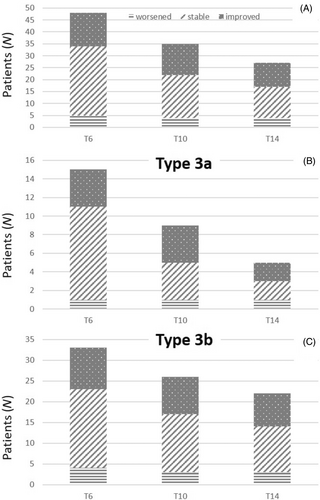
Data on the improvement, stability, and worsening of 6MWT distance during the follow-up are summarized in Table 2 and Figure 2A. At T14, 23 of 27 (85.2%) patients improved or remained stable. No difference in terms of improvement, stability or worsening at T14 was found between patients with SMA type 3a (onset before 3 years) and 3b (onset after 3 years) (Figure 2B,C).
| T0–T14 | T0–T10 | T0–T6 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | Δ | N | % | Δ | N | % | Δ | |
| Improved | 10 | 37 | +75.9 ± 40.9 | 13 | 37.1 | +55.8 ± 19.0 | 14 | 29.2 | +56.5 ± 34.9 |
| Stable | 13 | 48.2 | +7.7 ± 12.1 | 18 | 51.4 | + 7.1 ± 13.1 | 29 | 60.4 | +6.0 ± 11.4 |
| Worsened | 4 | 14.8 | −47.5 ± 18.4 | 4 | 11.4 | −40.7 ± 11.6 | 5 | 10.4 | −48.0 ± 26.1 |
No significant change of 6MWT distance between the baseline and the different time points was observed between longer and shorter walkers (Table 3).
| Longer walkers | Shorter walkers | |
|---|---|---|
| No. of patients | 28 | 20 |
| Type 3a/3b | 5/23 | 10/10 |
| Age at baseline (years) | 34.4 ± 12.7 | 36.0 ± 12.1 |
| Age at symptoms onset (years) | 10.0 ± 5.0 | 6.1 ± 5.3 |
| Improved at T14 | N = 5 Δ 53.4 ± 25.4a | N = 5 Δ 98.4 ± 43.0a |
| Stable at T14 | N = 8 Δ 14.6 ± 8.4a | N = 5 Δ 3.5 ± 8.1a |
| Worsened at T14 | N = 4 Δ 47.5 ± 18.4a | N = 0 |
- Note: In the last three rows: number of subjects (N) and average differences (Δ) of 6MWT walked between T14 with T0 in the two sub-groups.
- a Not statistically significant.
In the Longer-walker subgroup, 17 patients were followed-up for 14 months (Table 3); in this subgroup, the age at the beginning of nusinersen therapy was significantly inversely correlated with the clinically meaningful improvement after 14 months of treatment (p = 0.016), whereas no significant correlation was found with the age at presentation. The 13 of 17 patients who remained stable or experienced improvement at T14, had a mean age at onset of 8.9 ± 3.9 years and started therapy at a mean age of 33.2 ± 12.6 years. In the Shorter-walker subgroup, the 14-month follow-up data were available only for 10 patients (Table 3); none of them showed 6MWT worsening. In this small subgroup, no significant correlation was detected between the 6MWT changes over the follow-up period and the age at onset or at the beginning of nusinersen.
3.2 Fatigability analysis
Meters walked per each minute were available in 38 (79.2%) patients (respectively 38 patients at T0 and T6, 28 at T10, and 23 at T14). The difference of meters walked at minutes 1 and 6 by 6MWT showed a statistically significant decline suggestive of fatigability both at baseline and at all times thereafter (p < 0.05) according to the definition of fatigability in adult and pediatric patients.15, 18 The average fatigability calculated at different time points is summarized in Table 4. In all SMA groups, fatigability did not significantly change across the different time points (p = 0.8). No significant difference in terms of fatigability at baseline was found between patients carrying 3 (n = 5/10) or 4 (n = 14/28) SMN2 copies.
| No. of patients | Distance walked in minute 1 (m) | Distance walked in minute 6 (m) | Fatigability (%) | |||
|---|---|---|---|---|---|---|
| All patients | SMA 3a patients | SMA 3b patients | ||||
| T0 | 38 | 62.8 ± 20.2 | 53.5 ± 22.3 | 17.3 | 26.8 | 14.3 |
| T6 | 38 | 65.5 ± 18.6 | 57.7 ± 21.1 | 16.5 | 17.9 | 12.6 |
| T10 | 28 | 64.5 ± 19.1 | 54.4 ± 23.0 | 17.9 | 29.9 | 15.9 |
| T14 | 23 | 65.6 ± 17.7 | 56.1 ± 21.9 | 18.6 | 25.7 | 15.0 |
| p | 0.7 | 0.6 | 0.8 | / | / | |
Patients presenting with fatigability at baseline were the majority of the sample (n = 26/38–68%), among which 20 were SMA type 3b and 6 SMA type 3a; 14 of them carried 4 SMN2 copies, and 5 patients had 3 copies. Half of these patients were females (13/26) and the mean age at onset was 9.4 ± 5.0 years. The remaining 12 patients, who did not show fatigability during the 6MWT at baseline, were mostly women (8 vs. 4), although no significant effect of sex was found; among them, 75% were SMA type 3b (9/12), nine with four SMN2 copies and three with unknown SMN2 copy number; the mean age at disease onset was 8.8 ± 5.4 years. In the cohort, there was no correlation between the fatigability at baseline and the probability of reaching clinically meaningful improvement during follow-up (p = 0.7). By Spearman's correlation test, there was no correlation between fatigability and age at disease onset (r = 0.33, p = 0.12), age at baseline (r = 0.01, p = 0.99), HFMSE values (r = 0.34, p = 0.28), or 6MWT distance (r = 0.38, p = 0.05) at baseline.
4 DISCUSSION
In our study, SMA3 walker patients demonstrated fatigability at baseline, but we did not find a significant change in fatigability during the treatment period with nusinersen. This may be explained by lack of effect of nusinersen on fatigability in SMA or may be related to other factors such as motor compensatory mechanisms or energy-conserving strategies during ambulation.
Earlier disease onset as in SMA3a has been associated with greater fatigability18; in our study, we did not find a statistically significant difference of fatigability between SMA 3a and SMA 3b. No significant association was found between fatigability rate and SMN2 copy number, gender, age, and motor function at baseline. Hence, to date, factors predicting fatigability in SMA3 walker patients still need to be clarified.
Unfortunately, the few studies focusing on fatigability available in literature include different methods and tools and there is currently no international consensus on the methodology to assess fatigability in adult and pediatric SMA patients.
Contrary to other reports,7, 8, 15 we did not find any correlation between the 6MWT total distance at baseline and fatigability; this discrepancy may be explained by the different age ranges of the study populations, the different cohort sizes, and the different methods used to evaluate the fatigability.
Recently, Pechman et al. reported a study on a large cohort of adult SMA3 patients and did not show any fatigability at baseline or during the treatment period.14 Conversely, fatigability at baseline was found in approximately two-thirds of the patients in our cohort, similar to other studies4, 18 suggesting that fatigability assessed through 6MWT represents a frequent feature of SMA3 walker patients. Again, contrasting results are probably related to different baseline features and different methods of defining fatigability by the 6MWT.14
Data from literature and our study still fail to define whether nusinersen may be effective in reducing fatigability in walker SMA patients. Persistence of fatigability in our SMA patients treated with nusinersen is probably related to multiple factors affecting the NMJ function and involving other pathological mechanisms, such as muscle fiber metabolism and mitochondrial function.
NMJ impairment was observed in an SMA mouse model21 and reported in a relevant proportion of SMA3 patients performing the 6MWT and undergoing repetitive nerve stimulation, further suggesting a crucial role of fatigability in SMA patients.17 To this purpose, symptomatic drugs targeting the NMJ have been recently tested in pharmacological clinical trials, with little or no beneficial effect.22-24 However, these few trials present some limitations in the form of small sample sizes, the use of outcome measures not specific for fatigability, short observation periods, and the inclusion of patients with heterogeneous phenotypes in terms of motor function. Data on large cohort of patients, with stratification according to disease severity, would be helpful to verify the efficacy of these symptomatic treatments as add-on therapies in association with disease-modifying drugs.
Our data showed an improvement of 6MWT in 37% of patients after 14 months of treatment in agreement with previous studies.13, 25 In addition, about half of the patients showed stable performance on the 6MWT. The improvement we observed may be an effect of the drug considering the natural history of SMA, according to which a worsening of 7.8 m per year is expected.26 This further supports a potential effect of nusinersen in walker patients.
Notably, the increase in 6WMT distance during the treatment period (Figure 1), as already reported,12, 13 suggested that the whole distance covered in 6MWT is not generally related to fatigability, but to global motor function.18
This study has some limitations, including the relatively short follow-up period and the decreasing number of patients with available fatigability data over time.
These limitations are related to the retrospective nature of the protocol and warrant further studies in adult SMA patients to better investigate the presence of fatigability, its predictive factors, progression, and response to treatments. Moreover, our study focused on the analysis of fatigability as a correlate of peripheral fatigue; in this regard, further analysis, such as interpolated twitch technique studies, will be necessary in order to investigate the presence of central fatigue and its variations in response to new therapies in SMA patients.27, 28
AUTHOR CONTRIBUTIONS
Alessandra Govoni: Conceptualization; investigation; writing – original draft; writing – review and editing. Giulia Ricci: Conceptualization; investigation; writing – original draft; writing – review and editing. Silvia Bonanno: Investigation. Luca Bello: Investigation. Francesca Magri: Investigation. Megi Meneri: Investigation. Francesca Torri: Investigation. Claudia Caponnetto: Investigation; writing – review and editing. Luigia Passamano: Investigation; writing – review and editing. Marina Grandis: Investigation; writing – review and editing. Francesca Trojsi: Investigation. Federica Cerri: Investigation; writing – review and editing. Giulio Gadaleta: Investigation. Giuliana Capece: Investigation. Luca Caumo: Investigation. Raffaella Tanel: Investigation. Elena Saccani: Investigation. Veria Vacchiano: Investigation. Gianni Sorarù: Investigation. Eustachio D'Errico: Investigation. Irene Tramacere: Investigation. Sara Bortolani: Investigation. Enrica Rolle: Investigation. Cinzia Gellera: Investigation. Riccardo Zanin: Investigation. Mauro Silvestrini: Investigation; writing – review and editing. Luisa Politano: Investigation; writing – review and editing. Angelo Schenone: Investigation; writing – review and editing. Stefano Carlo Previtali: Investigation; writing – review and editing. Angela Berardinelli: Investigation; writing – review and editing. Mara Turri: Investigation. Lorenzo Verriello: Investigation. Michela Coccia: Investigation; writing – review and editing. Renato Mantegazza: Investigation; writing – review and editing. Rocco Liguori: Investigation; writing – review and editing. Massimiliano Filosto: Investigation; writing – review and editing. Maria Antonietta Maioli: Investigation. Isabella Laura Simone: Investigation. Tiziana Mongini: Investigation; writing – review and editing. Stefania Corti: Investigation; writing – review and editing. Maria Laura Manca: Methodology; writing – review and editing. Elena Pegoraro: Investigation; writing – review and editing. Gabriele Siciliano: Investigation; writing – review and editing. Giacomo Pietro Comi: Investigation; writing – review and editing. Lorenzo Maggi: Conceptualization; investigation; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
We would like to thank all the patients and their families. Eustachio D'Errico, Massimiliano Filosto, Marina Grandis, Rocco Liguori, Lorenzo Maggi, Luisa Politano, Stefano C. Previtali, Angelo Schenone, Gabriele Siciliano, and Veria Vacchiano are members of the European Reference Network for Neuromuscular Diseases (ERN-NMD). The authors wish to thank Rosalind Hendricks, Assistant Biomedical Librarian of Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy, for correcting the English manuscript.
FUNDING INFORMATION
This work was supported/partially supported by the Italian Ministry of Health (RRC).
CONFLICT OF INTEREST STATEMENT
Michela Coccia has received honoraria for speaking, advisory boards and compensation for congress participations from Roche Biogen and Novartis outside the submitted work. Giacomo Pietro Comi attended to advisory boards of Sanofi, Novartis, Roche, Sarepta and PTC Therapeutics. Stefania Corti has participated to scientific advisory board of Novartis, not related to submitted work. Lorenzo Maggi has received honoraria for speaking, advisory boards and compensation for congress participations from Sanofi Genzyme, Roche and Biogen, Amicus Theraputics, Alexion, Janssen, Lupin, outside the submitted work. Rocco Liguori has received honoraria for speaking, advisory boards and compensation for congress participations from Argenx BV, Alexion, UCB Pharma, Amicus Therapeutics, Editree, Summeet, Edra S.p.A., Med Stage. None of the other authors has any conflict of interest to disclose.
ETHICS STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.