Gelsolin variant amyloidosis mimicking progressive bulbar palsy
Funding information National Research Foundation of Korea, Grant/Award Number: 2018M3C7A1056512
Hereditary gelsolin amyloidosis, a rare autosomal dominant hereditary disease, is caused by pathogenic variants in the gelsolin (GSN) gene.1 It is characterized by slowly progressive cranial and peripheral nerve degeneration, corneal lattice dystrophy, and loss of skin turgor (cutis laxa).1 However, it may be misdiagnosed as slowly progressive motor neuron disease (MND) or progressive bulbar palsy (PBP) owing to symptomatic similarity.2 Here, we describe two patients with GSN amyloidosis with a long-lasting undiagnosed MND-mimicking presentation that was eventually confirmed by genetic testing.
Patient A had visited our MND clinic 2 y ago. She was a 58-y-old woman with a 6 y history of progressive facial diplegia, slurred speech, difficulty swallowing water, and tongue atrophy. Neurological examination revealed bilateral lower facial weakness, myokymia, tongue atrophy, and dysarthria. There were no sensory abnormalities, and reflexes were normal in all extremities. There was no family history of neuromuscular disease. She had dry eye syndrome, but no other past medical history. Blood tests for thyroid disease, autoimmune disease, vasculitis, paraneoplastic syndrome, paraproteinemia, and infectious diseases were normal or negative. Sensory and motor nerve conduction studies of the upper and lower extremities were normal. Positive sharp waves and fibrillation potentials were noted in the orbicularis oris and masseter muscles on needle electromyography (EMG). Brain magnetic resonance imaging was unremarkable. Genetic testing using the TruSight One Sequencing Panel (Illumina Inc., San Diego, CA), which contains 4811 genes related to MND, did not reveal a known pathogenic variant.
Patient B was a 60-y-old woman with a 4 y history of dysarthria, tongue atrophy, and facial twitching. Her family members had similar features without a diagnosis. Neurological examination revealed facial diplegia with ptosis, decreased skin turgor, twitching of facial muscles, tongue atrophy, and dysarthria with normoactive reflexes in all extremities (Figure 1). There were no pathologic upper motor neuron signs. No sensory or visual symptoms were reported. Motor and sensory nerve conduction studies of the right upper and lower extremities were normal. Needle EMG revealed fibrillation potentials, positive sharp waves, and large motor unit potentials with reduced recruitment in the masseter and tongue muscles. Her grandmother (I-2), mother (II-2), and elder brother (III-1) showed similar symptoms (Figure 2B). We performed whole-exome sequencing and detected a pathogenic variant, c.640G > T (p.D214Y), in the GSN gene of the patient and sibling III-1. Sibling III-3 was asymptomatic and had no pathological variants (Figure 2D).
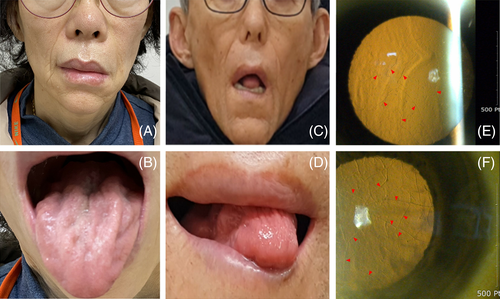
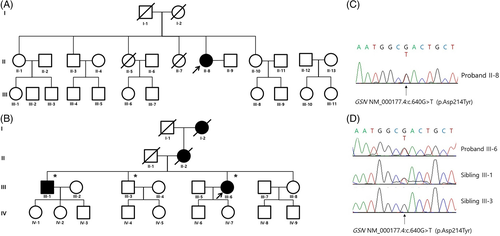
After noting the genetic findings in patient B, we performed whole-exome sequencing on patient A. The GSN variant (p.D214Y) was identified (Figure 2A and C). Subsequently, both patients were diagnosed with corneal lattice dystrophy by an ophthalmologist using slit-lamp examination with the retro-illumination technique (Figure 1E and F).
To date, several pathogenic GSN variants have been reported, including peripheral neuropathy, corneal lattice dystrophy (c.640G > A, c.640G > T, c.1631T > G), and chronic kidney disease (c.580G > A, c.633C > A). Recently reported rare phenotypes are seizures (c.100dupG), dermatomyositis-like symptom (c.1375C > G), and cardiac involvement (c.1783G > A, c.1732G > C). Most of the reported cases of GSN variants are familial.3 In diagnosing hereditary gelsolin amyloidosis, positive family history and characteristic ophthalmic findings, such as amyloid deposition on the cornea, are important early diagnostic clues.1 However, as observed in patient A, it is difficult to diagnose even a known GSN variant if there is no family history. Corneal lattice dystrophy is another unique ophthalmic characteristic of hereditary GSN amyloidosis and is reported to be an initial manifestation of gelsolin amyloidosis. However, patients A and B were not diagnosed with corneal lattice dystrophy through routine ophthalmologic examination before the genetic confirmation of GSN amyloidosis.
The absence of family history or subtle eye problems may obscure the diagnosis of GSN amyloidosis in patients with lower cranial motor neuropathies or PBP. Notably, Caress et al. mentioned that hereditary gelsolin amyloidosis is easily omitted in the differential diagnosis of amyotrophic lateral sclerosis or bulbar MND.4 Furthermore, it has not been listed as a disease mimicking MND according to consensus guidelines and some research articles.5
Genetic testing is now widely available. This has allowed detection of de novo mutations in patients without a family history.6 In patient A, we could not establish an accurate diagnosis without genetic testing. Therefore, whole-exome sequencing should be considered for patients with slowly PBP, even in the absence of family history, to prevent misdiagnosis.
ACKNOWLEDGMENTS
This study was supported by the National Research Foundation (NRF) of Korea funded by Korea's Ministry of Science (2018M3C7A1056512).
CONFLICT OF INTEREST
None of the authors have any conflicts of interest to disclose.
ETHICS STATEMENT
We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
Data openly available in a public repository that issues datasets with DOIs.
REFERENCES
Abbreviations
-
- EMG
-
- electromyography
-
- GSN
-
- gelsolin
-
- MND
-
- motor neuron disease
-
- PBP
-
- progressive bulbar palsy