Comparison between surface electrodes and ultrasound monitoring to measure TMS evoked muscle contraction
Funding information: Reta Lila Weston Trust
Abstract
Introduction
Transcranial magnetic stimulation (TMS) is widely used to explore cortical physiology in health and disease. Surface electromyography (sEMG) is appropriate for superficial muscles, but cannot be applied easily to less accessible muscles. Muscle ultrasound (mUS) may provide an elegant solution to this problem, but fundamental questions remain. We explore the relationship between TMS evoked muscle potentials and TMS evoked muscle contractions measured with mUS.
Methods
In 10 participants, we performed a TMS recruitment curve, simultaneously measuring motor evoked potentials (MEPs) and mUS in biceps (BI), first dorsal interosseous (FDI), tibialis anterior (TA), and the tongue (TO).
Results
Resting motor threshold (RMT) measurements and recruitment curves were found to be consistent across sEMG and mUS.
Discussion
This work supports the use of TMS-US to study less accessible muscles. The implications are broad but could include the study of a new range of muscles in disorders such as amyotrophic lateral sclerosis.
Abbreviations
-
- ALS
-
- amyotrophic lateral sclerosis
-
- BI
-
- biceps
-
- FDI
-
- first dorsal interosseous
-
- MEP
-
- motor evoked potential
-
- MSO
-
- maximum stimulator output
-
- mUS
-
- muscle ultrasound
-
- RMT
-
- resting motor threshold
-
- sEMG
-
- surface electromyography
-
- SICF
-
- short interval intracortical facilitation
-
- SICI
-
- short interval intracortical inhibition
-
- TA
-
- tibialis anterior
-
- TMS
-
- transcranial magnetic stimulation
-
- TO
-
- tongue
1 INTRODUCTION
Transcranial magnetic stimulation (TMS) is a non-invasive cortical stimulation technique that explores cortical physiology in health and a broad range of diseases.1 At the core of nearly all TMS protocols is the measurement of timing and magnitude of evoked muscle contractions using surface electromyography (sEMG). This defines the resting motor threshold (RMT), a measure of corticospinal excitability, on which several TMS protocols are based.
TMS output is typically recorded using sEMG and is therefore limited to accessible muscles while excluding potentially important harder to access muscles. For instance, surface electrodes are insufficient for monitoring motor evoked potentials (MEPs) in muscles of interest in the bulbar region, notably the tongue. Monitoring the cortical physiology of the tongue may be important in amyotrophic lateral sclerosis (ALS) because about 20%-30% of people present primarily with bulbar involvement and many others who present initially with symptoms elsewhere later go on to develop bulbar symptoms.2 Tongue (TO) monitoring during TMS is not usually performed as the sEMG is inconvenient for the subject, and it is impossible to discriminate individual TMS-evoked muscle contractions of the bulbar region from elicited electric patterns.3 Furthermore, the cross-talk phenomenon, where potentials from unselected muscles contribute to the evoked EMG signal through volume conduction, could be avoided using ultrasound in TMS studies aiming to isolate responses of small muscles, such as first dorsal interosseous (FDI).
Muscle ultrasonography (mUS) is a well-established muscle visualization tool that may overcome sEMG limitations of accessibility to certain muscles. mUS has the ability to register muscle movement in real-time, making it an excellent tool for detecting small intra-muscular movements, such as muscle fasciculations.4-6 Studies indicate that mUS may have advantages over sEMG examination in some muscles, particularly the TO.7, 8 Recently, there have been further developments in robust computational methods for detecting and analyzing fasciculations using mUS.9 Interestingly, ultrasound of the TO has been used previously to study TO kinematics, and more specifically TMS evoked tongue movement synergies.3 Ultrasound has become a powerful tool that can provide a broader view of typically difficult to access muscles, although it is unknown whether mUS can be used to measure TMS-evoked muscle contraction.
In this proof of concept study, we aim to understand the relationship between sEMG and mUS to investigate whether we can use mUS to measure TMS-evoked muscle contraction. We expect that for difficult to access muscles, such as the TO, mUS recordings are a more reliable measure of MEPs than traditional sEMG electrodes. We hypothesize that sEMG and mUS TMS evoked measurements will be highly correlated across a range of muscles. This will provide a foundation to use TMS-mUS to explore less accessible muscles. In a healthy population, we expect a positive correlation between sEMG and mUS measures.
2 METHODS
2.1 Subjects
Inclusion criteria were healthy, right-handed, consenting adults of either gender. Participants were excluded from the study if they had been diagnosed with recent trauma, such as fractures or structural pathologies of the right FDI, the right biceps (BI), the right tibialis anterior (TA), or the TO. Implanted metal objects or devices (cochlear implant or deep brain stimulator) in the brain or skull were prohibited. Individuals taking pro-epileptogenic medication or a history of spinal surgery were also excluded from the study. No subject had contraindications to TMS or ultrasound, which was assessed by a screening questionnaire. The study was approved by the University College London Ethics Committee.
2.2 Experimental setup
The FDI, BI, TA, and TO were studied. Standard recruitment curves were acquired in each muscle using both sEMG and mUS. Participants were seated and subjected to 100 single pulses of TMS (10 blocks of 10 single-pulse TMS simulations at 10% intervals 10%-100% maximum stimulator output) over the optimal point for stimulation, that is typically referred to as the “hotspot,” corresponding to their right FDI, BI, TA, and TO. In an effort to reduce pulse anticipation, stimulation intensity was randomized and stimulation intervals included random jitter, derived from a stochastic Gaussian process that randomly adjusted the time interval of 6-8 s between TMS pulses.
2.3 TMS
TMS was carried out with a Magstim 2002 (Magstim Co., Whitland, Dyfed, UK) magnetic stimulator using a standard commercial figure-of-eight coil (double 70 mm alpha coil). The coil was placed over the M1, tangentially to the scalp, 45° from the midsagittal line, approximately perpendicular to the central sulcus with current direction in a posterior-anterior direction. Stimulation target “hotspot” was determined by varying the coil direction and intensity over the motor homunculus, marking locations corresponding to maximum MEP output of the four desired muscles (BI, FDI, TA, TO).
2.4 sEMG
sEMG was monitored by disposable surface electrodes (WhiteSensor 40713, Ambu®, Denmark). Electrodes were placed bilaterally on the muscle bellies of BI, FDI, and TA (as seen on Supporting Information Figure S1, which is available online). For TO recordings, the recording electrode was placed along the median sulcus of the TO, and the reference electrode was attached to the posterior-medial aspect, proximal to the frenulum of the tongue. Electrodes were connected to an isolated amplifier system model D360 (Digitimer Ltd, Welwyn Darden City, Hertfordshire, UK), which recorded MEP data on Signal software (Version 7, Cambridge Electronic Design Ltd, Cambridge, UK).
RMT on the non-dominant (right) hemisphere was established. In efforts to standardize international guidelines, an individual RMT was defined as the stimulator output at which at least 5 out of 10 consecutive trials produced an MEP amplitude of at least 50 μV.
2.5 mUS
Ultrasound recordings were acquired using a Telemed LS128 (Telemed, Vilnius, Lithuania) ultrasound imaging system. Imaging was carried out by securing the ultrasound transducer parallel to muscle fibers of the FDI, TA, and BI, and under the chin in a cross-sectional orientation to image TO fibers (Figure S2). The transducer (HL9.0/40/128Z-4) had a real-time imaging rate of ~63 fps at time of recording and a frequency range set to 7 MHz for skeletal muscle analysis. The system was set in B-mode with harmonics on and focal depth, focus, and gain defined per muscle to optimise image quality. These settings were kept consistent between muscle types. Spectra 360 electrode gel (Parker Laboratories Inc., Fairfield, NJ, USA) was used as an acoustic medium when applying the ultrasound transducer over each of the muscles, or under the chin for tongue recordings.
2.6 Data processing and analysis
2.6.1 MEP data processing
MEP peaks were extracted from the evoked sEMG signal using Signal software (Cambridge Electronic Design Ltd, Cambridge, UK) and analyzed offline, using R (Version 4.0.2) in RStudio (Version 1.1.463, RStudio, Inc., Boston, MA, USA). Individual and group mean MEPs were calculated at each stimulation frequency.
2.6.2 mUS data processing
Computational image analysis approaches were used to quantify TMS evoked twitches in recorded ultrasound image sequences. For the BI, FDI, and TA a Lucas-Kanade feature tracking10 based approach was used to capture the muscle tissue displacements evoked by TMS, as described elsewhere.11, 12 Briefly, this involved placing an evenly spaced 80 × 100 feature grid on the image. An iterative search to identify the position of each feature in the subsequent image was then completed and the total movement of all features between the two images calculated. The process was repeated for all recorded images in a sequence. The resulting total displacement values were smoothed (lowpass butterworth filter 5 Hz cutoff) and peaks greater than the threshold (signal mean + 0.25 × SD) identified. The time of each peak, identified from the individual frame timestamps,13 and magnitude (pixels) were recorded for statistical analysis.
Underlying movements related to breathing and swallowing were captured in the ultrasound image sequences of the TO. This caused displacement in the feature tracking results that influenced magnitude measures of evoked muscle contractions. Therefore, for the TO, a recent foreground detection based approach was used.9 This approach assumes that the intensity value of each pixel hardly varies across images of the muscle at rest, but when a twitch is evoked there is a local, transient variation in the intensity value of the pixels in the area of the image in which the muscle twitch occurred. The intensity value of each pixel in the first 500 images is, therefore, used to construct a Gaussian mixture model.14 Here three distributions were used. The distributions were weighted, based on the proportion of the image sequence in which its intensities occur. Intensities in more highly weighted distributions occur more commonly (ie, when the muscle is at rest), while intensities in less weighted distributions occur less commonly (ie, only during an evoked twitch). The mixture model was then used to categorise pixels in all subsequent images (>500) as either background or foreground, with the mixture model updating to adapt to any repetitive changes in pixel intensity value (eg, related to breathing patterns). Images recorded during an evoked twitch therefore, contained dense clusters of foreground pixels located in the area the muscle tissue displacement occurred. Connective components were used to analyze the density of foreground pixels in each image, and more sparsely distributed foreground pixels (ie, resulting from noise) were discarded.15 The final result was, therefore, a 1D signal of the number of foreground objects in each image frame, with greater numbers of foreground objects indicating large muscle tissue displacement. Pearson correlations determined the relationship between sEMG and mUS.
3 RESULTS
3.1 Subjects
Ten right-handed participants (5 females, mean age 23.0 years, SD 1.6 years) were recruited. The TO was studied in a subset of eight participants.
3.2 RMT
The results are presented in Figure 1. In BI, FDI, TA, and TO the MEP amplitude, as measured by sEMG, and the tissue displacement, as measured by mUS, increased with stimulation intensity, after reaching a resting motor threshold intensity. RMT intensity varied among individuals. Average RMT intensities were lowest in FDI, where most individuals responded with changes in amplitude at 30%-50% maximum stimulator output (MSO). Individual BI and TO RMT ranged from as low as 40% MSO and in most cases reach threshold intensity by 70% MSO. RMTs were highest in TA. With the exception of one individual, whose threshold was below 60% MSO, other individuals had an RMT of 70%-90% MSO in TA. RMT intensity measurements appeared consistent across MEP and mUS methods on an individual and a group level.
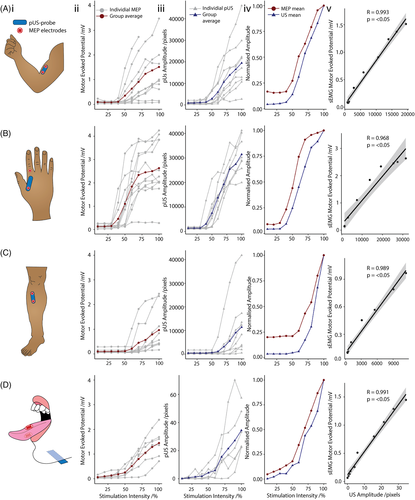
3.3 Amplitude pattern
Upon visual inspection of Figure 1v, changes in MEP amplitude and mUS tissue displacement were closely aligned, following a similar sigmoidal pattern in BI, FDI, TA, and TO with increasing TMS stimulation intensity. The MEP amplitude curve flattened out more towards the high stimulation intensities, whereas this effect was not apparent in the mUS tissue displacement curves. At high intensities of TMS, the sEMG response was polyphasic so that measuring peak power did not quantify the total muscle activity very well. Hence, there was saturation even though the twitch became larger and larger. This is particularly highlighted in FDI and BI muscles. All tested muscles showed a very high correlation in the changes in amplitudes with respect to stimulation intensity, depicted by sEMG and mUS.
4 DISCUSSION
In this study, we demonstrate that recruitment curves for TMS evoked mUS muscle contraction strongly relate to sEMG. Importantly, the motor evoked threshold defined by mUS is virtually the same as that defined by sEMG. This study shows that mUS is feasible in the TO and may be feasible in other muscles that are difficult to measure using sEMG. While applications of mUS could be broad, it could be applied to examine the TO in stroke or ALS. This may be particularly useful in clinical studies involving the TO when repeated measures are required and sEMG may be considered challenging. This work, therefore, provides a novel alternative to sEMG to measure TMS evoked muscle contraction.
The close relationship of sEMG output and mUS supports the use of mUS in TMS studies. Future experiments need to explore the relationship of more complex TMS protocols, such as short-interval intracortical inhibition (SICI), although we expect the relationship to be conserved. Adapting mUS for use in these types of protocols will enable us to probe excitatory and inhibitory corticomotoneuronal integrity in tracts corresponding to a broader range of muscles. Further studies could additionally examine correlations between MEP duration, latency, and changes in muscle contractions as measured by muscle ultrasound. There are very few studies that have explored TMS of difficult-to-access muscles, such as the TO.
TMS-mUS may be particularly useful in clinical disorders that affect the TO, such as ALS and stroke. There are very few studies that have explored TMS of the TO, and none that have characterized corticospinal tracts relating to the bulbar region in ALS. We suspect this is largely due to the difficulty in recording from this region using sEMG electrodes. ALS patients often experience bulbar symptoms and monitoring its progression is currently left to clinical assessments. Understanding the interaction of excitatory and inhibitory networks in the corticomotoneuronal pathways corresponding to bulbar regions may be crucial in the understanding of ALS neurophysiology. In stroke, TMS-mUS could allow studies to explore the longitudinal cortical reorganisation that occurs within the TO region, similar to those studies that map cortical reorganisation in the hand.16
Our findings suggest that mUS is a real alternative to sEMG. The main advantages for using mUS, for the patient, is that unlike sEMG, setting up mUS to view the TO, or indeed other muscles, is painless, comfortable, and non-invasive. We found that TO sEMG electrodes were not only uncomfortable for our participants, making them more likely to move their TO during the study, but that throughout the TMS session, saliva builds up, causing difficulties with electrode adhesion and recording evoked sEMG signals. In many conditions, swallowing ability and effectiveness are reduced, which can lead to excess saliva, which would make the use of electrodes even more challenging. mUS is a good solution to removing discomfort from TMS experiments in the TO.
We propose this technique may have additional applications in less accessible muscles. We recognize, in spite of advances in tissue harmonics of high frequency transducers,17 mUS accuracy for deep muscles is not as good as for superficial muscles due to overlying connective tissue, bones, and muscles. A lower frequency transducer (3-5 Hz) would be a suitable alternative for monitoring deeper structures. There are many examples where deeper muscles, such as the tibialis posterior18 and the soleus,19, 20 have been studied using ultrasound, both to capture fascicle mechanics and monitor changes in muscle thickness for running. While neither of these examples relate to TMS-evoked movement, they demonstrate that quantifiable information can be obtained using mUS recordings from deeper structures.
There are technical challenges and limitations with TMS-mUS. Ultrasound processing methods are currently carried out offline. This presents challenges when determining the RMT using mUS alone. In TMS experiments in which RMT is used as a reference point for inhibitory or excitatory protocols, such as SICI or short-interval intracortical facilitation (SICF) among others, RMT is measured at the start of a testing session. In order for TMS-mUS to be implemented for use instead of sEMG in such protocols, either a fast-offline mUS processing method, or an online mUS processing method must be developed. Second, the mUS probe is a manual device with which it may be difficult to achieve reproducible views of a muscle, simply due to area, angle of placement, the pressure applied to the probe, or quantity of gel used. Additionally, TMS-mUS samples only one plane of muscles at one time, whereas sEMG can sample multiple superficial muscles.
In summary, this study suggests that TMS-mUS is a novel technique that closely mirrors the evoked sEMG signal. Future work needs to establish its use in deeper muscles, and in paired-pulse and repetitive TMS paradigms. Offline processing may limit the use of TMS-mUS in some studies, but this is surmountable. Importantly, there are a number of potential applications of TMS-mUS in neuromuscular diseases and stroke.
ACKNOWLEDGEMENTS
We thank Paul Hammond for help with equipment design.
FUNDING
This research was supported and funded by a grant from the Reta Lila Weston Trust (NS). NS was supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
5 CONFLICTS OF INTEREST
All authors report no disclosures.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.