Controlling sharpness, SNR, and specific absorption rate for 3D fast-spin echo at 7T by end-to-end learning
Abstract
Purpose
To non-heuristically identify dedicated variable flip angle (VFA) schemes optimized for the point-spread function (PSF) and SNR of multiple tissues in 3D FSE sequences with very long echo trains at 7T.
Methods
The proposed optimization considers predefined specific absorption rate (SAR) constraints and target contrast using an end-to-end learning framework. The cost function integrates components for contrast fidelity (SNR) and a penalty term to minimize image blurring (PSF) for multiple tissues. By adjusting the weights of PSF/SNR cost-function components, PSF- and SNR-optimized VFAs were derived and tested in vivo using both the open-source Pulseq standard on two volunteers as well as vendor protocols on a 7T MRI system with parallel transmit extension on three volunteers.
Results
PSF-optimized VFAs resulted in significantly reduced image blurring compared to standard VFAs for T2-weighted while maintaining contrast fidelity. Small white and gray matter structures, as well as blood vessels, were more visible with PSF-optimized VFAs. Quantitative analysis shows that the optimized VFA yields 50% less deviation from a reference PSF (sinc) than the standard VFA. The SNR-optimized VFAs yielded images with significantly improved SNR in a white and gray matter region relative to standard (77.1 vs. 40.7, respectively) as trade-off for elevated image blurring.
Conclusion
This study demonstrates the potential of end-to-end learning frameworks to optimize VFA schemes in very long echo trains for 3D FSE acquisition at 7T in terms of PSF and SNR. It paves the way for fast and flexible adjustment of the trade-off between PSF and SNR for 3D FSE.
1 INTRODUCTION
The fast-spin echo (FSE, also known as turbo-spin echo [TSE], or rapid acquisition with relaxation enhancement [RARE])1 is an echo train sequence that is the backbone of many modern, time-efficient MR applications. It is robust to magnetic field inhomogeneities and provides images with relevant contrast and high SNR. Thus, it is widely used in clinical examinations. However, a major limitation is the high RF power deposition caused by the 180° flip angles (FAs), which often limits FSE examinations at high field strengths. The specific absorption rate (SAR) characterizes the RF power deposition and depends on the square of the main magnetic field and the square of the FAs.2 SAR limits exist to prevent patient heating, however, even FSE examinations at 3T can exceed these limits. A state-of-the-art approach for SAR-reduced 2D multi-slice imaging is the Transitions Between Pseudo Steady States (TRAPS) method.3 In TRAPS, the use of reduced variable FA (VFA) schemes significantly reduces the SAR while maintaining the desired contrast and high signal strength.
Another major limitation is the reduced time efficiency of 3D acquisitions. In 2D FSE, multiple slices can be excited and acquired in an interleaved manner during each TR. In 3D imaging, however, the entire volume is excited; therefore, slice interleaving is not possible. Instead, long echo trains are preferred to acquire as much data as possible during each TR. As a drawback, the transverse magnetization (i.e., the MR signal) decays during the acquisition in the transient state within a TR, resulting in k-space data that are inherently modulated by a multi-component relaxation decay. The modulation is mainly determined by the respective tissue T2 relaxation parameters, and is represented by the modulation transfer function (MTF). In a fast Fourier transform (FFT) reconstruction, a non-uniform MTF results in pronounced T2-dependent image blurring in the phase- and partition encoding direction, which is a major cause of image degradation and corrupts valuable image information.
To counteract T2 signal decay, VFA schemes are typically assigned to the refocusing pulses in the echo train.4, 5 By using dedicated VFA schemes, the magnetization at each echo is no longer refocused solely in the transverse plane. Instead, a significant portion of the magnetization is stored along the longitudinal axis. The subsequent use of this stored magnetization results in pseudo-steady states and enhanced signal amplitudes at relatively long TEs. This results in a more uniform MTF. For such approaches, the FA trains are optimized by performing a signal simulation to produce the desired signal response.
However, the desired signal responses are chosen heuristically. Although there are guidelines for the design of the VFA schemes, the user must define parameters and shapes for the ramp FAs, for example.3, 4, 6 For T2-weighted (T2w) imaging, the desired signal response is divided into three parts, with an asymptotic approach to a pseudo-steady state, a constant signal evolution until the k-space center is reached, and a linear FA increase until a maximum FA is reached.4, 5 While this standard approach produces clinical T2 contrast, the resulting VFAs may not be the optimal choice in terms of maximizing SNR or minimizing image blurring, which are important goals for specific imaging scenarios. Furthermore, the VFA design is typically limited to one target tissue and does not consider sampling schemes or spatial multi-tissue image content. Thus, while VFA schemes for 3D FSE are well-established approaches, potential improvements in terms of flexible, robust tailoring of VFA schemes to maximize SNR or minimize image blurring remain to be elucidated.
For ultrahigh field (UHF) MRI, the tailoring of dedicated VFA schemes is a particularly important aspect. While UHF MRI at 7T offers several advantages, such as higher baseline SNR, higher spectral resolution and, in many cases, better contrast between different tissues or between tissue and vessels, both SAR limits and image blurring due to faster T2 decay are serious issues. This is because the SAR depends on the square of the main magnetic field strength. In addition, reduced T2 relaxation parameters cause more signal loss for very long echo trains. Furthermore, MRI at 7T is strongly affected by inhomogeneous transmit B1 fields resulting in signal variations throughout the FOV, which can be mitigated with parallel transmit (pTx).
Compared to previous approaches,7-11 we suggest an end-to-end optimization framework for the flexible, non-heuristic identification of VFA schemes designed to maximize SNR or minimize image blurring of 3D FSE sequences with very long echo trains at 7T. Our framework considers multiple tissues, including their spatial distributions and sampling characteristics for clinical contrasts. VFA schemes are automatically identified to minimize image blurring or maximize SNR. An open-source 3D FSE sequence via the Pulseq12, 13 standard is employed to test optimized VFA schemes and to quantify the reduction in image blurring and the improvement in SNR. Furthermore, the compatibility with clinical vendor sequences at 7T with pTx extension is shown and 3D acquisitions with very long echo trains lengths up to 220 are presented.
2 METHODS
First, the optimization framework utilized to obtain VFA schemes tailored to PSF- or SNR-optimization is described. Afterward, the experimental evaluation is elaborated using an open-source, vendor-agnostic sequence development platform for imaging. It is then described how to quantify the benefits of PSF- and SNR-optimization. Finally, the VFA schemes were tested using clinical vendor sequences.
2.1 Standard VFA scheme
In this work, the standard VFA scheme4, 5 uses a prospective extended phase graph (EPG)14 algorithm to calculate the FA plan based on a target signal. The target signal is divided into three parts. First, an asymptotic approximation to a constant target signal level is computed. The target signal is kept constant until the k-space center is reached, and then a linear FA increase is applied until a maximum FA is reached. The VFA scheme that produces the defined signal response for a given T1 and T2 is then calculated. In this work, T1 = 1500 ms and T2 = 50 ms were chosen to optimize for white matter (WM).
2.2 Optimization framework
Although there exist other frameworks for VFA optimization such as MR-zero,10 we focus on a simplified model to reduce the computational complexity.
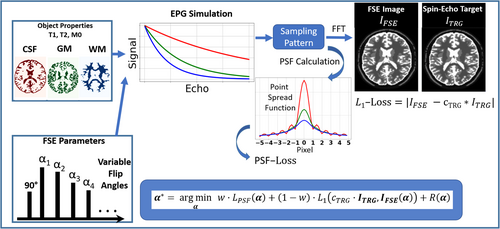
This parameter is used to provide the basis for a fair comparison of several sequences with different VFAs.
2.2.1 Contrast/SNR loss
2.2.2
Using the of all tissue types, images are generated for each echo number by mapping the signal amplitude of each tissue type to its corresponding spatial region, represented by tissue masks. In that way, multi-contrast images are generated. The next steps involve transforming these images into the k-space domain using FFT. View ordering information (i.e., information about the phase- and partition-encoding locations for the individual echoes, or sampling table) is used to form a single combined k-space representing the FSE acquisition. To achieve this, a 2D binary mask is assigned to each echo number, indicating the specific k-space locations populated by signals from that echo. In accelerated acquisitions, not all k-space points are directly sampled. In such cases, the missing k-space points are assigned the echo number of the nearest neighboring sampled k-space point. Finally, the combined FSE k-space data are subjected to an inverse FFT to reconstruct the simulated FSE image.
2.2.3 PSF loss
The is obtained using and the sampling table.
Thus, starting with FAs on the hypersphere, can be projected onto the hypersphere after each gradient descent step simply by global rescaling, thereby ensuring that satisfies the SAR constraint in each optimization step. This optimization procedure is also known as projected gradient descent. The FAs were initialized by . The optimization framework was implemented in PyTorch,17 and is fully differentiable relative to the FAs. Optimization was stopped when the loss of the following steps changed below a stopping criterion .
As 2D simulation phantom, we used the simulated brain dataset (SBD)18, 19 to initialize the object descriptor with three different tissue types: WM, gray matter (GM), and cerebrospinal fluid (CSF). The physical properties used in this study were taken from reference.20
2.3 Experiments
2.3.1 Test case 1: T2w 3D FSE with Pulseq standard
The first imaging scenario is a 3D FSE with T2w. A 3D FSE sequence was implemented within the open source sequence development platform PyPulseq12, 13 (interpreter version 1.3.1post1). In the spirit of reproducible research, all optimized sequences are accessible at our Github repository: https://github.com/MRsources/hyperSpace. The sequence parameters were as follows: TR = 3200 ms, echo spacing (ESP) = 3.8 ms, effective TE (TEeff) = 380.0 ms, FOV =300 × 200 × 50 mm3, resolution = 1.5 × 1 × 1 mm3, echo train length (ETL) = 200, rel.SAR cSAR = 9.0%, bandwidth = 234 Hz/pixel.
Linear view ordering was used in the phase-encoding direction, and partitions were encoded linearly with one shot per partition (i.e., one TR per partition). The total acquisition time was 160.0 s. The standard VFA was calculated as described above in paragraph “Standard VFA scheme”.
Two optimization schemes were considered: VFA-optimization to minimize image blurring and to maximize SNR, that is respectively in Eq. (1). All tissues were equally weighted in the PSF loss (i.e., in Eq. (4) for T = WM, GM, and CSF). The spin echo target image was assigned TE = 45 ms and TR = 6000 ms. The choice of TR and TE was made to minimize influences on the signal, ensuring that the results were primarily driven by contrast. The selected TR is slightly lower than five times the relaxation time of GM, which reduces the influence of on the image contrast and allows us to focus on -weighted imaging. At 3T, typical TEs for -weighted sequences range from 80 to 100 ms, but at 7T, the reduced values in brain tissue necessitate a shorter TE, such as the 45 ms chosen in this study. We set .
In vivo measurements were performed on two healthy volunteers using a 7T whole-body MR system (Magnetom Terra.X, Siemens Healthineers, Erlangen, Germany) with a commercial 8Tx/32Rx RF head coil. All MRI scans were under approval of the local ethics board, and were performed after written informed consent was obtained.
2.3.2 Quantitative PSF comparison
2.3.3 Quantitative SNR comparison
2.3.4 Contrast-to-noise quantification
2.3.5 Test case 2: T2w 3D FSE with vendor sequence
To evaluate the applicability of the PSF- and SNR-optimized FA schemes in clinical routine, they were tested beyond the Pulseq standard in a vendor sequence. The vendor sequence was modified so that an external list of VFAs can be loaded. The vendor sequence was assigned the same imaging parameters for contrast setting as for the Pulseq sequence in test case 1 and the same and . The FOV and resolution were set to (interpolated to double resolution from the acquired data). In addition, parallel imaging was performed using 23 with a total of six-fold acceleration (PE:3, 3D:2, Caipi shift:1). The total acquisition time was 279.0 s. Furthermore, a significant difference to the Pulseq sequence is that this vendor sequence utilizes optimized pTx pulses to mitigate signal variations throughout the FOV due to B1 inhomogeneities.24, 25
In vivo measurements were performed on two healthy volunteers using the same experimental setup described in test case 1.
2.3.6 Test case 3: 3D FLAIR with vendor sequence
In the third test case, a 3D vendor FSE with T2w-FLAIR weighting was considered. The imaging parameters were as follows: TR = 8000 ms, ESP = 4.04 ms, TEeff = 300.0 ms, TI = 2250 ms, FOV = 230 × 230 × 180 mm3, resolution = 0.4 × 0.4 × 0.5 mm3 (interpolated to double resolution from the acquired data), ETL = 220, rel. SAR cSAR = 6.0%, bandwidth = 651 Hz/pixel, linear reordering scheme, acceleration with CAIPIRINHA (total factor 6, PE: 3, 3D: 2, Caipi shift: 1). The total acquisition time was 353.0 s.
During optimization, the CSF compartment in the spin-echo target image was suppressed in this imaging scenario to account for fluid suppression. Correspondingly, an additional inversion pulse was included in the EPG simulation with a time delay of TI to the 90° excitation pulse.
VFA-optimization to minimize image blurring and to maximize SNR, that is w = 1 and w = 0 in Eq. (1), respectively, was considered. All tissues were equally weighted in the PSF loss (i.e., = 1 in Eq. (4) for T = WM and GM). The spin echo target image was assigned a TE = 45 ms and a TR = 6000 ms, and we set
In vivo measurements were performed on one healthy volunteer using the same experimental setup described in test case 2.
3 RESULTS
3.1 Test case 1: VFA schemes
Figure 2 shows the VFA schemes and the corresponding signal response simulations and PSFs for the standard (Figure 2A) and the PSF-, trade-off, and SNR-optimizations (Figure 2B–D respectively). The PSF-optimization results in a VFA scheme that is similar to the standard scheme, however, with significant differences. The FAs in the first three quarters of the FA train are reduced, and elevated for the last quarter. Consequently, the pseudo-steady state signal responses of WM and GM are elongated compared to the signal response of the standard VFA scheme. This results in corresponding PSFs with greatly reduced sidebands compared to the corresponding PSFs of the standard VFA scheme. While the PSFs of CSF, WM, and GM deviate in the standard, they are equalized by the PSF-optimization. Note that the resulting signal gap from CSF to WM and GM at the effective TE is reduced. This allows for images with more regularized CSF signal relative to WM and GM compared to the standard, while still preserving contrast (see next section, Figure 3).
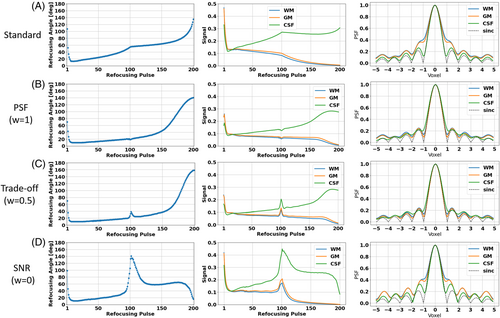

On the other hand, the SNR-optimization yields a VFA scheme that is comparable to the PSF-optimized VFA in the first two quarters. However, it shows a characteristic peak at the effective TE. This results in elevated signal responses at the echoes assigned to the k-space center, which are accompanied by elevated sidebands in the corresponding PSFs that exceed those of the standard, which results in enhanced image blurring (see next section, Figure 3). The trade-off case incorporates characteristics from both extreme cases: It reveals a peak in the FA train and signal response at the echoes assigned to the k-space center, as well as elongated, constant signal response parts in the k-space periphery. It should be noted that the PSF sidebands are comparable to those of the PSF-optimization, which predicts a similar PSF error to that in the solely PSF-optimized case.
3.2 Test case 1: Pulseq 3D FSE imaging
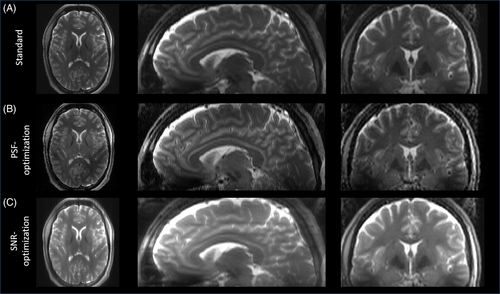
Figure 3 shows an exemplary transversal partition of the images obtained with the Pulseq sequences using the standard and the optimized VFA schemes shown in Figure 2 A for one subject. Compared to the standard, the PSF-optimized VFA scheme results in greatly reduced image blurring in the phase-encoding direction (anterior > posterior), while providing only a slightly altered contrast. In particular, WM/GM structures are sharper and WM/GM edges are better resolved. In addition, blood vessels are more pronounced, as shown in the zoomed region (see Figure 3, insets). This is partly due to a more regularized CSF signal relative to WM and GM due to the PSF-optimization, but can also be explained by less GM/WM blur into low intense vessel regions. On the other hand, the SNR-optimized VFA scheme provides increased signal values, however, at the cost of increased visual image blurring while providing same contrast setting. It is worth noting that the relative SAR is equal for all VFA schemes.
Figure 4 shows a 3D view of this test case on subject 2. It is worth noting that the PSF-optimized variant also shows reduced image blurring in the axial and sagittal planes. Note this sequence did not incorporate optimized pTx pulses to mitigate B1 inhomogeneities. Thus, signal variations throughout the brain are present. However, the compatibility of the optimized VFA schemes with optimized pTx pulses is demonstrated using vendor sequences in test case 2 and 3. Please note that for the Pulseq-based sequences, the signals at the beginning of the echo train, during the rapid transition, were used as is. These signals were not discarded or filtered.
3.3 Quantitative PSF comparison
After visual inspection, a quantitative comparison of PSF-induced image blurring is shown in Figure 5. The sequence from test case 1 was applied without partition and phase encoding gradients on subject 1 using the standard VFA and both PSF- and SNR-optimized VFAs. The resulting unencoded signals are shown in Figure 5 A. It is worth noting that the experimental, in vivo signal responses are consistent with the simulated signal response of WM and GM in all cases (see Figure 5 A and signal responses in Figure 2). This is due to the minimization of CSF signal contributions using an inversion pulse with a TI that was adjusted to suppress the CSF signal.
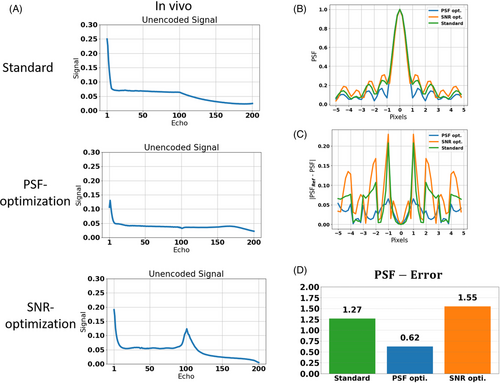
The corresponding PSFs are depicted in Figure 5B. The PSF-optimization results in a PSF with strongly reduced sidebands compared to the PSF of the standard VFA. On the other hand, the SNR-optimization shows elevated sidebands. In Figure 5 C, the error to the reference PSF (sinc) are depicted. Using Eq. 8, the error was quantified (Figure 5C). While the error of the standard amounts to 1.27, the PSF-optimization yields a minimum error reduced by 51.2% (0.62). On the other hand, the SNR-optimization reveals a maximum error (1.55) which increases the standard by 22.0%.
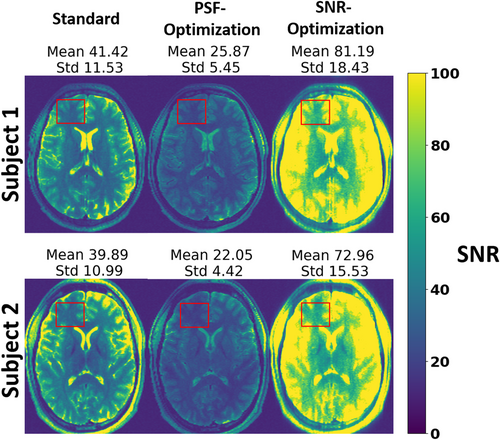
3.4 Quantitative SNR comparison
In addition to the PSF quantification, the SNR was analyzed quantitatively using Monte Carlo simulations to obtain SNR maps for both subjects (see Figure 6). Within a WM/GM ROI, the PSF-optimization yields an average SNR which is decreased by 37.4% relative to the standard for subject 1 (25.9 ± 5.5 vs. 41.4 ± 11.5, respectively). On the other hand, the SNR-optimization yields an average SNR that is increased by 96.1% relative to the standard (81.2 ± 18.4 vs. 41.4 ± 11.5, respectively). Note that similar observations were obtained for subject 2: The PSF-optimization shows decreased average SNR reduced by 44.6% relative to standard (22.1 ± 4.4 vs. 39.9 ± 11.0, respectively), while the SNR-optimization yields an average SNR elevated by 83.0% relative to the standard (73.0 ± 15.6 vs 39.9 ± 11.0, respectively).
3.5 CNR comparison
The contrast between the nucleus caudatus (GM) and the surrounding WM in Figure 3 was quantified using CNR, with the ROIs illustrated in Figure S1. The GM-WM CNR was highest for the SNR-optimized case (14.5), followed by the standard approach (12.2), while the PSF-optimized case exhibited a lower CNR (11.4). For CSF-WM CNR, the SNR-optimized scheme also demonstrates the highest CNR (99.1), followed by the standard approach (79.3), while the PSF-optimized scheme exhibits the lowest CNR (37.1).
3.6 Trade-off optimization
In another experiment, the trade-off optimized VFA scheme was compared to the standard and to the PSF- and SNR-optimized schemes. An exemplarly transversal slice is shown in Figure S2. The visual impression indicates that the trade-off case preserves the enhanced visibility of small structures of the PSF-optimized case, while providing strongly enhanced SNR in WM and GM. Quantitatively, the SNR in the trade-off case surpasses the SNR of the standard, as shown in the SNR maps (mean SNR in ROI for trade-off optimization 44.4 ± 6.7 vs. 41.4 ± 13.2 in standard, see Figure S3). The PSF-error of the trade-off optimization is only slightly worse than for the PSF-optimized case (0.62 vs. 0.57, respectively, while standard yields 1.28, see Figure S4). It should be noted that the GM-WM CNR is best for the SNR-optimized case (14.0), while the trade-off optimization shows the worst CNR (10.6), which is slightly lower than for the PSF-optimization (10.9). Standard yields a CNR of 12.7 (see Figure S5).
3.7 Test case 2: T2w 3D FSE with vendor sequence
The PSF- and SNR-optimized VFA schemes from test case 1 were used in the corresponding vendor sequence with pTx extension. The results for subject 3 and subject 4 are shown in Figures 7 and 8, respectively. In general, the observations from the images obtained via the Pulseq standard in test case 1 were validated for both subjects: In both examinations, the PSF-optimized VFA scheme shows strongly reduced image blurring relative to the standard, in particular for WM/GM structures. The blood vessels in axial view (Figure 7 insets), and small structures in the cerebellum in sagittal view (Figure 8 insets) are better resolved. Note that the blurring of the cochlea is strongly reduced, too, which is particularly helpful for segmentation tasks (Figure 8 insets). The contrast fidelity in comparison to the standard is well preserved. It is worth noting that this imaging scenario employs an echo train duration of 760.0 ms, and the PSF-optimization still yields highly resolved WM and GM structures with a reasonable SNR, which is worth emphasizing as WM and GM typically have T2 relaxation times of 40–50 ms and 50–60 ms at 7T, respectively.
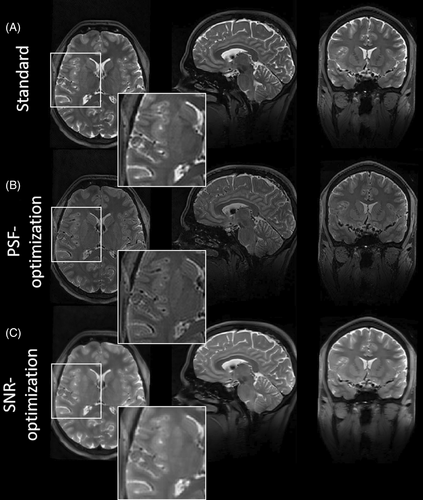

The SNR-optimization, on the other hand, strongly amplifies signal magnitudes, while maintaining contrast settings and relative SAR. However, in accordance to the visual inspections via the Pulseq sequence in test case 1 and quantitative analysis of image blurring, the SNR-enhanced images show elevated image blurring in this case, too. It should be noted that all VFA schemes have equal relative SAR.
The used pTx RF pulses yield homogenous signal throughout the brain, and signal variations caused by the B1 inhomogeneities, as present in the Pulseq acquisition (Figure 4), are strongly mitigated by the vendor acquisition. As we observe the same effects of VFA, as with the 1Tx Pulseq version, we can conclude that our VFAs are compatible with pTx pulses.
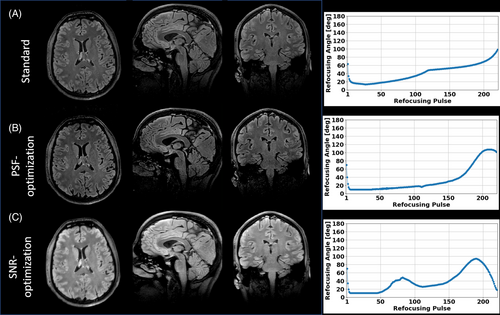
3.8 Test case 3: 3D FLAIR with vendor sequence
In the third test case, FAs were optimized for a T2-FLAIR sequence. The resulting images obtained using a vendor sequence with pTx RF pulses are shown in Figure 9 along with the corresponding VFA schemes (Figure 9, right hand side), with zoomed in images in Figure 10. This experiment shows that the T2-FLAIR sequence also benefits from the optimizations: The PSF-optimized images display highly resolved WM and GM structures and reduced image blurring while providing high contrast fidelity and reasonable SNR. Note that the total echo train length is 220 in this case, resulting into an echo train duration of 888.8 ms. Highly amplified signal magnitudes are provided by the SNR-optimized VFA scheme. Note that the k-space center is assigned the 75th echo, and the SNR-optimized VFA scheme reveals elevated FAs around this echo number. It should be noted that all VFA schemes have equal relative SAR.
4 DISCUSSION
The proposed framework builds on the initial results of conference contributions15, 27 and introduces a flexible and robust method for the automatic optimization of VFA schemes in 3D FSE sequences, addressing key challenges related to SNR and PSF penalties in case of very long echo trains at 7T. By adjusting the weighting of cost function components, VFA schemes were derived automatically that favor sharpness or SNR at equal relative SAR. PSF-flavored schemes provided better-resolved WM and GM structures in both T2w and FLAIR sequences in trade-off for SNR, while SNR-flavored schemes provided increased signal levels in trade-off for sharpness.
Automatic parameter optimization for FSE sequences has been investigated in previous works. Dang et al. optimized VFA schemes end-to-end within the MR–zero framework9, 10 for single-shot 2D FSE sequences with PDw and T2w at 3T. T2 blurring was significantly reduced and the optimized VFA schemes outperformed schemes proposed by Zhao et al., where FA trains were optimized by performing signal simulation to produce a desired signal response,11 and TRAPS.3 However, while this work showed the benefit of end-to-end VFA-optimization over standard approaches, the considered sequences were limited to 2D acquisitions at 3T with echo train lengths of 64. This work makes principal contributions by extending toward 3D acquisitions at 7T with very long echo trains, for which a dedicated optimization framework is proposed. It should be noted that Dang et al. used a convolutional neural network as a deblurring network,28 and the magnitude FSE image was assigned as input. It was trained jointly to the VFA-optimization. In this work, however, we did not employ a deblurring network, as the aspects of network generalization and the substantial demand for training data and time remain to be elucidated. Instead, the objective of reducing image blurrig was achieved via an explicit, dedicated loss term, which is minimized in the VFA-optimization.15 While this approach eliminates the computational burden associated with CNN training for 3D data, incorporating a deblurring network into the proposed framework is possible as Dang et al. proposed. Optimizing VFAs with explicit PSF loss term and a deblurring network jointly promises further enhancements for the visibility of small structures.
De Buck et al. employed a comprehensive DANTE-SPACE simulation framework for the optimization of T2w DANTE-SPACE at both 7T and 3T.8 The simulation-based optimization of the DANTE parameters facilitated improved T2w DANTE-SPACE contrast at 7T. However, the sequence optimization was limited to DANTE parameters, and optimizing both the VFA scheme and DANTE parameters jointly remains to be elucidated, and could lead to further improvements.
This work addresses a critical gap in prior research by optimizing the PSF across multiple tissue types. Previous VFA schemes often focused on single tissue optimization where single relaxation parameters are considered.3, 4, 7, 11, 29, 30 In contrast, by incorporating the optimization of the PSF for multiple tissue types simultaneously, more balanced signal levels of WM/GM and CSF were achieved in the T2w sequences. This significantly reduces CSF contamination, a common issue that obscures structural details, particularly at tissue interfaces. Indeed, in the PSF-optimized T2w images, enhanced visibility of blood vessels was achieved for both the Pulseq- and the vendor sequence. The improved visibility of small structures in the T2w images by PSF-optimized VFAs may facilitate and improve common segmentation tasks where T2w 3D FSE sequences are used.31, 32
While the proposed framework provides a flexible tool for optimizing both PSF and SNR, the balance between these two objectives should be carefully adjusted based on clinical priorities. A higher weighting on PSF-optimization improves spatial resolution but may come at the cost of reduced SNR, potentially affecting the visibility of fine details in certain tissues. Conversely, prioritizing SNR-optimization can be beneficial where the base-line SNR is low, for example in thorax imaging, as it enhances signal intensity but may introduce blurring artifacts. The SNR-optimization yielded also the best CNR, although the quality of the visual impression may be deteriorated due to the enhanced blurring artifacts. As expected, the SNR-optimized case, where the L1 error to the spin echo target is minimized, shows the best agreement with the target contrast (see Figure S6). This case matches the desired contrast relatively well. On the other hand, GM and WM signals progressively decrease as the L1 error weighting shifts toward the PSF error term.
Therefore, determining the optimal trade-off between PSF and SNR is not purely a technical decision but requires input from a radiologist. It is essential to ensure that the resulting images align with the clinical objectives of the scan, whether that be sharper delineation of tissue boundaries or maximized signal intensity for better contrast. As shown in this work, a trade-off optimization may even yield improved visibiliy of small structures with a SNR similar to the standard approach. The GM-WM CNR, however, was found to be slightly worse, but could be also controlled by adding additional loss terms, or adjusting the target spin echo image, to find a VFA scheme that surpasses the standad approach in PSF, SNR and CNR.
One of the strengths of this framework lies in its versatility, allowing the optimization process to be tailored to specific tissues or anatomical regions. In applications where high-resolution depiction of small structures is required, like inner ear or cochlea imaging,33 the optimization can be weighted to prioritize the PSF for that particular region. This capability is especially useful in cases where certain tissues demand higher resolution than others due to their small size or critical diagnostic importance. By fine-tuning the optimization for specific tissues or ROIs, clinicians can extract the maximum diagnostic value from each scan. The image quality of other regions can still be controlled, but there can of course be compromises.
Another advantage is the scalability to include additional tissue types or even pathological structures. For example, multiple sclerosis (MS) lesions, which can be difficult to visualize due to their small size and diffuse nature, can be included in the optimization process through the object descriptor. This extension would allow for tailored FA schemes that prioritize the visualization of pathological areas. It is relatively straightforward to adapt it for imaging protocols targeting other pathologies, such as tumors or ischemic regions, where clear differentiation from surrounding tissue is critical.
In this study, a consistent SAR constraint was maintained across all optimizations in the respective testcases to ensure comparability and adherence to practical limits in clinical applications. At this stage, we have not explicitly investigated the effects of relaxing or removing the SAR constraint on the optimization results. However, it is plausible that allowing higher SAR levels could provide additional degrees of freedom in the optimization process, potentially influencing the trade-off between PSF sharpness and SNR. Future work will explore the impact of varying SAR constraints through dedicated simulations to assess their role in shaping the optimal VFA design. This analysis would provide deeper insight into the trade-offs between SAR, SNR, and image resolution, further refining the applicability of the proposed framework.
An interesting extension of our framework, particularly in the context of PSF-optimization, would be the use of a half-Fourier acquisition strategy,34 wherein echoes are acquired only up to—or slightly beyond—the center of k-space. This approach could relax the requirement of maintaining adequate signal levels across the entire k-space trajectory, potentially enhancing PSF sharpness while improving scan efficiency. While we have not yet incorporated half-Fourier acquisition in the current study, future work could investigate its integration within our optimization framework. One challenge in doing so lies in the implementation of partial Fourier (PF) reconstruction techniques, such as projection onto convex sets (POCS),35, 36 in a manner that maintains differentiability for gradient-based optimization. Exploring the feasibility of such methods could further refine the flexibility of the proposed optimization strategy.
4.1 Limits
The model employed in this work was deliberately simplified to focus on the core objective of FA-optimization. More complex effects such as magnetization transfer (MT) were not incorporated. While this allowed for faster computation, we acknowledge that MT effects, particularly in multi-slice acquisitions, can have a substantial impact on image contrast and are also dependent on the VFA scheme. These effects should not be overlooked, as MT can alter the effective relaxation times and contribute to variations in contrast, especially in tissues with high macromolecular content. This can be achieved by extending the EPG with MT effects.37
Scanner imperfections such as B0 and B1 inhomogeneities can degrade the performance of VFA-optimized sequences, particularly at higher field strengths where these inhomogeneities are more pronounced. To address these limitations, we plan to incorporate the MR-zero framework10 into the optimization pipeline to consider realistic B1+ levels that affects the signal intensities. However, in this work the signal characteristics induced by the FA train were maintained even without considering B1+ variations, as shown in Figure S7. This is valid for both the standard train and the optimized trains. To achieve practical computational performance, a simplified optimization model was used that operates on a “tissue-wise” rather than a pixel-wise basis. By focusing on representative tissue properties rather than simulating every individual pixel, the computational cost was significantly reduced while still maintaining a high degree of accuracy in predicting optimal FAs. Additionally, by employing 2D simulations instead of full 3D modeling, we further streamline the process, enabling rapid computation of VFA schemes.
4.2 Future works
The incorporation of more sophisticated loss terms such as the CNR could provide further improvements in contrast optimization. The framework could more effectively balance image contrast and noise characteristics, particularly in sequences that require high contrast differentiation between tissues (e.g., GM and WM in neuroimaging or cartilage and bone in musculoskeletal imaging). Furthermore, incorporating tissue-wise relative contrast constraints (e.g., lesion-to-WM/GM) could prove beneficial for imaging crucial diagnostic structures.
In knee imaging, optimizing VFAs could enhance the visualization of cartilage and meniscal structures. Cochlea imaging is another subject, where the highspatial resolution and tissue contrast provided by optimized FSE sequences could contribute to better characterization of inner ear structures. It is worth noting that slab-selective FSE.5, 38 is typically used for inner ear imaging.39 In this work, whole-brain imaging was considered. However, future works will investigate combining the flexible VFA scheme optimization with slab-selective excitation, including dedicated considerations about transmit field inhomogeneities. Furthermore, integration of kT spokes optimization40 for further refinement of spatial selectivity and resolution could prove beneficial. In this regard, hippocampal imaging is another promising subject where the PSF- or SNR-optimized VFA schemes in T2w sequences could provide better resolved structures for manual segmentation.41
The fast and flexible adjustment of the VFA flavor in real time at the scanner, depending on the task and the tissues of interest, is another important aspect. In this work, one iteration step costs approximately 20.3 s, and the total iteration number were 252 for the T2w test case and 191 for the FLAIR test case. Future studies will focus on significantly reducing the computation time to make the proposed framework more practical for clinical use. One promising strategy involves leveraging deep learning techniques to provide an initial estimate of the FA train, serving as a prior for the optimization process.42 The network takes protocol parameters as inputs and outputs a near-optimal VFA train, which can be used to initialize the optimization process, requiring significantly fewer iterations to converge to the final solution.
5 CONCLUSIONS
This study demonstrates the potential of end-to-end learning frameworks to optimize VFA schemes in very long echo trains for 3D FSE acquisition at 7T in terms of PSF and SNR. By adjusting the weighting of cost function components, VFA schemes were obtained which favor sharpness (PSF), or increased signal levels (SNR). It paves the way for the fast and flexible adjustment of the trade-off between PSF and SNR for 3D FSE imaging, depending on the specific task. PSF-like VFAs are more beneficial for the optimized imaging of small details, while SNR-like VFAs are more beneficial where low base-line SNR deteriorates image quality.
CONFLICT OF INTEREST STATEMENT
Jürgen Herrler and Patrick Liebig are employees of Siemens Healthineers (Erlangen, Germany).
ACKNOWLEDGEMENT
Open Access funding enabled and organized by Projekt DEAL.
Open Research
DATA AVAILABILITY STATEMENT
In the spirit of reproducible research, all optimized sequences are accessible at our Github repository: https://github.com/MRsources/hyperSpace.




