Robust dual-module velocity-selective arterial spin labeling (dm-VSASL) with velocity-selective saturation and inversion
Click here for author-reader discussions
Funding information: National Institutes of Health (NIH), Grant/Award Number: R01EB033210
Abstract
Purpose
Compared to conventional arterial spin labeling (ASL) methods, velocity-selective ASL (VSASL) is more sensitive to artifacts from eddy currents, diffusion attenuation, and motion. Background suppression is typically suboptimal in VSASL, especially of CSF. As a result, the temporal SNR and quantification accuracy of VSASL are compromised, hindering its application despite its advantage of being delay-insensitive.
Methods
A novel dual-module VSASL (dm-VSASL) strategy is developed to improve the SNR efficiency and the temporal SNR with a more balanced gradient configuration in the label/control image acquisition. This strategy applies for both VS saturation (VSS) and VS inversion (VSI) labeling. The dm-VSASL schemes were compared with single-module labeling and a previously developed multi-module schemes for the SNR performance, background suppression efficacy, and sensitivity to artifacts in simulation and in vivo experiments, using pulsed ASL as the reference.
Results
Dm-VSASL enabled more robust labeling and efficient backgroud suppre across brain tissues, especially of CSF, resulting in significantly reduced artifacts and improved temporal SNR. Compared to single-module labeling, dm-VSASL significantly improved the temporal SNR in gray (by 90.8% and 94.9% for dm-VSS and dm-VSI, respectively; P < 0.001) and white (by 41.5% and 55.1% for dm-VSS and dm-VSI, respectively; P < 0.002) matter. Dm-VSI also improved the SNR of VSI by 5.4% (P = 0.018).
Conclusion
Dm-VSASL can significantly improve the robustness of VS labeling, reduce artifacts, and allow efficient background suppression. When implemented with VSI, it provides the highest SNR efficiency among VSASL methods. Dm-VSASL is a powerful ASL method for robust, accurate, and delay-insensitive perfusion mapping.
1 INTRODUCTION
Velocity-selective arterial spin labeling (VSASL)1 is a category of arterial spin labeling (ASL)2, 3 methods that label arterial blood based on its velocity. Compared to the other 2 ASL categories relying on spatial labeling, and therefore sensitive to arterial transit time (ATT) effects,4-7 that is, pulsed ASL (PASL)8-11 and (pseudo-) continuous ASL ([P-]CASL),3, 12, 13 VSASL is insensitive to ATT effects1, 14 and has an SNR advantage15 when arterial blood supply is significantly delayed.
VSASL can be performed with VS saturation (VSS) or VS inversion (VSI) labeling. In VSS,1, 16, 17 ASL signal is created by saturating the magnetization of spins moving above a cutoff velocity (Vcut) under the label condition and leaving the same population of spins at equilibrium (relaxed) under the control condition. In VSI,18-20 the blood moving above the Vcut is inverted under the control condition and is relaxed under the label condition. The velocity selectivity is realized by the combined effects of RF and flow-sensitive gradient pulses and a physical mixing process. In both VSS- and VSI-based VSASL, a vascular crushing module (VCM) with the same Vcut is required to define the trailing edge of the bolus by removing intravascular signal moving faster than Vcut and unwanted venous signal for quantification. A post-labeling delay (PLD) is the time between the VCM and the image acquisition. In practice, PLD in VSASL is typically set to a minimal value, such as zero, to reduce the T1 decay.
Despite recent advancement in VSASL method development,15 2 major challenges remain: (1) the labeling efficiency is relatively low; and (2) artifacts compromise the robustness and the quantification accuracy of VSASL.
For labeling efficiency, the VSS-based VSASL has a maximal labeling efficiency of 0.5 theoretically, lower than typical values of PASL (0.97) and pseudo-continuous ASL (PCASL) (0.85) in practice,21 resulting in compromised SNR. To improve the SNR, 2 strategies have been developed including: (1) using multiple VSS modules to re-label relaxed ASL signal and generate a larger labeling bolus14; and (2) using a VSI preparation, which has a maximal labeling efficiency of 1 in theory.20 In practice, both strategies can improve the SNR by 20%–30% compared to single-module VSS-based VSASL.22 Despite these achievements, further improvement of the SNR of VSASL is desired.
The artifacts in VSASL mainly come from the fact that the application of flow-sensitive gradient pulses differs under the label and control conditions. Typically, under the label condition, gradient pulses with zero zeroth moment and non-zero first moment are applied; whereas under the control condition, zero zeroth and zero first moment are required, either by turning off the gradient pulses or using flow-compensated gradient pulses. Typically, the gradient pulses under the label and control conditions have small but different diffusion attenuation1, 14 and different sensitivity to eddy current (EC) effects.16, 17 Such difference makes the labeling sensitive to processes that are irrelevant to blood flow, such as diffusion attenuation, or undesired labeling of tissues caused by ECs; that is, artifactual ASL “signals” are generated, resulting in compromised robustness and quantification accuracy. For example, cerebral blood flow (CBF) may be significantly overestimated if EC effects are not reduced or properly matched in the label and control images.16, 17 Methods have been developed to improve the preparation and quantification accuracy of VSASL, such as reducing sensitivity to EC effects,16, 17 and correction of artifactual ASL signal due to diffusion attenuation effects, especially in voxels containing CSF.14 Despite these development and research efforts, the temporal SNR (tSNR) of existing VSASL methods is still low in practice.20, 22, 23
In this study, a novel dual-module labeling strategy is developed to address the 2 major challenges in VSASL described above. It is applicable with VSS and VSI labeling modules and their combinations. In addition, it also enables better background suppression (BS)24 than existing VSASL methods, further enhancing the SNR performance. To differentiate the new dual-module labeling method from the previous VSS-based multi-module VSASL (mm-VSASL) method,14 we refer to the new dual-module labeling strategy as dual-module VSASL (dm-VSASL), though the mm-VSASL can be (and typically is) implemented with 2 VSS modules.
The principles of the dm-VSASL scheme are first introduced and followed by the modeling of dm-VSASL signal and the optimization for maximal SNR efficiency. The practical performance, including the ASL signal strength, labeling robustness or tSNR, BS performance, and CBF quantification, was examined and compared with existing VSASL methods and PASL in in vivo experiments.
2 THEORY
As described above, traditional VSASL (single-module VSASL, or sm-VSASL) has different gradient layouts, and thus unbalanced diffusion and EC sensitivities, in the acquisition of label and control images; and mm-VSASL has improved SNR efficiency, but the diffusion and EC sensitivities are higher; that is, 2 VS modules under the label condition (with flow-sensitizing gradients) are used to acquire label images. To tackle this, the dm-VSASL design rearranges the flow-sensitizing gradients in the acquisition of label and control images such that the diffusion and EC sensitivities are better balanced in the 2; therefore, the associated artifacts can be reduced or canceled after subtraction. Similar to mm-VSASL, dm-VSASL uses more than 1 VS labeling module in preparation, but they differ in a few important aspects: (1) mm-VSASL is applicable with VSS labeling only, whereas dm-VSASL can use both VSS and VSI labeling and their combinations; (2) dm-VSASL uses a different gradient configuration to acquire label and control images; (3) dm-VSASL requires the first VS module to invert the static spins, whereas mm-VSASL does not.
Below we start with the implementation of dm-VSASL to demonstrate the design principles as illustrated in Figure 1. Briefly, a global saturation is first applied to reset the magnetization of all spins to a known state (). After a delay time of (e.g., 2 s) for the arterial spins to recover, 2 VS modules are applied consecutively with a delay (on the order of 1 s) after each to allow arterial inflow to deliver. The types of the VS modules (VSS vs. VSI) and the conditions (label vs. control) depend on the specific implementation and the image type being acquired (label vs. control), as described in the details below. Then a VCM is applied to remove undelivered (i.e., with V > Vcut) spins and followed by image acquisition after a short PLD (close to zero). Additional BS pulses can be applied between the second VS module and the VCM.
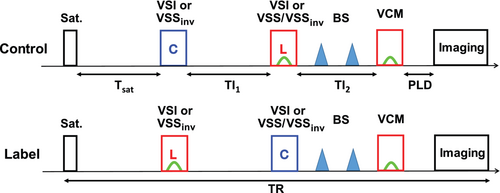
2.1 Dm-VSASL using VSI only
To acquire a control image, the first VSI module is applied under the control condition, that is, without flow-sensitive gradients. After a delay of TI1, the second VSI module is applied under the label condition, that is, with the flow-sensitive gradient pulses. After a second delay time TI2, the VCM can be applied and followed by PLD and image acquisition. To acquire a label image, the first VSI module is applied under the label condition, and the second module under the control condition. Background tissue signals are partially suppressed by the inversion effect of the VSI modules, and the SNR is improved.22 Additional global BS pulses can be applied after the second VSI module to further improve the SNR.
2.2 Dm-VSASL using VSS only
Unlike the previous mm-VSASL, where 2 VSS modules under the same condition are applied consecutively to acquire a label or a control image, dm-VSASL using VSS only obtains a control image with the first VSS module under the control condition and the second module under the label condition, and a label image with the first VSS module under the label condition and the second module under the control condition. In addition, the first VSS module has to be modified to invert the magnetization of static spins. To differentiate it from the unmodified VSS module, we refer it to as VSSinv. The second VSS module can be either VSS or VSSinv.
VSSinv can be implemented in 2 ways: (1) applying an inversion pulse immediately after the VSS module, or (2) modifying the phase of the RF pulses in the VSS module to induce a built-in inversion effect as described in Ref. 25; for example, a phase of π can be added to the last RF pulse in a double-refocused hyperbolic secant/tangent1 or a symmetric 8-segment B1 insensitive rotation (sBIR8)16 module to tip the static spins down instead of up. The VSSinv module with built-in inversion is preferred because: (1) it does not increase the specific absorption rate; and (2) no addition signal reduction is introduced. Like VSI, VSSinv effectively serves as a BS pulse whose inversion effect should be accounted for in BS timing calculation.
2.3 Dm-VSASL using both VSS and VSI
Combinations of VSS and VSI modules, such as VSI + VSS/VSSinv and VSSinv + VSI, are also feasible. For example, a VSI module followed by a VSS or VSSinv module, or a VSSinv followed by a VSI module, would also work under the principles of dm-VSASL.
Note that for the dm-VSASL implementations described above, the label/control condition switching is required for proper accumulation of ASL signal (see below). Otherwise, the ASL signal created by the 2 VS modules will have opposite signs, resulting in signal reduction or even cancellation, as in the examples shown in Supporting Information Figure S1.
Compared to sm-VSASL and mm-VSASL, dm-VSASL has a more balanced gradient configuration between the label and the control image acquisition. This arrangement should mitigate the eddy current and the diffusion attenuation effects that are typically observed in VSASL, as well as reducing its sensitivity to motion, potentially reducing artifacts and improving quantification accuracy. In addition, the inversion effects at an early time allow more flexible and efficient BS. All these should contribute to improving the labeling stability, the tSNR, and the quantification accuracy of VSASL.
2.4 Dm-VSASL signal modeling and blood flow quantification
Similar to the previous mm-VSASL signal modeling,14 3 groups of arterial spins are considered in dm-VSASL: (1) group 1 being labeled by only the first VS module, that is, it is in the transmit field of the RF coil and moves above Vcut at the application of the first VS module and has decelerated below Vcut (delivered) at the application of the second VS module; (2) group 2 being labeled by both VS modules, that is, in the range of the RF coil and moving above Vcut at the application of both VS modules; and (3) group 3 being labeled only by the second VS module, that is, moving into the transmit field of the RF coil after the first VS module. Since group 3 is not likely to contribute to the measured ASL signal when ( is the maximal bolus duration, on the order of 2 s) and including it complicates the quantification,14 only the first 2 groups are included in the following modeling. The evolution of the magnetization of the 2 groups is shown in Figure 2. Note that the label/control condition switching in the second VS module is necessary to ensure the ASL signals from the 2 groups are of the same sign.
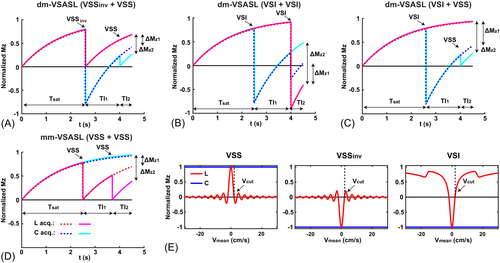
Under such definition, group 1 is first affected by the labeling efficiency of the first VS module , and then only scaled by the T2 relaxation of the second VS module since the arterial blood has decelerated and reached the capillary bed when the second VS module is applied; whereas group 2 is affected by both VS modules as it is in the arterial space at the application of both VS modules. Following Equation 1, the magnetization difference of the 2 groups can be approximated (see Supporting Information for detailed derivation) as:
Note the sign difference between different dm-VSASL implementations. Since is affected by the T2 relaxation of both VS modules, it is therefore beneficial to use VS modules with short . For example, sinc-shaped VSI (sinc-VSI)22 is preferred over rectangular-shaped VSI (rect-VSI)20 in dm-VSI for its shorter (29.4 ms versus 37.6 ms with the same pulse duration of ˜64 ms).
3 METHODS
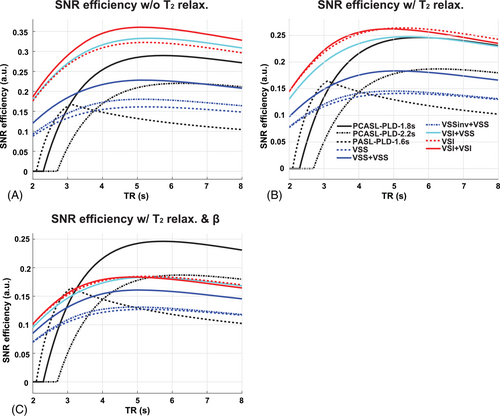
3.1 Optimizing SNR efficiency of VSASL
One of the major goals of designing dm-VSASL is to improve its SNR efficiency (SNR per unit time), defined as . A kinetic ASL signal model26 was used to model and compare the SNR efficiency of different VSASL schemes, along with PASL and PCASL for reference.14 The SNR efficiency was evaluated with ideal or realistic labeling efficiencies, at different TR ranging from 2 to 8 s. At each TR, the maximal SNR efficiency was calculated with different values of (for sm-VSASL), or and (for mm-VSASL and dm-VSASL) by grid searching under the constraint or , respectively. is the maximal bolus duration: 2 s for VSASL,14 1 s for PASL, and unlimited for PCASL. Other parameters included: = 1.66 s, = 0.5 s, = 1.6 s, = 1.8 s, = 2.2 s, , and 21 When T2 relaxation was considered for VSASL: = 150 ms, = 20 ms, = 30 ms; when realistic β was considered: = 0.9 and = 0.7 were assumed.22
3.2 In vivo experiments
Six young healthy subjects (2 female, age 21–38 years) were studied on a 3 Tesla scanner (Siemens Prisma, Erlangen, Germany) under University of California Riverside's Internal Review Board approval and written consent from the subjects. Among the dm-VSASL implementations, 2 dm-VSASL with VSS labeling only and VSI labeling only were implemented and tested. sBIR816 and sinc-VSI22 pulses were used for VSS and VSI, respectively. There are other VS pulses, such as BIR-427 and double-refocused hyperbolic secant/tangent1 for VSS and rect-VSI20 for VSI labeling. sBIR8 VSS was chosen for its B0/B1 insensitivity and robustness against EC effects16 and sinc-VSI was chosen for its higher labeling efficiency (shorter effect TE) and smoother velocity-labeling profile compared to rect-VSI.22
Single-module VSASL using VSS and sinc-VSI labeling, and mm-VSASL using 2 VSS modules, were compared. A PASL scan was also included as the reference for its robust labeling efficiency compared to PCASL in the presence of off-resonance and blood velocity difference.12, 28 The following ASL scans with BS were performed in a randomized order in each subject: (1) PASL: FAIR9, 29 with Q2TIPS,30 TI1 = 0.8 s, TI = 2.4 s (PLD = 1.6 s), 2 BS pulses applied at 1.4 s and 0.42 s before imaging; (2) sm-VSASL using VSS (VSSinv or VSS for simplicity): TI = 1.4 s, 1 BS pulse at 0.48 s before imaging; (3) mm-VSASL (VSS + VSSinv or mm-VSS): TI1/2 = 1.15/0.82 s, 1 BS pulse 0.26 s before imaging; (4) dm-VSASL using VSS (VSSinv + VSS or dm-VSS, BS1): TI1/2 = 1.45/0.54 s, 1 BS pulse at 0.28 s before imaging; (5) sm-VSASL using VSI (VSI): TI = 1.4 s, 1 BS pulse at 0.48 s before imaging; (6) dm-VSASL using VSI (dm-VSI, BS1): TI1/2 = 1.45/0.54 s, 2 BS pulses at 0.47 s and 0.14 s before imaging. To explore the flexibility and the effectiveness of BS in dm-VSASL, additional scans with different BS timings were performed: (7) dm-VSASL using VSS (VSSinv + VSSinv or dm-VSS, BS2), and (8) dm-VSASL using VSI (dm-VSI, BS2), both with the same timings: TI1/2 = 1.45/0.54 s, 2 BS pulses at 0.37 s and 0.25 s before imaging.
Other imaging parameters included: 2-segmented (along the slice-encoding direction) 3D gradient and spin echo (GRASE) EPI readout with 180° refocusing RF pulses; an in-plane FOV of 220 × 220 mm and a matrix size of 64 × 64; 24 slices and 4 mm thickness to cover the whole brain; TR = 4 s (PASL) and 5 s (VSASL); TE = 36.1 ms; 15 and 12 label/control pairs for PASL and VSASL, respectively. In VSASL, a VCM using sBIR8 VSS module was applied about 100 ms (PLD) before image acquisition. The Vcut was 2 cm/s along the superoinferior direction. The total scan time was 4 min for each ASL scan. Additional fully relaxed proton-density-weighted reference images were acquired for quantification. 3D T1w anatomical images were collected using MP-RAGE sequence with TR/TE = 2.4 s/2.72 ms, TI = 1.06 s, an isotropic resolution of 0.8 mm, and an acquisition time of 6.5 min.
3.3 Data processing
To ensure the quality of ASL images, the first pair of ASL acquisition was discarded. The BS level (tissue signal) maps were calculated by dividing the mean of control/label images by the relaxed reference images, and expressed in percentage. ASL signal was produced with pairwise subtraction. The signal reductions due to additional BS pulses were corrected, assuming 5% reduction per BS pulse. Normalized mean ASL signal was calculated as percentage relative to the reference image for comparison across subjects. The average ASL signal across time was divided by its temporal SD to calculate the tSNR31 in each scan. CBF was quantified with the modeling and parameters provided earlier. Gray and white matter (GM and WM) and CSF regions of interest (ROIs) were identified after registering the T1w anatomical images to the ASL images and segmentation using the FSL toolbox.32 The normalized ASL signal, tSNR, and CBF were compared between different labeling schemes.
3.4 Statistical analysis
The mean tissue signal (BS level), normalized ASL signal, tSNR, and CBF in the ROIs across subjects were tested for normality (Jarque-Bera test). All values were normally distributed except the tissue signals acquired using PASL, and Wilcoxon signed rank test was used when needed. ASL signal, tSNR, and CBF in the GM and WM ROIs were compared using 1-way analysis of variance (with Tukey–Kramer adjustment) and multiple pairwise t tests. Significant differences were identified with P < 0.05 (uncorrected). Bonferroni correction was applied on the threshold when multiple pairwise comparisons (reported as n) were performed, and uncorrected P value are reported.
4 RESULTS
4.1 SNR efficiency optimization
The results of SNR efficiency simulation are shown in Figure 3. The SNR efficiencies of VSASL methods generally peaked around TR = 5 s. Under ideal conditions, that is, the labeling efficiencies were 1 for all methods, the maximal SNR efficiency of dm-VSASL (TI1/2 = 1.52/0.48 s) was increased by 12.0% compared to sm-VSASL (TI = 1.4 s) for both VSS and VSI. For dm-VSS, the increase was smaller than that of mm-VSS (TI1/2 = 1.18/0.82 s, 41.6%). Note the SNR efficiency advantage of VSASL methods compared to PCASL and PASL in this ideal scenario. When T2 relaxation was included (β = 1), the maximal SNR efficiency of dm-VSI (TI1/2 = 1.46/0.54 s) was almost the same as VSI (TI = 1.4 s) labeling; dm-VSS had a smaller increase (3.4%), whereas mm-VSS (TI1/2 = 1.16/0.84 s) still had an increase of 30.2%, compared to VSS labeling. When realistic VS pulses were considered (with T2 relaxation and β < 1), the SNR efficiencies of VSASL methods would further decrease. Compared to VSI (TI = 1.4 s), dm-VSI (TI1/2 = 1.46/0.54 s) still had a comparable SNR efficiency (0.4% less), and the hybrid dm-VSASL (VSI + VSS, TI1/2 = 1.43/0.57 s) had a slightly lower SNR efficiency (2.7% less). Compared to VSS, dm-VSS (TI1/2 = 1.48/0.52 s) had a slightly higher SNR efficiency (3.4% more), and mm-VSS (TI1/2 = 1.18/0.82 s) had an increase of 28.5%. Note that under this realistic scenario, PCASL had a higher SNR efficiency than VSASL methods when PLD was not long (1.8 s); when a longer PLD (e.g., 2.2 s) was used in PCASL due to prolonged ATTs, dm-VSASL using at least 1 VSI module would have a comparable SNR efficiency and would be more SNR-efficient if the PLD in PCASL had to be further increased.
4.2 In vivo results
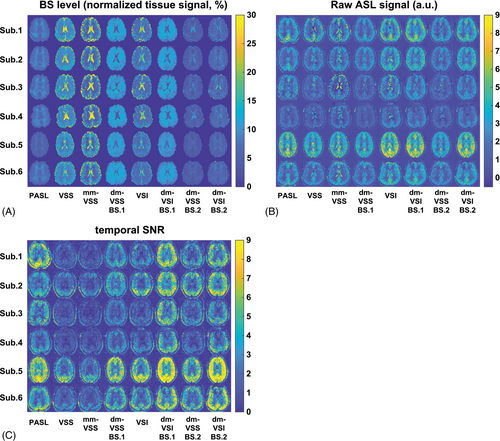
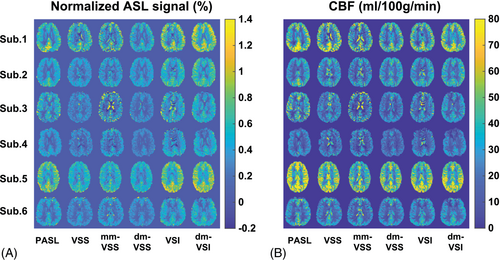
The BS level maps are shown in Figure 4A. The averaged BS levels in different tissue ROIs and across subjects are summarized in Table 1. Different and consistent BS levels were achieved using different ASL methods across subjects. In VSS, mm-VSS, and VSI, the CSF signal could not be sufficiently suppressed (≥ 15.7%) despite the effort in BS timing optimization. In contrast, the new dm-VSASL strategy achieved sufficient suppression across all brain tissues in both BS1 and BS2, including excellent suppression of CSF compared to VSS, mm-VSS, and VSI (≤ 7.1%, P < 0.001, n = 3).
| ASL schemes (n = 6) | PASL | VSS | mm-VSS | dm-VSS (BS1) | VSI | dm-VSI (BS1) | dm-VSS (BS2) | dm-VSI (BS2) | |
|---|---|---|---|---|---|---|---|---|---|
| Mean BS level (mean ± SEM, %) | GM | 5.8 ± 0.5 | 13.4 ± 0.9 | 15.7 ± 2.2 | 9.8 ± 0.4 | 10.6 ± 1.3 | 9.1 ± 0.2 | 4.7 ± 0.4 | 5.7 ± 0.5 |
| WM | 8.5 ± 0.7 | 8.6 ± 0.2 | 5.1 ± 0.2 | 11.4 ± 0.3 | 6.7 ± 0.3 | 11.5 ± 0.4 | 5.2 ± 0.2 | 7.1 ± 0.2 | |
| CSF | 5.4 ± 0.4 | 20.4 ± 2.9 | 23.7 ± 3.7 | 5.2 ± 1.3 | 15.7 ± 2.1 | 5.5 ± 0.9 | 7.1 ± 0.8 | 6.3 ± 0.5 | |
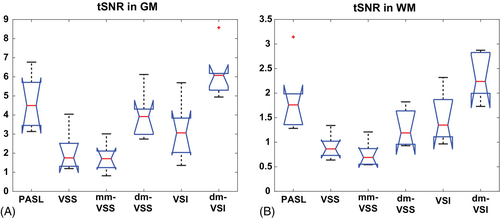
| Mean tSNR (mean ± SEM) | GM | 4.7 ± 1.3 | 2.1 ± 1.0 | 1.8 ± 0.7 | 4.0 ± 1.1 | 3.2 ± 1.4 | 6.2 ± 1.2 | 3.7 ± 1.4 | 5.6 ± 2.0 |
| WM | 1.9 ± 0.6 | 0.9 ± 0.2 | 0.8 ± 0.2 | 1.3 ± 0.3 | 1.5 ± 0.5 | 2.3 ± 0.4 | 1.3 ± 0.4 | 2.2 ± 0.5 | |
- Note: dm-VSS (BS1) and dm-VSI (BS1) were referred to as dm-VSS and dm-VSI for simplicity in later analyses.
- Abbreviations: ASL, arterial spin labeling; BS, background suppression; dm-VSI, dual-module velocity-selective inversion; dm-VSS, dual-module velocity-selective saturation; GM, gray matter; mm-VSS, multi-module velocity-selective saturation; PASL, pulsed ASL; SEM, standard error of the mean; tSNR, temporal SNR; VSS, velocity-selective saturation; VSI, velocity-selective inversion; WM, white matter.
Of the 2 BS settings using dm-VSS and dm-VSI, BS1 had higher GM (P < 0.0006) and WM (P < 1.3 × 10−6) tissue signal than BS2 but lower CSF tissue signal (though not significant, P = 0.11 and 0.17 for dm-VSS and dm-VSI, respectively). BS1 produced ASL images with higher quality than BS2, that is, with higher (though not significantly) tSNR (P = 0.22 for dm-VSS, and P = 0.27 for dm-VSI, respectively), and should provide a reasonable representation of the performance of dm-VSASL. Therefore, further analyses focused on the measurements using the BS1 setting for comparisons between different labeling schemes. For simplicity, dm-VSS with BS1 was referred to as dm-VSS and dm-VSI with BS1 as dm-VSI unless specified.
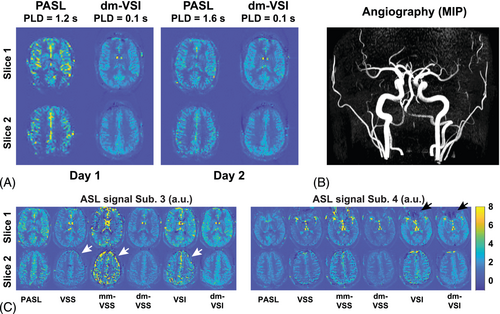
Raw ASL signal maps are shown in Figure 4B. CSF artifacts were noticeable using VSS, mm-VSS, and VSI; whereas dm-VSASL methods yielded markedly improved quality with almost no CSF artifact, especially around the ventricles. These observations were confirmed by the tSNR maps shown in Figure 4C, where obvious tSNR improvement was observed in both the cortical GM and the deep GM regions when comparing dm-VSS versus VSS and mm-VSS, and dm-VSI versus VSI. Note that there was some regional ASL signal reduction in the frontal area in subjects 4 and 6 with VSI-based labeling but not with VSS-based labeling, likely due to its sensitivity to field inhomogeneities (see discussion).
Significantly improved temporal stability using dm-VSASL schemes can be appreciated in an example of the raw ASL signal time series shown in Supporting Information Figure S2. High signal fluctuations were observed in regions where CSF signals were not sufficiently suppressed with VSS, mm-VSS, and VSI labeling. Even though some of the fluctuations were averaged out, there were erroneous ASL signals in voxels with dominant CSF signals, for example, around ventricles and sulci. In contrast, both dm-VSS and dm-VSI produced ASL signals with high temporal stability throughout the brain and had a better performance than PASL.
Averaged tSNR in GM and WM ROIs across subjects is shown in the boxplots in Figure 5A,B, respectively, and summarized in Table 1. Compared to their single-module counterparts, dm-VSS significantly improved the tSNR by 90.8% in GM (P = 1.9 × 10−5) and 41.5% in WM (P = 1.8 × 10−3); and dm-VSI improved the tSNR by 94.9% in GM (P = 1.4 × 10−4) and 55.1% in WM (P = 8.1 × 10−4). Dm-VSS had a significantly higher tSNR in GM (25.9% higher, P = 0.0083) but a lower tSNR in WM (13.8% lower, P = 0.036) than VSI. Compared to VSS, mm-VSS had a similar tSNR in GM (P = 0.11) but a lower tSNR in WM (P = 4.5 × 10−4). In GM, dm-VSI had the highest tSNR among all methods (P < 0.006, n = 8); in WM, dm-VSI had the highest tSNR among the VSASL methods (P < 8.1 × 10−4, n = 8) and tended to have a higher tSNR than PASL (P = 0.048, not significantly after correction, n = 8).
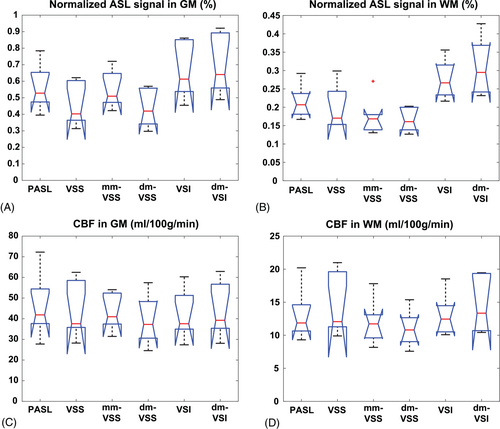
Normalized ASL signal and quantified CBF maps are shown in Figure 6. The averaged values in GM and WM ROIs across subjects are shown in Figure 7 and summarized in Table 2. For GM ASL signal, dm-VSI increased the ASL signal by 5.4% (P = 0.018) compared to VSI labeling; mm-VSS increased ASL signal by 21.1% (P = 0.0009) compared to VSS, consistent with previous results14, 22; whereas dm-VSS had a comparable ASL signal compared to VSS (P = 0.27). Compared to VSS, VSI and dm-VSI increased the ASL signal by 45.1% (P = 0.0002, n = 4) and 53.0% (P = 0.0001, n = 4), respectively. For WM ASL signal, compared to VSS, mm-VSS (P = 0.33) and dm-VSS (P = 0.15) had similar ASL signal, VSI and dm-VSI increased the ASL signal by 39.1% (P = 0.012, n = 4) and 56.3% (P = 0.0006, n = 4), respectively. Compared to VSI, dm-VSI yielded 12.4% higher signal in WM, though not significantly (P = 0.15).
| ASL schemes (n = 6) | PASL | VSS | mm-VSS | dm-VSS | VSI | dm-VSI | |
|---|---|---|---|---|---|---|---|
| Normalized ASL signal (mean ± SEM, %) | GM | 0.56 ± 0.13 | 0.45 ± 0.12 | 0.55 ± 0.10 | 0.43 ± 0.11 | 0.66 ± 0.15 | 0.69 ± 0.17 |
| WM | 0.22 ± 0.04 | 0.20 ± 0.05 | 0.18 ± 0.05 | 0.16 ± 0.03 | 0.28 ± 0.05 | 0.31 ± 0.07 | |
| CBF (mean ± SEM, ml/100 g/min) | GM | 46.0 ± 14.2 | 43.4 ± 12.6 | 42.9 ± 8.1 | 39.2 ± 11.0 | 41.5 ± 11.0 | 43.6 ± 12.3 |
| WM | 13.1 ± 3.6 | 14.3 ± 4.3 | 12.0 ± 3.1 | 11.0 ± 2.6 | 13.1 ± 3.0 | 14.4 ± 3.9 | |
| Gray/white ratio | 3.51 | 3.03 | 3.57 | 3.55 | 3.17 | 3.02 | |
- Note: The signal attenuation from additional background suppression pulses was corrected in ASL signal and CBF calculation.
- Abbreviations: CBF, cerebral blood flow; ROI, region of interest.
There was no significant difference in CBF measured using different ASL methods in GM (P = 0.97) or WM (P = 0.62) ROIs according to 1-way analysis of variance. Averaged gray/white ratios were within the range of 3.02 ˜ 3.57. These values are reported in Table 2.
5 DISCUSSION
The novel dm-VSASL strategy offers a few distinctive advantages compared to existing VSASL methods: (1) the label/control condition switching in the second VS module creates a more balanced distribution of VS (motion-sensitizing) gradients and diffusion weighting in the label/control acquisition, reducing artifacts and errors from sources such as diffusion attenuation, ECs, and possibly pulsatile motion such as in CSF; (2) the inversion effect from the first VS module at an early time point enables more flexible and effective BS, especially of CSF, resulting in further noise reduction; (3) dual-module labeling can increase the SNR efficiency, improving the quality and/or the efficiency of VSASL scans. These features significantly enhanced the accuracy and the robustness of VSASL. Combined with its insensitivity to ATT artifacts and SNR advantage in presence of delayed blood flow, VSASL is particularly suited for perfusion imaging applications such as in vascular disease cohorts or in aging population. VSASL's insensitivity to ATT effects has been demonstrated in healthy subjects and patients.14, 33, 34 An interesting case was encountered in this study and is shown in Figure 8A,B, where ATT artifacts were accidentally observed in a young healthy subject using PASL with a PLD of 1.2 s (corresponding to a TI of 2 s, already longer than recommended TI value of 1.8 s), and were mostly gone (ASL images still a little grainy) after increasing the PLD to 1.6 s; whereas VSASL (only dm-VSI is shown) yielded consistent and ATT–artifact-free perfusion maps.
In principle, dm-VSASL is applicable with any even number of VS modules (e.g., more than 2); however, as each additional VS labeling module results in higher signal reduction due to imperfect labeling, it may not be beneficial to use more than 2 VS module in practice. The SNR efficiency simulation results also emphasized the need for robust VS modules of higher β and shorter effect TE.
The constraint of was adopted for accurate quantification of VSASL in the brain, based on estimation from a few healthy subjects with a whole-body RF coil for labeling.14 may vary in different situations, such as applications in different organs, using different RF coils or in subjects with abnormal arterial velocities. If is smaller, the timing optimization for optimal SNR efficiency should be adjusted accordingly; if is larger, for example, in vascular disease patients with slow and delayed flow, the constraint remains valid and the timings reported in this study are directly applicable, with a slightly suboptimal SNR efficiency. For example, if increases to 2.5 s, using the timings derived under will still achieve 95% of the optimal SNR efficiency.
Compared to PASL, VSASL methods yielded comparable CBF values, suggesting the β values used in this study were reasonable. This is also consistent with the results from a study performed on a different scanner and using PCASL as the reference.22 Compared to VSI, dm-VSI yielded a 5.4% increase of GM ASL signal in in vivo experiments despite the possibility of VSI having artificially higher signal due to diffusion attenuation effect from CSF. This is higher than predicted, possibly due to an improved overall labeling robustness using the dual-module strategy, that is, a slightly higher averaged β in dual-module labeling (e.g., an improved β in the second VS module) than in single-module labeling. On the other hand, the ASL signal improvement of dm-VSS with respect to VSS was lower than that predicted by simulation, suggesting either reduced averaged β in dm-VSS labeling or more severe CSF contamination in VSS labeling. The latter is more likely, judging from the ASL signal maps compared to PASL and the fact that the β of VSS is already close to 1. Nevertheless, accurate measurement of the labeling efficiencies (especially β) of different VS modules and under different labeling strategies is needed for further improved quantification accuracy using VSASL.
Dm-VSASL with BS1 (higher GM/WM and lower CSF signals) had a better tSNR performance than with BS2 (lower GM/WM but slightly higher CSF signals), indicating that CSF contributed much higher noise than GM or WM. This is also supported by the observation that the tSNR improvement with dm-VSASL in GM is higher than that in WM (90.8% vs. 41.5% with VSS and 94.9% vs. 55.1% with VSI) compared with sm-VSASL, likely due to a generally higher partial volume of CSF in “GM” voxels. In PASL and PCASL, the labeling and imaging volumes are separated, and the labeling mechanisms hardly interact with CSF. Therefore, CSF is typically not a significant source of signal variations in PASL and PCASL and can be well suppressed using existing methods.24, 35 In contrast, the labeling in VSASL is global and interacts with tissues in the imaging volume in a more complex way. In sm-VSASL and mm-VSASL, effective suppression of CSF is difficult and limited by the timing constraints for optimizing the SNR, resulting in insufficient BS levels (typically >20%) and high noise from CSF. In addition, the diffusion attenuation effect from CSF is also a greater source of error than that from GM and WM in VSASL. Therefore, good suppression of CSF should be prioritized in VSASL (additional examples of CSF artifacts can be found in Figure 8C). Previous studies had emphasized its importance with efforts to directly suppress CSF signal at the cost of SNR36 or to correct for its erroneous signal with additional post-processing steps.14 With the new dm-VSASL schemes, the BS optimization in VSASL, especially for CSF, is much more amiable. For example, both sm-VSS and dm-VSS had the same number (2) of effective BS pulses, including 1 from the built-in inversion in VSSinv, and dm-VSS had better BS and tSNR performance. The 2 sets of BS parameters used in this study were for demonstration of the flexibility and effectiveness of BS with dm-VSASL; its optimization was not a focus and should be investigated further. In general, more additional BS pulses may provide more flexibility in BS, but the associated signal reduction and increased specific absorption rate should also be taken into consideration in pulse sequence design. In addition, complex reconstruction would be beneficial as it allows more aggressive timings for improved BS while avoiding rectification errors.
Aside from the effective suppression of CSF, the more balanced VS gradient application in label/control images also contributed significantly to the tSNR improvement with dm-VSASL. This is more evident when comparing the tSNR in WM, where the partial volume fraction of CSF is much smaller than in GM. The tSNR increased by 41.5% (dm-VSS vs. VSS) and 55.1% (dm-VSI vs. VSI) despite higher tissue signals (less BS) in WM with dm-VSASL than with sm-VSASL.
In addition to the application in baseline perfusion measurement, VSASL is useful in fMRI studies for its insensitivity to ATT effects.37 And a recent study demonstrated the SNR advantage of VSI-based VSASL in fMRI.23 With almost doubled tSNR in GM compared to existing VSASL methods, dm-VSI should be an excellent tool for imaging functional changes of blood flow.
Despite the excellent SNR of VSI-based labeling, its current implementations (rect-VSI and sinc-VSI) are still somewhat susceptible to field inhomogeneities as predicted by Bloch simulation and shown in vivo.20, 22 For example, as shown in Figure 8C, insufficient labeling and artifacts were observed in regions with compromised homogeneity in B0 and B1 fields. Improving the robustness to field inhomogeneities is highly desired for VSI-based labeling and should be studied further. In addition, better shimming should also help improve the performance. In contrast, sBIR8 VSS-based labeling did not generate such artifacts, suggesting dm-VSS may be more suitable for applications with higher field inhomogeneities. Dual-module labeling increases the specific absorption rate compared to single-module labeling. Though dm-VSI has slightly higher specific absorption rate than dm-VSS, both were within the normal safety limit and can be reduced with pulse optimization.
6 CONCLUSION
The dm-VSASL strategy can significantly reduce noise and artifacts that are typically encountered with existing VSASL methods, offering dramatically enhanced tSNR in both GM and WM. It is achieved by utilizing a more balanced VS gradient configuration in control and label image acquisition and enabling more efficient suppression of background tissue signals, especially of CSF. A slight SNR improvement is also achieved with dm-VSI compared to VSI. With enhanced labeling robustness and reduced artifacts, dm-VSASL can measure perfusion more reliably and accurately, especially in applications where ATT effects are concerned.
ACKNOWLEDGMENT
The author thanks Dr. Eric Wong for inspiration of this project, Dr. Divya Bolar for sharing code, Dr. Sinyoeb Ahn for help in sequence implementation, and Dr. Jason Langley for help in data collection.
FUNDING INFORMATION
This work is supported by the National Institutes of Health (NIH), grant R01EB033210.




