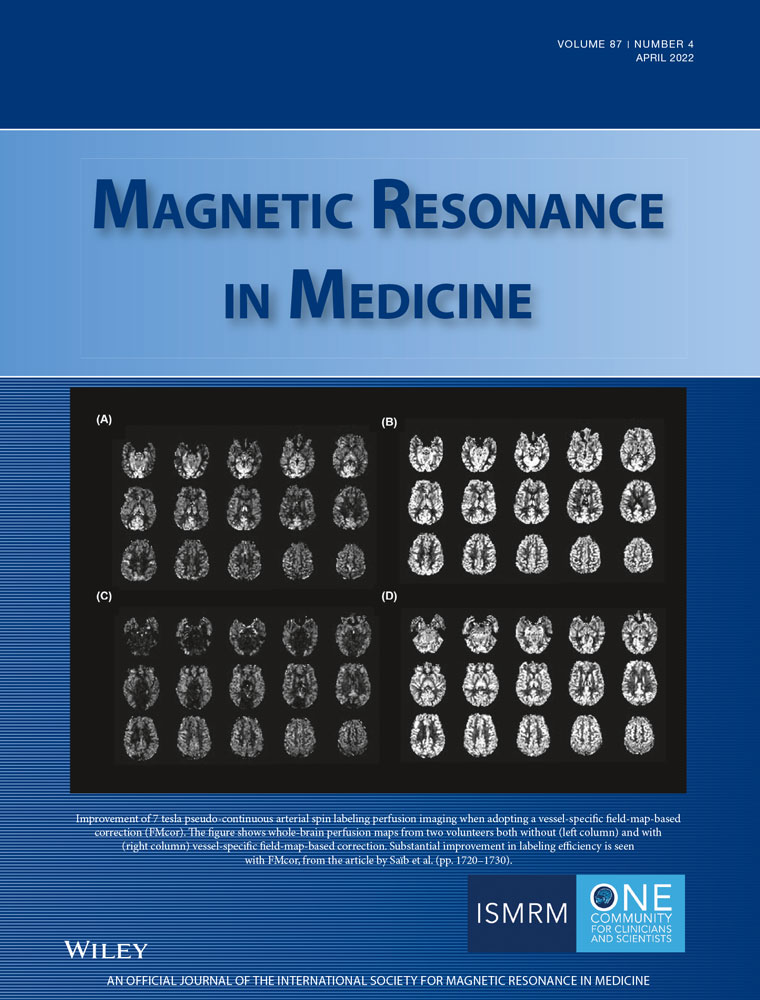
Simultaneous comprehensive liver T1, T2, 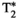 , T1ρ, and fat fraction characterization with MR fingerprinting
, T1ρ, and fat fraction characterization with MR fingerprinting
Funding information
EPSRC (EP/P032311/1, EP/P007619/1, EP/P001009/1, EP/V044087/1); the Wellcome/EPSRC Center for Medical Engineering (WT 203148/Z/16/Z); Fondecyt (1210637); and Department of Health through the National Institute for Health Research (NIHR) comprehensive Biomedical Research Center Award to Guy’s & St. Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust and by the NIHR MedTech Co-operative for Cardiovascular Disease at Guy’s and St. Thomas’ NHS Foundation Trust. This research was funded in whole, or in part, by the Wellcome Trust (WT 203148/Z/16/Z). For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Abstract
Purpose
To develop a novel simultaneous co-registered T1, T2, 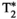 , T1ρ, and fat fraction abdominal MR fingerprinting (MRF) approach for fully comprehensive liver-tissue characterization in a single breath-hold scan.
, T1ρ, and fat fraction abdominal MR fingerprinting (MRF) approach for fully comprehensive liver-tissue characterization in a single breath-hold scan.
Methods
A gradient-echo liver MRF sequence with low fixed flip angle, multi-echo radial readout, and varying magnetization preparation pulses for multiparametric encoding is performed at 1.5 T. The 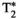 and fat fraction are estimated from a graph/cut water/fat separation method using a six-peak fat model. Water/fat singular images obtained are then matched to an MRF dictionary, estimating water-specific T1, T2, and T1ρ. The proposed approach was tested in phantoms and 10 healthy subjects and compared against conventional sequences.
and fat fraction are estimated from a graph/cut water/fat separation method using a six-peak fat model. Water/fat singular images obtained are then matched to an MRF dictionary, estimating water-specific T1, T2, and T1ρ. The proposed approach was tested in phantoms and 10 healthy subjects and compared against conventional sequences.
Results
For the phantom studies, linear fits show excellent coefficients of determination (r2 > 0.9) for every parametric map. For in vivo studies, the average values measured within regions of interest drawn on liver, spleen, muscle, and fat are statistically different from the reference scans (p < 0.05) for T1, T2, and T1⍴ but not for 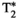 and fat fraction, whereas correlation between MRF and reference scans is excellent for each parameter (r2 > 0.92 for every parameter).
and fat fraction, whereas correlation between MRF and reference scans is excellent for each parameter (r2 > 0.92 for every parameter).
Conclusion
The proposed multi-echo inversion-recovery, T2, and T1⍴ prepared liver MRF sequence presented in this work allows for quantitative T1, T2, 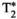 , T1⍴, and fat fraction liver-tissue characterization in a single breath-hold scan of 18 seconds. The approach showed good agreement and correlation with respect to reference clinical maps.
, T1⍴, and fat fraction liver-tissue characterization in a single breath-hold scan of 18 seconds. The approach showed good agreement and correlation with respect to reference clinical maps.
1 INTRODUCTION
Chronic liver disease is defined as a progressive destruction of liver tissue over a period greater than 6 months. One of the main causes of chronic liver disease, with a 25% prevalence in the world’s population,1 is nonalcoholic fatty liver disease. This disease can progress through different stages, from inflammation (nonalcoholic steatohepatitis or NASH) to hepatocellular carcinoma, passing through liver fibrosis and liver cirrhosis.2 Liver biopsy, despite its invasiveness, is considered to be the current gold standard for staging and assessment of liver fibrosis. Still, many factors such as sampling errors and interrater variability can affect the accuracy and diagnostic value of this technique.3-5 To overcome the limitations of liver biopsy, many noninvasive alternatives have been proposed.6, 7 Among these, quantitative MRI arises as a promising imaging tool for the assessment and follow-up of liver disease. Particularly, quantitative maps of T1 and T2 (for fibrosis and inflammation detection8, 9), 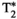 (for hepatic iron content visualization10), and fat fraction (FF, for hepatic lipid content assessment11, 12) are promising imaging biomarkers for the assessment and follow-up of liver disease. Multiparametric information is conventionally obtained by performing sequential scans during different breath-holds,13 which leads to long scan times, bias due to inter-parameter dependencies, patient discomfort, and potential multiparametric image misalignments due to subject’s bulk and respiratory motion. Many efforts have been undertaken to develop multiparametric methods that provide joint parameter mapping in a single scan.14, 15 In recent years, MR fingerprinting (MRF)16 has shown to be a powerful technique to provide multiparametric information in abdominal scans.17-20 Particularly, T1, T2, and B1 maps have been obtained with MRF in a single breath-hold scan of about 19 seconds.17 We recently proposed an MRF framework able to quantify T1, T2,
(for hepatic iron content visualization10), and fat fraction (FF, for hepatic lipid content assessment11, 12) are promising imaging biomarkers for the assessment and follow-up of liver disease. Multiparametric information is conventionally obtained by performing sequential scans during different breath-holds,13 which leads to long scan times, bias due to inter-parameter dependencies, patient discomfort, and potential multiparametric image misalignments due to subject’s bulk and respiratory motion. Many efforts have been undertaken to develop multiparametric methods that provide joint parameter mapping in a single scan.14, 15 In recent years, MR fingerprinting (MRF)16 has shown to be a powerful technique to provide multiparametric information in abdominal scans.17-20 Particularly, T1, T2, and B1 maps have been obtained with MRF in a single breath-hold scan of about 19 seconds.17 We recently proposed an MRF framework able to quantify T1, T2, 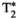 , and fat fraction (FF) from a single 14-second breath-hold scan,20 whereas simultaneous T1, T2, and T1ρ MRF relaxation mapping was recently proposed by Wyatt et al.18 T1⍴ mapping has been shown to be of importance in liver fibrosis assessment21, 22 and function.23 T1⍴ relaxation correlates with physical processes that take place within the low-frequency range (0.1-1 kHz),24 which are related to interactions between bulk water and extracellular matrix macromolecules, such as collagen or proteoglycans.25-27 Therefore, it could provide additional information to standard T1 and T2 imaging regarding macromolecular composition of liver tissue, especially when these three relaxation parameters are measured in the same scan. A comprehensive scan that is able to simultaneously quantify not only T1, T2, and T1ρ (as proposed by Wyatt et al18), but also providing
, and fat fraction (FF) from a single 14-second breath-hold scan,20 whereas simultaneous T1, T2, and T1ρ MRF relaxation mapping was recently proposed by Wyatt et al.18 T1⍴ mapping has been shown to be of importance in liver fibrosis assessment21, 22 and function.23 T1⍴ relaxation correlates with physical processes that take place within the low-frequency range (0.1-1 kHz),24 which are related to interactions between bulk water and extracellular matrix macromolecules, such as collagen or proteoglycans.25-27 Therefore, it could provide additional information to standard T1 and T2 imaging regarding macromolecular composition of liver tissue, especially when these three relaxation parameters are measured in the same scan. A comprehensive scan that is able to simultaneously quantify not only T1, T2, and T1ρ (as proposed by Wyatt et al18), but also providing  and FF quantification, is desired.
and FF quantification, is desired.
In this work, we build on our previous work20 to propose a simultaneous co-registered T1, T2, , T1ρ, and FF abdominal MRF approach to enable a fully comprehensive liver-tissue characterization in a single breath-hold scan.
, T1ρ, and FF abdominal MRF approach to enable a fully comprehensive liver-tissue characterization in a single breath-hold scan.
2 METHODS
2.1 Magnetic resonance fingerprinting acquisition scheme
An eight-echo gradient-echo (GRE) liver MRF sequence for simultaneous quantification of T1, T2,  , T1⍴, and FF is proposed (Figure 1), based on our previous work.20 The acquisition includes two inversion-recovery (IR) (TI = {1,100} ms) pulses, five T2-prepared pulses (TEs = {30,60} ms) with adiabatic refocusing pulses, five spin-lock (SL) pulses (SL times [SLT] = {30,60} ms) of SL amplitude (SLA) = 350 Hz, and six additional blocks in which no magnetization preparation is applied, leading to a total of 18 acquisition blocks of 507-ms duration each. Acquisition blocks are spaced regularly every 1 second, allowing for magnetization recovery before the next block. The T1ρ preparation pulses robust to both B1 and B0 inhomogeneities were considered.28, 29 Acquisition parameters included golden-angle radial (~111°) readout; fixed low flip angle (15°); TR/TE1/ΔTE = 16.9/1.6/1.95 ms (in which all of the echoes are sampled along the same radial spoke); FOV = 350 × 350 mm2; voxel size = 2 × 2 mm2; slice thickness = 8 mm; number of readouts per block = 30; one time-point image per radial readout, leading to a total of 540 timepoints; and scan time of 18 seconds. Acquisitions were performed on phantoms and 10 healthy subjects on a 1.5T scanner (Ingenia, Philips Healthcare).
, T1⍴, and FF is proposed (Figure 1), based on our previous work.20 The acquisition includes two inversion-recovery (IR) (TI = {1,100} ms) pulses, five T2-prepared pulses (TEs = {30,60} ms) with adiabatic refocusing pulses, five spin-lock (SL) pulses (SL times [SLT] = {30,60} ms) of SL amplitude (SLA) = 350 Hz, and six additional blocks in which no magnetization preparation is applied, leading to a total of 18 acquisition blocks of 507-ms duration each. Acquisition blocks are spaced regularly every 1 second, allowing for magnetization recovery before the next block. The T1ρ preparation pulses robust to both B1 and B0 inhomogeneities were considered.28, 29 Acquisition parameters included golden-angle radial (~111°) readout; fixed low flip angle (15°); TR/TE1/ΔTE = 16.9/1.6/1.95 ms (in which all of the echoes are sampled along the same radial spoke); FOV = 350 × 350 mm2; voxel size = 2 × 2 mm2; slice thickness = 8 mm; number of readouts per block = 30; one time-point image per radial readout, leading to a total of 540 timepoints; and scan time of 18 seconds. Acquisitions were performed on phantoms and 10 healthy subjects on a 1.5T scanner (Ingenia, Philips Healthcare).
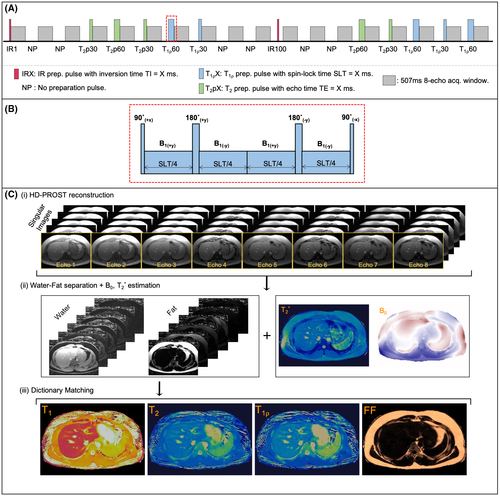
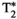 , T1ρ, and fat fraction (FF) quantification. (B) Schematic depiction of spin-lock (SL) pulse used18, 28 as T1⍴ preparation pulse. The SL pulse is equally divided into four blocks with alternating phase, all of them at SL amplitude (SLA) = 350 Hz. (C) Reconstruction framework: (i) A set of singular images for each echo is obtained by low-rank inversion (LRI) reconstruction and regularized with a multicontrast patch-based higher-order low-rank reconstruction (HD-PROST)30; (ii) the first singular image of each echo is used for water/fat separation (B0 and
, T1ρ, and fat fraction (FF) quantification. (B) Schematic depiction of spin-lock (SL) pulse used18, 28 as T1⍴ preparation pulse. The SL pulse is equally divided into four blocks with alternating phase, all of them at SL amplitude (SLA) = 350 Hz. (C) Reconstruction framework: (i) A set of singular images for each echo is obtained by low-rank inversion (LRI) reconstruction and regularized with a multicontrast patch-based higher-order low-rank reconstruction (HD-PROST)30; (ii) the first singular image of each echo is used for water/fat separation (B0 and 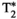 maps are also obtained in this step); and (iii) T1, T2, and T1⍴ maps are obtained by matching water/fat singular images to an extended phase graph–based dictionary. The FF map is calculated from water and fat M0 maps. Abbreviations: IRX, inversion-recovery prep pulse with TI – X ms; NP, no preparation pulse; SLT, SL time
maps are also obtained in this step); and (iii) T1, T2, and T1⍴ maps are obtained by matching water/fat singular images to an extended phase graph–based dictionary. The FF map is calculated from water and fat M0 maps. Abbreviations: IRX, inversion-recovery prep pulse with TI – X ms; NP, no preparation pulse; SLT, SL time2.2 Magnetic resonance fingerprinting reconstruction
Reconstruction of the highly undersampled MRF data is performed using a multicontrast patch-based higher-order low-rank reconstruction30 with dictionary-based temporal compression (obtained from a truncated singular value decomposition to a rank R of the MRF dictionary31). The rank value R = 10 of the temporal compression is determined by the minimum value that captures 96% of the matrix energy ratio of the singular value decomposition, and the reconstruction problem is solved using the alternating direction method of multipliers.32 Coil sensitivity maps needed for the reconstruction are derived from ESPIRiT33 using all of the acquired data. Additional details on the reconstruction, including reconstruction parameters and times, are given in Supporting Information S1.
2.3 T1, T2, 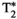 , T1⍴, and FF map estimation
, T1⍴, and FF map estimation
A set of singular images for each echo is obtained after the reconstruction scheme described previously. Following previous work,20, 34 water/fat separation and 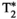 , B0 estimation is performed using a graph/cut algorithm described by Hernando et al,35 using a six-peak fat model.36 The model assumes the same T1 and T2 values of the fat peaks.
, B0 estimation is performed using a graph/cut algorithm described by Hernando et al,35 using a six-peak fat model.36 The model assumes the same T1 and T2 values of the fat peaks.
Water/fat singular images obtained are then used to match a previously calculated extended phase graph–based dictionary.37 Additional details on dictionary generation are provided in Supporting Information S1. Water-specific and fat-specific T1, T2, T1⍴, and apparent M0 maps are obtained from this inner-product matching. The FF map is subsequently estimated from water and fat M0 maps.38
2.4 Phantom study
The proposed MRF framework was evaluated in two custom-made phantoms. One of them consisted of a set of sample tubes filled with different concentrations of agar (for T2 and T1⍴ variation) and NiCl2 (for T1 variation) following the relaxation model provided in Captur et al.40 The other phantom consisted of a set of vials of different concentrations of peanut oil (from 0% to 100%) and was used exclusively for FF validation. The phantoms were scanned with the proposed MRF framework and the corresponding conventional mapping sequences.
The MRF measurements on the agar-based phantom were compared against IR spin echo (SE) for T1, multi-echo SE for T2, eight-echo GRE for  , and a T1⍴-prepared SE (T1⍴-SE) with SLT = {0,10,25,40,50,60,70,80} ms at SLA = 350 Hz for T1⍴ (where maps were obtained from a voxel-wise linear fitting of the logarithm of the signal intensity measured in every T1⍴ prepared acquisition28). The FF reference maps were obtained in the oil-based phantom with a six-echo GRE. Additional details of the conventional mapping sequences are provided in Supporting Information Table S1. Additionally, T1-modifed Look-Locker inversion recovery (MOLLI)41 and T2–gradient and SE (GRASE)42 were used as clinical conventional reference maps.
, and a T1⍴-prepared SE (T1⍴-SE) with SLT = {0,10,25,40,50,60,70,80} ms at SLA = 350 Hz for T1⍴ (where maps were obtained from a voxel-wise linear fitting of the logarithm of the signal intensity measured in every T1⍴ prepared acquisition28). The FF reference maps were obtained in the oil-based phantom with a six-echo GRE. Additional details of the conventional mapping sequences are provided in Supporting Information Table S1. Additionally, T1-modifed Look-Locker inversion recovery (MOLLI)41 and T2–gradient and SE (GRASE)42 were used as clinical conventional reference maps.
2.5 In vivo study
Ten healthy subjects (age = 31 ± 4 years, 6 females) were scanned with the same protocol as used in the phantom. Two of the subjects (age = 34 ± 1 years, 2 males) declared previously diagnosed mild hepatic steatosis. The study was approved by the institutional review board, and written informed consent was obtained from all subjects according to institutional guidelines. Two-dimensional axial acquisitions were performed in the abdominal region.
Conventional 2D T1-MOLLI41 and T2-GraSE42 acquisitions were performed for T1 and T2 reference measurements, respectively. A single breath-held spoiled GRE sequence T1⍴-GRE was performed for T1⍴ reference measurement. This scan consisted of five consecutive single-shot spoiled GRE acquisitions (SLTs = {0, 10, 20, 40, and 60 ms}, at SLA = 350 Hz) with a 4.5-second gap between SLT acquisitions to allow for magnetization recovery (total scan time ~20 seconds).  and FF in vivo quantification were obtained with the same reference multi-echo GRE scans used in the phantom study.
and FF in vivo quantification were obtained with the same reference multi-echo GRE scans used in the phantom study.
2.6 Statistical analysis
Circular regions of interest (ROIs) of about 16-mm diameter were drawn inside each sample tube of the phantoms for all parametric maps. Measurements for each ROI were obtained as mean ± SD. Coefficients of determination and best fits are reported for MRF against SE-based reference maps for the phantom study. Bland-Altman plots43 along with the calculated bias and limits of agreement (defined as mean bias ±1.96 SD) are also reported.
For in vivo studies, ROIs were manually drawn inside liver (avoiding blood vessels), spleen, and erector spinae muscle. Additionally, FF was also measured in the fat maps within a ROI manually drawn in the subcutaneous fat. Mean ± SD values for each parameter are reported for MRF and reference mapping sequence; measurement distributions are shown with violin plots. Correlation between MRF and conventional measurements is assessed with their respective scatter plots, coefficients of determination, and best fits.
3 RESULTS
3.1 Phantom study
Correlation analyses for the average T1, T2,  , T1⍴, and FF maps of MRF against their respective reference measurements (i.e., T1-IRSE, T2-multi-echo SE, eight-echo GRE, T1⍴-prepared SE, and six-echo GRE) are shown in Figure 2A. The corresponding Bland-Altman plots are shown in Figure 2B. Linear fits show excellent coefficients of determination (r2 > 0.9) for each parameter, while a zero bias is always included in the 95% confidence interval of their respective Bland-Altman analyses. Best fits, coefficients of determination, biases, and limits of agreement are reported in Supporting Information Table S2. Mean ± SD values measured inside each vial are reported in Supporting Information Table S3. Mean ± SD T1 and T2 MRF values compared with T1-MOLLI and T2-GRASE are reported in Supporting Information Table S4.
, T1⍴, and FF maps of MRF against their respective reference measurements (i.e., T1-IRSE, T2-multi-echo SE, eight-echo GRE, T1⍴-prepared SE, and six-echo GRE) are shown in Figure 2A. The corresponding Bland-Altman plots are shown in Figure 2B. Linear fits show excellent coefficients of determination (r2 > 0.9) for each parameter, while a zero bias is always included in the 95% confidence interval of their respective Bland-Altman analyses. Best fits, coefficients of determination, biases, and limits of agreement are reported in Supporting Information Table S2. Mean ± SD values measured inside each vial are reported in Supporting Information Table S3. Mean ± SD T1 and T2 MRF values compared with T1-MOLLI and T2-GRASE are reported in Supporting Information Table S4.
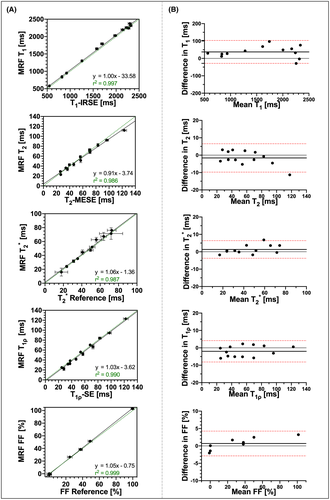
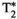 , T1⍴, and FF liver MRF quantification compared against their reference values obtained from T1 inversion-recovery spin echo (IRSE), T2 multi-echo spin echo (MESE), eight-echo gradient-echo (GRE)
, T1⍴, and FF liver MRF quantification compared against their reference values obtained from T1 inversion-recovery spin echo (IRSE), T2 multi-echo spin echo (MESE), eight-echo gradient-echo (GRE) 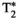 , T1⍴ SE, and six-echo GRE FF, respectively. Black straight lines depict the best linear fit. Green dashed lines depict identity lines. Coefficients of determination and best fits are shown for each individual plot. (B) Corresponding Bland-Altman plots of their scatter plots on the left. Black thick straight lines depict mean bias, and dashed red lines depict the limits of agreement (±1.96 SD)
, T1⍴ SE, and six-echo GRE FF, respectively. Black straight lines depict the best linear fit. Green dashed lines depict identity lines. Coefficients of determination and best fits are shown for each individual plot. (B) Corresponding Bland-Altman plots of their scatter plots on the left. Black thick straight lines depict mean bias, and dashed red lines depict the limits of agreement (±1.96 SD)3.2 In vivo study
A comparison between the maps obtained with the proposed MRF framework and reference maps is shown in Figure 3 for 2 representative healthy subjects and in Figure 4 for 2 subjects who presented with previously diagnosed mild hepatic steatosis. This mild elevation of fat percentage in liver tissue can be observed in the proposed FF-MRF map (13.3 ± 4.4% and 20.5 ± 2.7% for each subject, respectively) and was confirmed by the reference six-echo GRE in both cases (16.4 ± 5.3% and 18.6 ± 10.5% for each subject, respectively). The quantitative analysis of MRF-derived T1, T2, 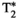 , T1⍴, and FF values measured within ROIs manually drawn inside the liver, spleen, erector spinae muscle, and subcutaneous fat is shown in Figure 5A, and the corresponding values in Supporting Information Table S5. The average values measured within each ROI are statistically different from the reference scans (p < 0.05) for T1, T2, and T1⍴ but not for
, T1⍴, and FF values measured within ROIs manually drawn inside the liver, spleen, erector spinae muscle, and subcutaneous fat is shown in Figure 5A, and the corresponding values in Supporting Information Table S5. The average values measured within each ROI are statistically different from the reference scans (p < 0.05) for T1, T2, and T1⍴ but not for 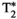 and FF, whereas correlation between MRF and reference values (shown in Figure 5B) is excellent for each parameter (r2 = 0.95, r2 = 0.97, r2 = 0.92, r2 = 0.98, and r2 = 0.99 for T1, T2,
and FF, whereas correlation between MRF and reference values (shown in Figure 5B) is excellent for each parameter (r2 = 0.95, r2 = 0.97, r2 = 0.92, r2 = 0.98, and r2 = 0.99 for T1, T2,  , T1⍴, and FF, respectively).
, T1⍴, and FF, respectively).
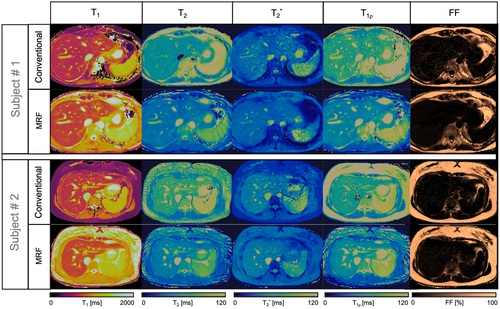
 , T1ρ, and FF quantitative maps in 2 representative healthy subjects. Conventional maps (top row for each subject) of T1 modified Look-Locker inversion recovery (MOLLI), T2 gradient and spin echo (GRASE), eight-echo GRE
, T1ρ, and FF quantitative maps in 2 representative healthy subjects. Conventional maps (top row for each subject) of T1 modified Look-Locker inversion recovery (MOLLI), T2 gradient and spin echo (GRASE), eight-echo GRE  , T1⍴-TFE, and six-echo GRE FF are compared against the proposed inherently co-registered T1, T2,
, T1⍴-TFE, and six-echo GRE FF are compared against the proposed inherently co-registered T1, T2, 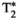 , T1ρ, and FF abdominal MRF maps (bottom row for each subject) obtained from a single 18-second scan. Average parametric values measured within a region of interest (ROI) in the liver are as follows: subject 1: T1 = 636 ± 25 ms, T2 = 54.5 ± 2.1 ms,
, T1ρ, and FF abdominal MRF maps (bottom row for each subject) obtained from a single 18-second scan. Average parametric values measured within a region of interest (ROI) in the liver are as follows: subject 1: T1 = 636 ± 25 ms, T2 = 54.5 ± 2.1 ms, 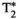 = 34.1 ± 4.9, T1⍴ = 65.9 ± 6.4 ms, and FF = 3.9 ± 5.8% for the conventional maps, and T1 = 756 ± 19 ms, T2 = 36.5 ± 1.1 ms,
= 34.1 ± 4.9, T1⍴ = 65.9 ± 6.4 ms, and FF = 3.9 ± 5.8% for the conventional maps, and T1 = 756 ± 19 ms, T2 = 36.5 ± 1.1 ms, 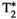 = 35.4 ± 2.8, T1⍴ = 54.2 ± 2.3 ms, and FF = 2.5 ± 2.8% for MRF; and subject 2: T1 = 677 ± 39 ms, T2 = 61.4 ± 5.5 ms,
= 35.4 ± 2.8, T1⍴ = 54.2 ± 2.3 ms, and FF = 2.5 ± 2.8% for MRF; and subject 2: T1 = 677 ± 39 ms, T2 = 61.4 ± 5.5 ms, 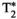 = 43.4 ± 5.2, T1⍴ = 64.2 ± 5.5 ms, and FF = 0.7 ± 6.5% for the conventional maps, and T1 = 731 ± 15 ms, T2 = 44.7 ± 1.6 ms,
= 43.4 ± 5.2, T1⍴ = 64.2 ± 5.5 ms, and FF = 0.7 ± 6.5% for the conventional maps, and T1 = 731 ± 15 ms, T2 = 44.7 ± 1.6 ms, 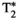 = 46.6 ± 4.7, T1⍴ = 52.9 ± 2.3 ms, and FF = 3.8 ± 2.0% for MRF
= 46.6 ± 4.7, T1⍴ = 52.9 ± 2.3 ms, and FF = 3.8 ± 2.0% for MRF
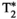 , T1ρ, and FF quantitative maps in 2 representative subjects with known mild hepatic steatosis. Conventional maps (top row for each subject) of T1 MOLLI, T2 GRASE, eight-echo GRE
, T1ρ, and FF quantitative maps in 2 representative subjects with known mild hepatic steatosis. Conventional maps (top row for each subject) of T1 MOLLI, T2 GRASE, eight-echo GRE 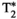 , T1⍴ turbo field echo (TFE), and six-echo GRE FF are compared against the proposed inherently co-registered T1, T2,
, T1⍴ turbo field echo (TFE), and six-echo GRE FF are compared against the proposed inherently co-registered T1, T2, 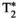 , T1ρ, and FF abdominal MRF maps (bottom row for each subject) obtained from a single 18-s scan. Average parametric values measured within an ROI in the liver are as follows: subject 1: T1 = 477 ± 36 ms, T2 = 62.7 ± 5.2 ms,
, T1ρ, and FF abdominal MRF maps (bottom row for each subject) obtained from a single 18-s scan. Average parametric values measured within an ROI in the liver are as follows: subject 1: T1 = 477 ± 36 ms, T2 = 62.7 ± 5.2 ms, 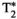 = 31.7 ± 8.9, T1⍴ = 54.1 ± 8.9 ms, and FF = 18.6 ± 10.5% for the conventional maps, and T1 = 683 ± 20 ms, T2 = 34.7 ± 2.3 ms,
= 31.7 ± 8.9, T1⍴ = 54.1 ± 8.9 ms, and FF = 18.6 ± 10.5% for the conventional maps, and T1 = 683 ± 20 ms, T2 = 34.7 ± 2.3 ms, 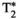 = 28.3 ± 2.8, T1⍴ = 40.8 ± 2.5 ms, and FF = 20.5 ± 2.7% for MRF; and subject 2: T1 = 455 ± 36 ms, T2 = 48.4 ± 4.1 ms,
= 28.3 ± 2.8, T1⍴ = 40.8 ± 2.5 ms, and FF = 20.5 ± 2.7% for MRF; and subject 2: T1 = 455 ± 36 ms, T2 = 48.4 ± 4.1 ms, 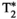 = 23.2 ± 3.0, T1⍴ = 50.4 ± 7.3 ms, and FF = 16.4 ± 5.3% for the conventional maps, and T1 = 624 ± 12 ms, T2 = 30.4 ± 1.9 ms,
= 23.2 ± 3.0, T1⍴ = 50.4 ± 7.3 ms, and FF = 16.4 ± 5.3% for the conventional maps, and T1 = 624 ± 12 ms, T2 = 30.4 ± 1.9 ms, 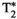 = 19.5 ± 1.1, T1⍴ = 36.8 ± 3.4 ms, and FF = 13.3 ± 4.4% for MRF
= 19.5 ± 1.1, T1⍴ = 36.8 ± 3.4 ms, and FF = 13.3 ± 4.4% for MRF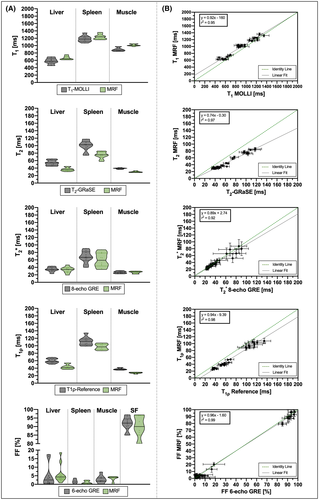
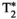 , T1ρ, and FF abdominal MRF quantification. Measurements were taken from circular ROIs drawn in liver, spleen, and erector spinae muscle. Additionally, FF was measured within an ROI drawn in subcutaneous fat (SF). The MRF values (green violins) are compared against conventional values (gray violins). Individual measurements (colored dots) as well as population medians (thick lines) and 25th/75th percentiles (fine-colored lines) are also depicted. (B) Scatter plots for all of the MRF abdominal measurements of each parameter against their reference. Best fits are denoted with black lines. Green dashed lines depict identity lines
, T1ρ, and FF abdominal MRF quantification. Measurements were taken from circular ROIs drawn in liver, spleen, and erector spinae muscle. Additionally, FF was measured within an ROI drawn in subcutaneous fat (SF). The MRF values (green violins) are compared against conventional values (gray violins). Individual measurements (colored dots) as well as population medians (thick lines) and 25th/75th percentiles (fine-colored lines) are also depicted. (B) Scatter plots for all of the MRF abdominal measurements of each parameter against their reference. Best fits are denoted with black lines. Green dashed lines depict identity lines4 DISCUSSION
An eight-echo MRF approach is proposed to enable simultaneous (inherently co-registered) and comprehensive water-specific T1, T2, 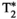 , and T1⍴ maps and FF liver maps in a single breath-hold scan. The framework relies on a radial multi-echo readout to estimate
, and T1⍴ maps and FF liver maps in a single breath-hold scan. The framework relies on a radial multi-echo readout to estimate 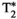 and B0 and separate the signal in water-based and fat-based fingerprints, and on IR, T2, and T1⍴ preparations to encode T1, T2, and T1⍴. The number of echoes was chosen based on previous optimization studies shown in Jaubert et al.20 The method provides a set of reconstructed multi-echo singular images, from where water-fat separation is performed. Subsequently, T1, T2, and T1⍴ parametric maps are estimated by matching the water-based fingerprints to a precalculated dictionary, whereas FF is estimated from the water and fat M0 maps. Because the multiparametric quantification is performed on several tissues, including liver, the dictionary T1, T2, and T1⍴ combinations were chosen to represent a broad range of combinations that covered those tissues. Different to our previous work,20 five SL pulses are applied here with different SLT in order to provide additional T1⍴ encoding. The SL pulses used in this work are totally balanced,29, 44, 45 which have been shown to be robust to B1 and B0 inhomogeneities.46-48 A SL amplitude of SLA = 350 Hz was chosen for these experiments. The SLA is known to have a positive correlation with maximum contrast achievable between healthy and fibrotic tissue49, 50; hence, the application of SL pulses at the highest possible SLA is desired. However, several factors limit the maximum SLA achievable, including the maximum power of the RF amplifiers and specific absorption rate restrictions, which are especially stringent in abdominal scans. To address these limitations, a compromise between maximum SLA and maximum SLT applied must be found. Therefore, an SLA = 350 Hz was chosen to make SLT = 60 ms feasible, despite scanner and specific absorption rate limitations.
and B0 and separate the signal in water-based and fat-based fingerprints, and on IR, T2, and T1⍴ preparations to encode T1, T2, and T1⍴. The number of echoes was chosen based on previous optimization studies shown in Jaubert et al.20 The method provides a set of reconstructed multi-echo singular images, from where water-fat separation is performed. Subsequently, T1, T2, and T1⍴ parametric maps are estimated by matching the water-based fingerprints to a precalculated dictionary, whereas FF is estimated from the water and fat M0 maps. Because the multiparametric quantification is performed on several tissues, including liver, the dictionary T1, T2, and T1⍴ combinations were chosen to represent a broad range of combinations that covered those tissues. Different to our previous work,20 five SL pulses are applied here with different SLT in order to provide additional T1⍴ encoding. The SL pulses used in this work are totally balanced,29, 44, 45 which have been shown to be robust to B1 and B0 inhomogeneities.46-48 A SL amplitude of SLA = 350 Hz was chosen for these experiments. The SLA is known to have a positive correlation with maximum contrast achievable between healthy and fibrotic tissue49, 50; hence, the application of SL pulses at the highest possible SLA is desired. However, several factors limit the maximum SLA achievable, including the maximum power of the RF amplifiers and specific absorption rate restrictions, which are especially stringent in abdominal scans. To address these limitations, a compromise between maximum SLA and maximum SLT applied must be found. Therefore, an SLA = 350 Hz was chosen to make SLT = 60 ms feasible, despite scanner and specific absorption rate limitations.
Phantom experiments showed excellent agreement between MRF and reference measurements for all parameters. The T1⍴ and T2 quantitative results in the agar-based phantom were similar between them for both reference and MRF measurements. This is in line with previous MRF-based studies18, 51, 52 and indicate that the mechanisms that model T1⍴ and T2 relaxations are the same in agar-based phantoms.
In vivo experiments demonstrated the feasibility of the proposed MRF approach to provide simultaneous quantification of T1, T2, 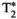 , T1⍴, and FF in a single breath-hold scan of 18 seconds. The MRF parametric maps (as those shown in Figures 3 and 4) presented similar, if not better, visual image quality than the conventional mapping approaches, with the added value of being inherently co-registered and obtained in a single scan, thus reducing considerably the scan time from five acquisitions (plus their respective preparation times) to just one. Considering the quantitative results,
, T1⍴, and FF in a single breath-hold scan of 18 seconds. The MRF parametric maps (as those shown in Figures 3 and 4) presented similar, if not better, visual image quality than the conventional mapping approaches, with the added value of being inherently co-registered and obtained in a single scan, thus reducing considerably the scan time from five acquisitions (plus their respective preparation times) to just one. Considering the quantitative results, 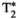 and FF showed a very good correlation and low bias between MRF and the conventional mapping techniques, with MRF measurements not significatively different from the reference values. Very good agreement was also found in FF quantification; this was also observed in the liver FF values measured with the proposed MRF and the conventional proton density FF approach in 2 subjects who presented with mild hepatic steatosis. T1 values obtained with MOLLI were slightly lower than those obtained with the proposed MRF framework, whereas T2 and T1⍴ values measured with the conventional mapping approaches were higher than those measured with the proposed MRF. The MOLLI technique has a known negative bias, and this underestimation has been previously reported.20, 53 This underestimation is also observed in the phantom study, as shown in Supporting Information Figure S1. The MRF underestimation bias for T2 has also been previously reported in several studies.18, 20, 34, 39, 54, 55 Diffusion effects56 or imperfect T2 relaxation during the adiabatic T2 preparation pulses could be some of the sources of this underestimation. A clinical reference technique for T1⍴ quantification does not currently exist in abdominal imaging. To enable in vivo liver T1⍴ validation, a fast, single-breath-hold T1⍴ reference scan, similar to the one used by Wyatt et al,18 was used as reference; however, this approach has its own limitations and may present a bias. Liver T1⍴ values obtained with this reference technique were 58.0 ms (54.1-65.8 ms) expressed as median (first to third quartiles), which are higher than those reported for healthy livers at 1.5 T by Allkemper et al22 (T1⍴ = 40.9 ± 2.2 ms) and Kritsaneepaiboon et al57 (T1⍴ = 54.7 [49.6-56.6]). The T1⍴ values measured with the proposed MRF approach were 41.8 ms (38.9-47.1 ms), which are in agreement with those reported by Allkemper et al.22 However, further studies are still required to determine the degree of bias of the proposed MRF approach with respect to the ground-truth T1⍴ values. Finally, T1⍴ values measured with the proposed MRF approach were higher than the T2-MRF values, as expected in liver tissue and observed18 in previous liver MRF studies.
and FF showed a very good correlation and low bias between MRF and the conventional mapping techniques, with MRF measurements not significatively different from the reference values. Very good agreement was also found in FF quantification; this was also observed in the liver FF values measured with the proposed MRF and the conventional proton density FF approach in 2 subjects who presented with mild hepatic steatosis. T1 values obtained with MOLLI were slightly lower than those obtained with the proposed MRF framework, whereas T2 and T1⍴ values measured with the conventional mapping approaches were higher than those measured with the proposed MRF. The MOLLI technique has a known negative bias, and this underestimation has been previously reported.20, 53 This underestimation is also observed in the phantom study, as shown in Supporting Information Figure S1. The MRF underestimation bias for T2 has also been previously reported in several studies.18, 20, 34, 39, 54, 55 Diffusion effects56 or imperfect T2 relaxation during the adiabatic T2 preparation pulses could be some of the sources of this underestimation. A clinical reference technique for T1⍴ quantification does not currently exist in abdominal imaging. To enable in vivo liver T1⍴ validation, a fast, single-breath-hold T1⍴ reference scan, similar to the one used by Wyatt et al,18 was used as reference; however, this approach has its own limitations and may present a bias. Liver T1⍴ values obtained with this reference technique were 58.0 ms (54.1-65.8 ms) expressed as median (first to third quartiles), which are higher than those reported for healthy livers at 1.5 T by Allkemper et al22 (T1⍴ = 40.9 ± 2.2 ms) and Kritsaneepaiboon et al57 (T1⍴ = 54.7 [49.6-56.6]). The T1⍴ values measured with the proposed MRF approach were 41.8 ms (38.9-47.1 ms), which are in agreement with those reported by Allkemper et al.22 However, further studies are still required to determine the degree of bias of the proposed MRF approach with respect to the ground-truth T1⍴ values. Finally, T1⍴ values measured with the proposed MRF approach were higher than the T2-MRF values, as expected in liver tissue and observed18 in previous liver MRF studies.
This study has several limitations. The proposed MRF approach is based on a previously introduced MRF sequence for T1, T2, 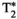 , and FF quantification, which has been extended to further include T1⍴ encoding. However, although simulations were performed (data not shown) to heuristically optimize SLT and the position of SL pulses in the sequence, an extensive optimization study (e.g., using Cramer-Rao lower bound) would be required to find the optimal configuration and will be investigated in future studies. Additionally, the proposed approach requires the subject to hold their breath for 18 seconds. Although this breath-holding duration is usually feasible, this may be challenging for some patients; thus, efforts to reduce the scan time will be considered in future studies. Regarding the amplitude of the SL pulses, it is well known that there is a positive correlation between SLA and T1⍴, known as T1⍴ dispersion. Although T1⍴ dispersion has been previously studied for cardiac,50 muscle,58 cartilage,59 and brain60 imaging, to the best of our knowledge, T1⍴ dispersion has not been studied on the human liver yet. Hence, the combination of SLA and SLTs used in this work (SLA = 350 Hz for maximum SLT = 60 ms) may be suboptimal, and a better compromise between RF and specific absorption rate limitations and T1⍴ encoding capability of the sequence could be yet to be found. In addition, this T1⍴ preparation approach fails to handle the chemical shift in fat, as it ignores the different behavior that each fat peak would have with respect to the SL pulse. However, the method proposed here is intended to provide water-specific T1, T2,
, and FF quantification, which has been extended to further include T1⍴ encoding. However, although simulations were performed (data not shown) to heuristically optimize SLT and the position of SL pulses in the sequence, an extensive optimization study (e.g., using Cramer-Rao lower bound) would be required to find the optimal configuration and will be investigated in future studies. Additionally, the proposed approach requires the subject to hold their breath for 18 seconds. Although this breath-holding duration is usually feasible, this may be challenging for some patients; thus, efforts to reduce the scan time will be considered in future studies. Regarding the amplitude of the SL pulses, it is well known that there is a positive correlation between SLA and T1⍴, known as T1⍴ dispersion. Although T1⍴ dispersion has been previously studied for cardiac,50 muscle,58 cartilage,59 and brain60 imaging, to the best of our knowledge, T1⍴ dispersion has not been studied on the human liver yet. Hence, the combination of SLA and SLTs used in this work (SLA = 350 Hz for maximum SLT = 60 ms) may be suboptimal, and a better compromise between RF and specific absorption rate limitations and T1⍴ encoding capability of the sequence could be yet to be found. In addition, this T1⍴ preparation approach fails to handle the chemical shift in fat, as it ignores the different behavior that each fat peak would have with respect to the SL pulse. However, the method proposed here is intended to provide water-specific T1, T2, 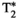 , and T1⍴ maps, considering fat tissues only through FF quantification. Hence, quantification of T1⍴ in fat is not performed. In the case in which a nonnegligible amount of fat co-exists with water in the same voxel (such as in hepatic tissue that presents steatosis), a small bias may still be introduced due to fat-signal distortion if the SL pulses used do not effectively lock the spins of the off-resonant frequencies of fat. Alternative strategies for SL, such as adiabatic SL T1⍴ preparation pulses, shall be explored to deal with chemical shift at higher fields and multipeak encoding61; and future studies are warranted in this direction. Spatial resolution in this study (2 × 2 mm2, slice thickness of 8 mm) may be considered in the lower end for liver imaging. Optimal sequence design for this acquisition could lead to more efficient parametric encoding/scan efficiency, thus allowing a higher resolution. In addition, further development of stronger regularizers or deep-learning reconstruction methods could also enable higher resolution. The use of a multi-echo SE sequence for reference T2 mapping in the phantom study is also acknowledged as a limitation, as this technique is sensitive to effects like stimulated echoes. In the future, single-echo SE sequences will be used instead to remove this possible source of bias. Another limitation of this proof-of-concept study is the evaluation in phantom and 10 healthy subjects only. Future studies should be performed in patients with liver disease to evaluate the ability of the proposed MRF approach to provide fully comprehensive liver characterization in a single-breath-hold scan.
, and T1⍴ maps, considering fat tissues only through FF quantification. Hence, quantification of T1⍴ in fat is not performed. In the case in which a nonnegligible amount of fat co-exists with water in the same voxel (such as in hepatic tissue that presents steatosis), a small bias may still be introduced due to fat-signal distortion if the SL pulses used do not effectively lock the spins of the off-resonant frequencies of fat. Alternative strategies for SL, such as adiabatic SL T1⍴ preparation pulses, shall be explored to deal with chemical shift at higher fields and multipeak encoding61; and future studies are warranted in this direction. Spatial resolution in this study (2 × 2 mm2, slice thickness of 8 mm) may be considered in the lower end for liver imaging. Optimal sequence design for this acquisition could lead to more efficient parametric encoding/scan efficiency, thus allowing a higher resolution. In addition, further development of stronger regularizers or deep-learning reconstruction methods could also enable higher resolution. The use of a multi-echo SE sequence for reference T2 mapping in the phantom study is also acknowledged as a limitation, as this technique is sensitive to effects like stimulated echoes. In the future, single-echo SE sequences will be used instead to remove this possible source of bias. Another limitation of this proof-of-concept study is the evaluation in phantom and 10 healthy subjects only. Future studies should be performed in patients with liver disease to evaluate the ability of the proposed MRF approach to provide fully comprehensive liver characterization in a single-breath-hold scan.
5 CONCLUSIONS
The proposed eight-echo IR, T2, and T1⍴ prepared liver MRF sequence presented in this work allows for quantitative T1, T2, 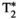 , T1⍴, and FF liver-tissue characterization in a single-breath-hold scan of 18 seconds. This approach has been investigated in phantoms and healthy subjects, showing good agreement and excellent correlation with reference SE measurements and conventional clinical maps. The additional information provided by T1⍴ quantification may improve the diagnostic power of multiparametric liver tissue characterization, especially in the presence of liver fibrosis. Future work will aim to validate the proposed approach in patients with liver disease.
, T1⍴, and FF liver-tissue characterization in a single-breath-hold scan of 18 seconds. This approach has been investigated in phantoms and healthy subjects, showing good agreement and excellent correlation with reference SE measurements and conventional clinical maps. The additional information provided by T1⍴ quantification may improve the diagnostic power of multiparametric liver tissue characterization, especially in the presence of liver fibrosis. Future work will aim to validate the proposed approach in patients with liver disease.



 , T1⍴ an FF respectively). Biases and limits of agreement (LoA = Mean bias ± 1.96 SD) are calculated from Bland-Altman analyses of difference vs. average between MRF and reference measurements
, T1⍴ an FF respectively). Biases and limits of agreement (LoA = Mean bias ± 1.96 SD) are calculated from Bland-Altman analyses of difference vs. average between MRF and reference measurements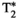 , T1⍴ and FF values (for MRF and their corresponding reference scans) reported for each vial of the phantom scans. Vials 1-13 correspond to different concentrations of agar and NiCl2 and were used for T1, T2,
, T1⍴ and FF values (for MRF and their corresponding reference scans) reported for each vial of the phantom scans. Vials 1-13 correspond to different concentrations of agar and NiCl2 and were used for T1, T2, 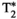 and T1⍴ validation. Vials A-H correspond to different peanut oil concentrations and were used for FF validation. Reference scans are IRSE, MESE, 8-echo GRE, T1⍴-prepared SE and 6-echo GRE for T1, T2,
and T1⍴ validation. Vials A-H correspond to different peanut oil concentrations and were used for FF validation. Reference scans are IRSE, MESE, 8-echo GRE, T1⍴-prepared SE and 6-echo GRE for T1, T2, 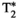 , T1⍴ and FF respectively
, T1⍴ and FF respectively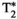 , T1⍴ and FF values measured inside ROIs drawn in liver, spleen, muscle and subcutaneous fat (SF). Values reported are from the proposed MRF sequence and their corresponding reference scans: MOLLI, T2-GraSE, 8-echo GRE, T1⍴-prepared GRE and 6-echo GRE for T1, T2,
, T1⍴ and FF values measured inside ROIs drawn in liver, spleen, muscle and subcutaneous fat (SF). Values reported are from the proposed MRF sequence and their corresponding reference scans: MOLLI, T2-GraSE, 8-echo GRE, T1⍴-prepared GRE and 6-echo GRE for T1, T2,  , T1⍴ and FF respectively
, T1⍴ and FF respectively