Specialized computational methods for denoising, B1 correction, and kinetic modeling in hyperpolarized 13C MR EPSI studies of liver tumors
Corresponding Author
Philip M. Lee
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Correspondence
Philip M. Lee, Department of Radiology and Biomedical Imaging, University of California, San Francisco, 1700 Fourth Street, Byers Hall Suite 102, San Francisco, CA 94158, USA.
Email: [email protected]
Search for more papers by this authorHsin-Yu Chen
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorJeremy W. Gordon
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorZihan Zhu
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorPeder E.Z. Larson
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorNicholas Dwork
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorMark Van Criekinge
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorLucas Carvajal
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorMichael A. Ohliger
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorZhen J. Wang
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorDuan Xu
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorJohn Kurhanewicz
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorRobert A. Bok
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorRahul Aggarwal
Department of Medicine, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorPamela N. Munster
Department of Medicine, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorDaniel B. Vigneron
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorCorresponding Author
Philip M. Lee
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Correspondence
Philip M. Lee, Department of Radiology and Biomedical Imaging, University of California, San Francisco, 1700 Fourth Street, Byers Hall Suite 102, San Francisco, CA 94158, USA.
Email: [email protected]
Search for more papers by this authorHsin-Yu Chen
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorJeremy W. Gordon
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorZihan Zhu
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorPeder E.Z. Larson
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorNicholas Dwork
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorMark Van Criekinge
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorLucas Carvajal
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorMichael A. Ohliger
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorZhen J. Wang
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorDuan Xu
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorJohn Kurhanewicz
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorRobert A. Bok
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorRahul Aggarwal
Department of Medicine, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorPamela N. Munster
Department of Medicine, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorDaniel B. Vigneron
UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, San Francisco and University of California, Berkeley, California, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
Search for more papers by this authorFunding information
National Institutes of Health, Grant/Award Numbers: R01CA183071, U01EB026412, R01DK115987, and P41EB013598
Abstract
Purpose
To develop a novel post-processing pipeline for hyperpolarized (HP) 13C MRSI that integrates tensor denoising and 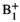 correction to measure pyruvate-to-lactate conversion rates (kPL) in patients with liver tumors.
correction to measure pyruvate-to-lactate conversion rates (kPL) in patients with liver tumors.
Methods
Seven HP 13C MR scans of progressing liver tumors were acquired using a custom 13C surface transmit/receive coil and the echo-planar spectroscopic imaging (EPSI) data analysis included B0 correction, tensor rank truncation, and zero- and first-order phase corrections to recover metabolite signals that would otherwise be obscured by spectral noise as well as a correction for inhomogeneous transmit (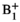 ) using a
) using a 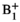 map aligned to the coil position for each patient scan. Processed HP data and corrected flip angles were analyzed with an inputless two-site exchange model to calculate kPL.
map aligned to the coil position for each patient scan. Processed HP data and corrected flip angles were analyzed with an inputless two-site exchange model to calculate kPL.
Results
Denoising averages SNR increases of pyruvate, lactate, and alanine were 37.4-, 34.0-, and 20.1-fold, respectively, with lactate and alanine dynamics most noticeably recovered and better defined. In agreement with Monte Carlo simulations, over-flipped regions underestimated kPL and under-flipped regions overestimated kPL. 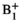 correction addressed this issue.
correction addressed this issue.
Conclusion
The new HP 13C EPSI post-processing pipeline integrated tensor denoising and 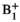 correction to measure kPL in patients with liver tumors. These technical developments not only recovered metabolite signals in voxels that did not receive the prescribed flip angle, but also increased the extent and accuracy of kPL estimations throughout the tumor and adjacent regions including normal-appearing tissue and additional lesions.
correction to measure kPL in patients with liver tumors. These technical developments not only recovered metabolite signals in voxels that did not receive the prescribed flip angle, but also increased the extent and accuracy of kPL estimations throughout the tumor and adjacent regions including normal-appearing tissue and additional lesions.
Supporting Information
| Filename | Description |
|---|---|
| mrm28901-sup-0001-Supinfo.docxWord document, 387.8 KB | FIGURE S1 (A) The simulated pyruvate and lactate signals. Following similar approaches,1 the inputless 2-site exchange model was used, an approach that only fits the lactate magnetization and not the pyruvate magnetization while the measured pyruvate magnetization is used as the input at each time point. The nominal simulation values were kPL = 0.03 s−1, T1 of pyruvate = 30 s, and T1 of lactate = 25 s. The noise standard deviation was set at 0.004 relative to a fixed total magnetization input normalized to 1. This value was chosen to approximately match the SNR of the simulated data with typical empirical data. The γ-distribution function to simulate the pyruvate and lactate signals had an arrival time of 4 s and a full width at half-maximum of 8 s. All excitations of each time point were consolidated into a single effective flip angle.1 (B) Monte Carlo simulations of the 2-site inputless model demonstrated an inverse relationship between the relative 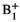 error and the fractional kPL error, the simulated kPL values (kPL,fit) against the input kPL of 0.03 s−1 (kPL,truth). The solid and dashed lines indicate the mean and ±1 standard deviation, respectively. Positive errors in error and the fractional kPL error, the simulated kPL values (kPL,fit) against the input kPL of 0.03 s−1 (kPL,truth). The solid and dashed lines indicate the mean and ±1 standard deviation, respectively. Positive errors in 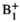 resulted in underestimations of kPL and negative errors resulted in overestimations, agreeing with acquired data. This relationship corresponded with the computed kPL maps before and after resulted in underestimations of kPL and negative errors resulted in overestimations, agreeing with acquired data. This relationship corresponded with the computed kPL maps before and after  correction. The selected voxels presented in Table 1 are indicated with blue circles and all but 1 are within the 1 SD bounds correction. The selected voxels presented in Table 1 are indicated with blue circles and all but 1 are within the 1 SD bounds |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Ardenkjær-Larsen JH, Golman K, Gram A, et al. Increase of signal-to-noise of more than 10,000 times in liquid state NMR. Discov Med. 2003; 100: 10158-10163.
- 2Cunningham CH, Lau JYC, Chen AP, et al. Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res. 2016; 119: 1177-1182.
- 3Rider OJ, Apps A, Miller JJJJ, et al. Noninvasive in vivo assessment of cardiac metabolism in the healthy and diabetic human heart using hyperpolarized 13C MRI. Circ Res. 2020; 126: 725-736.
- 4Abeyakoon O, Latifoltojar A, Gong F, et al. Hyperpolarised 13C MRI: a new horizon for non-invasive diagnosis of aggressive breast cancer. BJR Case Rep. 2019; 5: 20190026.
- 5Gallagher FA, Woitek R, McLean MA, et al. Imaging breast cancer using hyperpolarized carbon-13 MRI. Proc Natl Acad Sci USA. 2020; 117: 2092-2098.
- 6Park I, Larson PEZ, Gordon JW, et al. Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies. Magn Reson Med. 2018; 80: 864-873.
- 7Miloushev VZ, Granlund KL, Boltyanskiy R, et al. Metabolic imaging of the human brain with hyperpolarized 13C Pyruvate demonstrates 13C lactate production in brain tumor patients. Cancer Res. 2018; 78: 3755-3760.
- 8Autry AW, Gordon JW, Carvajal L, et al. Comparison between 8- and 32-channel phased-array receive coils for in vivo hyperpolarized 13 C imaging of the human brain. Magn Reson Med. 2019; 82: 833-841.
- 9Mammoli D, Gordon J, Autry A, et al. Kinetic modeling of hyperpolarized carbon-13 pyruvate metabolism in the human brain. IEEE Trans Med Imaging. 2020; 39: 320-327.
- 10Aggarwal R, Vigneron DB, Kurhanewicz J. Hyperpolarized 1-[13C]-pyruvate magnetic resonance imaging detects an early metabolic response to androgen ablation therapy in prostate cancer. Eur Urol. 2017; 72: 1028-1029.
- 11Granlund KL, Tee S-S, Vargas HA, et al. Hyperpolarized MRI of human prostate cancer reveals increased lactate with tumor grade driven by monocarboxylate transporter 1. Cell Metab. 2020; 31: 105-114.
- 12Chen H-Y, Aggarwal R, Bok RA, et al. Hyperpolarized 13C-pyruvate MRI detects real-time metabolic flux in prostate cancer metastases to bone and liver: a clinical feasibility study. Prostate Cancer Prostatic Dis. 2020; 23: 269-276.
- 13Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, et al. Hyperpolarized 13C MRI: path to clinical translation in oncology. Neoplasia (United States). 2019; 21: 1-16.
- 14Larson PEZ, Chen H-Y, Gordon JW, et al. Investigation of analysis methods for hyperpolarized 13C-pyruvate metabolic MRI in prostate cancer patients. NMR Biomed. 2018; 31:e3997.
- 15Crane JC, Gordon JW, Chen HY, et al. Hyperpolarized 13C MRI data acquisition and analysis in prostate and brain at University of California, San Francisco. NMR Biomed. 2021; 34:e4280.
- 16Wang ZJ, Ohliger MA, Larson PEZ, et al. Hyperpolarized 13C MRI: state of the art and future directions. Radiology. 2019; 291: 273-284.
- 17Day SE, Kettunen MI, Gallagher FA, et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007; 13: 1382-1387.
- 18Chaumeil MM, Ozawa T, Park IW, et al. Hyperpolarized 13C MR spectroscopic imaging can be used to monitor Everolimus treatment in vivo in an orthotopic rodent model of glioblastoma. Neuroimage. 2012; 59: 193-201.
- 19Radoul M, Chaumeil MM, Eriksson P, Wang AS, Phillips JJ, Ronen SM. MR studies of glioblastoma models treated with dual PI3K/mTOR inhibitor and temozolomide: metabolic changes are associated with enhanced survival. Mol Cancer Ther. 2016; 15: 1113-1122.
- 20Hesketh RL, Wang J, Wright AJ, et al. Magnetic resonance imaging is more sensitive than PET for detecting treatment-induced cell death–dependent changes in glycolysis. Cancer Res. 2019; 79: 3557-3569.
- 21Walker CM, Fuentes D, Larson PEZ, Kundra V, Vigneron DB, Bankson JA. Effects of excitation angle strategy on quantitative analysis of hyperpolarized pyruvate. Magn Reson Med. 2019; 81: 3754-3762.
- 22Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]Pyruvate. Sci Transl Med. 2013; 5: 198ra108.
- 23Chen HY, Larson PEZ, Gordon JW, et al. Technique development of 3D dynamic CS-EPSI for hyperpolarized 13 C pyruvate MR molecular imaging of human prostate cancer. Magn Reson Med. 2018; 80: 2062-2072.
- 24Larson PE, Bok R, Kerr AB, et al. Investigation of tumor hyperpolarized [1-13C]-pyruvate dynamics using time-resolved multiband RF excitation echo-planar MRSI. Magn Reson Med. 2010; 63: 582-591.
- 25Brender JR, Kishimoto S, Merkle H, et al. Dynamic imaging of glucose and lactate metabolism by 13 C-MRS without hyperpolarization. Sci Rep. 2019; 9: 3410.
- 26Chen HY, Autry AW, Brender JR, et al. Tensor image enhancement and optimal multichannel receiver combination analyses for human hyperpolarized 13C MRSI. Magn Reson Med. 2020; 84: 3351-3365.
- 27Stollberger R, Wach P, McKinnon G, et al. RF-filed mapping in vivo. In: Proceedings of the 7th Annual Meeting of SMRM, San Francisco, CA, USA. 1988, p. 106.
- 28Insko E, Bolinger L. B1 mapping. In: Proceedings of the 11th Annual Meeting of SMRM, Berlin, Germany. 1992, p. 4302.
- 29Cunningham CH, Pauly JM, Nayak KS. Saturated double-angle method for rapid B1+ mapping. Magn Reson Med. 2006; 55: 1326-1333.
- 30Hansen RB, Sánchez-Heredia JD, Bøgh N, et al. Coil profile estimation strategies for parallel imaging with hyperpolarized 13C MRI. Magn Reson Med. 2019; 82: 2104-2117.
- 31Jacobsen M. Absolute Orientation - Horn’s method. MATLAB Central File Exchange. Published 2020. https://www.mathworks.com/matlabcentral/fileexchange/26186-absolute-orientation-horn-s-method
- 32Ohliger MA, Gordon JW, Carvajal L, et al. 55Mn-based fiducial markers for rapid and automated RF coil localization for hyperpolarized 13C MRI. Magn Reson Med. 2021; 85: 518-530.
- 33Warburg O. On the origin of cancer cells. Science (80- ). 1956; 123: 309-314.
- 34Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017; 168: 657-669.
- 35Hu S, Balakrishnan A, Bok R, et al. 13C-pyruvate imaging reveals alterations in glycolysis that precede c-Myc-induced tumor formation and regression. Cell Metab. 2011; 14: 131-142.
- 36Keshari KR, Sriram R, Van Criekinge M, et al. Metabolic reprogramming and validation of hyperpolarized 13C lactate as a prostate cancer biomarker using a human prostate tissue slice culture bioreactor. Prostate. 2013; 73: 1171-1181.
- 37Sriram R, Van Criekinge M, Santos JD, et al. Non-invasive differentiation of benign renal tumors from clear cell renal cell carcinomas using clinically translatable hyperpolarized 13C pyruvate magnetic resonance. Tomography. 2016; 2: 35-42.
- 38Albers MJ, Bok R, Chen AP, et al. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res. 2008; 68: 8607-8615.
- 39Sriram R, Van Criekinge M, Hansen A, et al. Real-time measurement of hyperpolarized lactate production and efflux as a biomarker of tumor aggressiveness in an MR compatible 3D cell culture bioreactor. NMR Biomed. 2015; 28: 1141-1149.
- 40Serrao EM, Kettunen MI, Rodrigues TB, et al. MRI with hyperpolarised [1-13C]pyruvate detects advanced pancreatic preneoplasia prior to invasive disease in a mouse model. Gut. 2016; 65: 465-475.
- 41Grist JT, McLean MA, Riemer F, et al. Quantifying normal human brain metabolism using hyperpolarized [1– 13 C]pyruvate and magnetic resonance imaging. Neuroimage. 2019; 189: 171-179.
- 42Lee CY, Soliman H, Geraghty BJ, et al. Lactate topography of the human brain using hyperpolarized 13C-MRI. Neuroimage. 2020; 204: 116202.
- 43Gordon JW, Autry AW, Tang S, et al. A variable resolution approach for improved acquisition of hyperpolarized 13C metabolic MRI. Magn Reson Med. 2020; 84: 2943-2952.
- 44Gordon JW, Chen H-Y, Autry A, et al. Translation of Carbon-13 EPI for hyperpolarized MR molecular imaging of prostate and brain cancer patients. Magn Reson Med. 2019; 81: 2702-2709.
- 45Tran M, Latifoltojar A, Neves JB, et al. First-in-human in vivo non-invasive assessment of intra-tumoral metabolic heterogeneity in renal cell carcinoma. BJR|case reports. 2019; 5: 20190003.
- 46Stødkilde-Jørgensen H, Laustsen C, Hansen ESS, et al. Pilot study experiences with hyperpolarized [1-13C]pyruvate MRI in pancreatic cancer patients. J Magn Reson Imaging. 2020; 51: 961-963.
- 47Autry AW, Gordon JW, Chen H-Y, et al. Characterization of serial hyperpolarized 13C metabolic imaging in patients with glioma. NeuroImage Clin. 2020; 27: 102323.
- 48Tang S, Milshteyn E, Reed G, et al. A regional bolus tracking and real-time B1 calibration method for hyperpolarized 13C MRI. Magn Reson Med. 2019; 81: 839-851.