An optimal acquisition and post-processing pipeline for hybrid IVIM-DKI in head and neck
Funding information
This work was funded by a research grant from Elekta AB (Stockholm, Sweden). SP would like to acknowledge financial support from The Dutch Cancer Society project number 12141.
Click here for author-reader discussions
Abstract
Purpose
To optimize the diffusion-weighting b values and postprocessing pipeline for hybrid intravoxel incoherent motion diffusion kurtosis imaging in the head and neck region.
Methods
Optimized diffusion-weighting b value sets ranging between 5 and 30 b values were constructed by optimizing the Cramér-Rao lower bound of the hybrid intravoxel incoherent motion diffusion kurtosis imaging model. With this model, the perfusion fraction, pseudodiffusion coefficient, diffusion coefficient, and kurtosis were estimated. Sixteen volunteers were scanned with a reference b value set and 3 repeats of the optimized sets, of which 1 with volunteers swallowing on purpose.
The effects of (1) b value optimization and number of b values, (2) registration type (none vs. intervolume vs. intra- and intervolume registration), and (3) manual swallowing artifact rejection on the parameter precision were assessed.
Results
The SD was higher in the reference set for perfusion fraction, diffusion coefficient, and kurtosis by a factor of 1.7, 1.5, and 2.3 compared to the optimized set, respectively. A smaller SD (factor 0.7) was seen in pseudodiffusion coefficient. The sets containing 15, 20, and 30 b values had comparable repeatability in all parameters, except pseudodiffusion coefficient, for which set size 30 was worse. Equal repeatability for the registration approaches was seen in all parameters of interest. Swallowing artifact rejection removed the bias when present.
Conclusion
To achieve optimal hybrid intravoxel incoherent motion diffusion kurtosis imaging in the head and neck region, b value optimization and swallowing artifact image rejection are beneficial. The optimized set of 15 b values yielded the optimal protocol efficiency, with a precision comparable to larger b value sets and a 50% reduction in scan time.
1 INTRODUCTION
In the last decade, the apparent diffusion coefficient has been shown to be a promising parameter for response assessment of head and neck cancer treated with (chemo)radiotherapy.1, 2 More recently, the benefit of obtaining additional parameters from DWI by intravoxel incoherent motion (IVIM) and diffusion kurtosis imaging (DKI) has been acknowledged.3, 4 Combining these models in hybrid IVIM-DKI allows for the simultaneous assessment of the diffusion coefficient (D), perfusion fraction (f), pseudodiffusion coefficient (D*), and kurtosis (K). Both f and D* are related to perfusion and could therefore provide insight in abnormal tumor perfusion. The latter parameter, K, is associated with restricted diffusion and cellularity,5 which is commonly increased in tumors. However, full assessment of their potential as response biomarkers and, in the future, for application in radiotherapy treatment planning and treatment adaptation, requires robust and repeatable estimation of all IVIM-DKI parameters within a patient.6
IVIM-DKI parameter estimation is currently hampered by inefficient sampling of the DWI signal curve and motion artifacts. Inefficient sampling of the DWI signal curve, due to nonoptimal choice of b values, leads to unnecessary long scan times in order to achieve a similar precision as optimized sampling. Moreover, long scan times might increase the amount of motion corruption. Several efforts have been made to optimize b values for the monoexponential,7-13 the IVIM model with direct13-16 and segmented fitting,17 and the kurtosis model13 in a variety of tissues, although none of the mentioned papers address the head and neck region specifically. For complex models such as hybrid IVIM-DKI, optimizing b values is increasingly more difficult and, to the best of our knowledge, has not been done so far.
Additionally, the head and neck region is prone to several types of motion artifacts. Firstly, swallowing and coughing artifacts, which present themselves as severe signal dropout, could cause over- or underestimation of the DWI parameters. Current mitigation strategies mainly consist of specific patient instruction for not swallowing or coughing but are not always sufficient because both can be a reflex behavior. Secondly, head motion hampers parameter estimation by causing misalignment between scan volumes. This type of motion can be partially prevented using fixation of the patient, either in the form of padding, or in the case of radiotherapy-planning MRI, with an immobilization mask. Additionally, motion artifacts might be corrected after acquisition by registration; however, registration of high b value images can be problematic due to lower SNR.
Therefore, the goal of this study was to find an optimal acquisition and develop a postprocessing pipeline for hybrid IVIM-DKI DWI in the head and neck region. To this end, we optimized the b values of hybrid IVIM-DKI for the head and neck region, applied motion compensation, and investigated the effect of swallowing artifact rejection.
2 METHODS
Acquisition optimization and postprocessing of the hybrid IVIM-DKI DWI data consisted of 3 stages. In the first stage, described in Section 2.1, the set of b values was optimized for different b value set sizes based on simulated ground truth parameter sets. Next, these b value sets were scanned in healthy volunteers, as described in Section 2.2. In the second stage, intra- and intervolume registration were applied to the acquired data as described in Section 2.3. The third stage consisted of swallowing artifact image rejection (Section 2.4). Lastly, Section 2.5 describes assessment of the parameter estimation precision of the pipeline. In this paper, the term postprocessing refers to all processing done after acquisition but before parameter estimation.
2.1 Optimization of b values
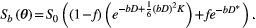 (1)
(1)where 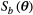 is the signal at a specific b value (amount of diffusion weighting) as function of the parameters
is the signal at a specific b value (amount of diffusion weighting) as function of the parameters 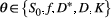 , which are described in Table 1.
, which are described in Table 1.
| Parameter | Description | Minimum | Maximum |
|---|---|---|---|
| S 0 | Signal intensity at b = 0 s/mm2 | 4275.8 | 7126.3 |
| D | Diffusion coefficient | 0.25·10–3 mm2/s | 3.41·10–3 mm2/s |
| f | Perfusion fraction | 0.09 | 0.42 |
| D* | Pseudodiffusion coefficient/apparent perfusion coefficient | 6.29·10–3 mm2/s | 237.39·10–3 mm2/s |
| K | Kurtosis | 0.1 | 2.81 |
Note
- S0 in this table is the estimated signal intensity without T2 decay effects.
- Abbreviation: IVIM-DKI, intravoxel incoherent motion diffusion kurtosis imaging.
A ground truth set 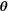 was created using a Halton sequence18 for the ground truth ranges shown in Table 1. A Halton sequence yields a sequence of low-discrepancy, pseudorandom numbers. These ranges were derived from the parameter values reported in several previous studies in head and neck tumors.2, 3, 19-21 From these ground truth parameter sets, sets showing a D* smaller than 6 times D were removed to be able to properly separate the 2 parameters. Some parameter combinations result in increased signal at high b values. To eliminate this nonphysical result, parameter sets in which the partial derivative of the model to the b value was larger than 0 at b = 1500 s/mm2 were removed. After exclusion, 272 of 576 ground truth
was created using a Halton sequence18 for the ground truth ranges shown in Table 1. A Halton sequence yields a sequence of low-discrepancy, pseudorandom numbers. These ranges were derived from the parameter values reported in several previous studies in head and neck tumors.2, 3, 19-21 From these ground truth parameter sets, sets showing a D* smaller than 6 times D were removed to be able to properly separate the 2 parameters. Some parameter combinations result in increased signal at high b values. To eliminate this nonphysical result, parameter sets in which the partial derivative of the model to the b value was larger than 0 at b = 1500 s/mm2 were removed. After exclusion, 272 of 576 ground truth 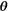 were left.
were left.
 (2)
(2)where C is the cost function value and b the set of b values; the bar with subscripts indicates mean over parameter  and
and 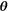 , where
, where  corresponds to f, D*, D, and K.
corresponds to f, D*, D, and K.
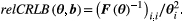 (3)
(3)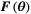 , the Fisher information matrix:
, the Fisher information matrix:
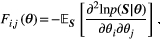 (4)
(4)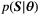 is the joint probability density function of the Rician-distributed measurements
is the joint probability density function of the Rician-distributed measurements 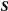 .
.The CRLB, 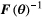 , gives a lower bound of the variance for the given parameter vector and is commonly used in experiment design.22 Therefore, it is used as a measure of precision in this paper. The optimization of
, gives a lower bound of the variance for the given parameter vector and is commonly used in experiment design.22 Therefore, it is used as a measure of precision in this paper. The optimization of 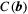 was constrained to avoid negative b values and b values higher than b = 1500 s/mm2. A maximum b value of 1500 s/mm2 was chosen due to limited SNR in the head and neck region at higher b values in healthy tissue at 1.5 tesla. The acquisition time was given by
was constrained to avoid negative b values and b values higher than b = 1500 s/mm2. A maximum b value of 1500 s/mm2 was chosen due to limited SNR in the head and neck region at higher b values in healthy tissue at 1.5 tesla. The acquisition time was given by 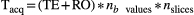 , with
, with 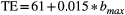 and RO the readout time. This relation between the maximum b value and TE was experimentally obtained from the sequence implementation. The readout time RO was determined to be 120 ms for the scan protocol used in this paper.
and RO the readout time. This relation between the maximum b value and TE was experimentally obtained from the sequence implementation. The readout time RO was determined to be 120 ms for the scan protocol used in this paper.
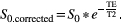 (5)
(5)The T2 was set to 80 ms. A maximum b value of 1500 s/mm2 resulted in a modeled signal intensity at b = 0 s/mm2 of 1500 to 2500. The noise level was set to 20, leading to an estimated intratumor SNR of 75-125 at b = 0 s/mm2. Initial sets were chosen containing b values along the range of 0-1500 s/mm2. These sets were optimized using the fminsearch algorithm in MatLab 2017a (MathWorks, Natick, MA). Optimization was carried out for a set size of 5, 6, 10, 15, 20, and 30 b values. The set of 20 b values was optimized first and initialized with approximately linearly distributed b values. Because the final b values were clustered, we subsequently restarted the optimization with varying initial distributions of b values over the 3 regimes (perfusion (b = ~0-200 s/mm2), free diffusion (b = ~200-800 s/mm2), and restricted diffusion b > ~800 s/mm2) to reduce the chance of ending in a local minimum. For each number of b values, the set with the lowest overall cost value was selected. The b value optimization code is available online at github.com/nsijtsema/IVIMDKI_b-value_optimization.
2.2 MR scanning
Seventeen healthy volunteers (14 females, 3 males, mean age 26, age range 19-59) were imaged on a 1.5 T GE Optima MR450w GEM imaging system (General Electric Medical Systems, Waukesha, WI) with a 16-channel head and neck coil (General Electric Medical Systems, Waukesha, WI). The study was approved by the institutional review board (protocol 2014-096), and written informed consent was obtained from all volunteers. The imaging protocol consisted of a T2 periodically rotated overlapping parallel lines with enhanced reconstruction (scan time 5 minutes 34 seconds) followed by a single shot spin-echo EPI IVIM-DKI DWI acquisition (FOV 26 × 26 cm2, 128 × 128 matrix, 2 × 2 × 4 mm voxels, 0.2 mm interslice gap, TE = 81.8 ms, TR = 6700 ms, SENSE parallel imaging acceleration factor 2, number of averages = 1, 3 orthogonal diffusion directions) with a reference b value set (scan time 6 minutes 35 seconds) of the geometrical form 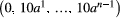 with a = 1.3 and the number of b values n = 20, similar to the approach used by Lu et al.3 Next, 3 repeats of the optimized single shot spin-echo EPI IVIM-DKI DWI sets (scan time 11 minutes 17 seconds) were scanned with the same imaging parameters mentioned for the reference b value set, with exception of the b values. Volunteers were asked to lie still, except for the last optimized IVIM-DKI acquisition. During that acquisition, volunteers were asked to swallow 4 times so that most optimized b value set had at least 1 swallowing artifact. For all DWI acquisitions, reversed readout gradient polarity for b = 0 s/mm2 (scan time 1 minute 7 seconds) was collected for the purpose of distortion correction using reversed gradient polarity blips,23, 24 which were applied to all images. The odd and even slices of the DWI were acquired in interleaved fashion to minimize slice crosstalk. Total scan time of the protocol was 55 minutes.
with a = 1.3 and the number of b values n = 20, similar to the approach used by Lu et al.3 Next, 3 repeats of the optimized single shot spin-echo EPI IVIM-DKI DWI sets (scan time 11 minutes 17 seconds) were scanned with the same imaging parameters mentioned for the reference b value set, with exception of the b values. Volunteers were asked to lie still, except for the last optimized IVIM-DKI acquisition. During that acquisition, volunteers were asked to swallow 4 times so that most optimized b value set had at least 1 swallowing artifact. For all DWI acquisitions, reversed readout gradient polarity for b = 0 s/mm2 (scan time 1 minute 7 seconds) was collected for the purpose of distortion correction using reversed gradient polarity blips,23, 24 which were applied to all images. The odd and even slices of the DWI were acquired in interleaved fashion to minimize slice crosstalk. Total scan time of the protocol was 55 minutes.
To fit the acquisition in the available scan time, the optimized b value sets containing 5, 6, 10, 15, 20, and 30 b values were combined into 1 set. This was done by adding the b values of the longer subsequent set that were not already present in the shorter set to the end of the acquisition. The set of 5 was thus expanded with 2 b values not initially present in that set to be able to form the optimized set of 6. The set was then consecutively expanded to 10, 15, 20, and 30 b values in the same manner. Consequently, the b value sets were grouped in time to ensure the effect of motion is representative for a patient scan. Due to overlap between the b values in the optimized sets, the total b value set encompassed 34 b values. Table 2 contains the acquired b value sets.
| b Values | |
|---|---|
| Reference | 0, 10, 1460, 20, 1120, 20, 870, 30, 670, 40, 510, 50, 390, 60, 300, 80, 230, 110, 180, 140 |
| Optimized | 0, 20, 780, 1500, 130, 790, 640, 80, 1500, 570, 770, 770, 80, 1500, 780, 1500, 10, 790, 1500, 790, 1500, 80, 750, 1500, 80, 760, 790, 80, 750, 280, 1500, 80, 790, 10 |
- Abbreviation: IVIM-DKI, intravoxel incoherent motion diffusion kurtosis imaging.
2.3 Registration
Two registration methods were compared with nonregistering: intervolume registration only and intra- and intervolume registration. The methods for both intra- and intervolume registration were obtained from Guyader et al.25 In case of intravolume registration, the odd and even slices from each b value image were separated into 2 separate volumes. Subsequently, the 2 volumes were aligned by group-wise registration because the odd and even slices were acquired interleaved. Intervolume registration was carried out by pair-wise registration of each b value image to the b = 0 s/mm2 image. Intravolume registration, if applicable, was performed before intervolume registration. All registrations were nonrigid and carried out with Elastix (version 4.9).26 The parameter files from the registration approach25 are available in the Elastix parameter file database. Registration was applied after distortion correction for all cases in the mentioned order. Intervolume registration errors were detected by manually assessing the imaging volumes and identifying the volumes with severe anatomical mismatches. To correct for registration errors, the transformations of the 2 well-registered b values that were closest in time were linearly interpolated and applied to the original image that contained the registration error.
Finally, the b = 0 s/mm2 scan of each acquisition was registered to the reference b = 0 s/mm2 acquisition using the same registration approach as used for the intervolume registration. The transformation of the b = 0 s/mm2 was propagated to the remaining images of the acquisition.
2.4 Swallowing artifact image rejection
Swallowing artifacts presented as severe signal dropout in the individual b value images were detected by visual inspection and subsequently rejected. Because the data were acquired using 3 sequential orthogonal gradient directions, artifacts present in 1 diffusion direction resulted in rejection of all 3 directions to maintain isotropic weighting in the fit.
2.5 Assessment
Regions of interest (ROIs) were drawn inside both tonsils of each volunteer based on the first acquired b = 0 s/mm2 volume. Subsequently, the ROIs were propagated to the other DWI images in the scanned series. For analysis, the tonsils were regarded as a single organ. Voxel-wise fitting was performed with an in-house fitting script in MatLab 2017a (MathWorks), which employs a variable starting point method before direct fitting of the biexponential with the most suitable starting point. The range in which starting points were chosen was the same as the ranges chosen for the optimization of f, D*, and D. For S0, the range was widened to 200 to 5000, and for K the upper bound was rounded to 3. Fitting constraints were set to prevent severe outliers but aiming to avoid skewing the distribution at the edge of the physiologically plausible parameter values. The used constraints were S0 in [0, 10 000] [arbitrary units], f in [−1, 1] [–], D* in [0, 0.8] mm2/s, D in [0, 0.02] mm2/s, and K in [−5, 5] [–] as a compromise between unconstrained and more strictly constrained fitting.
2.5.1 Comparison of the optimized set of 20 b values with the reference set
To assess the change in precision due to b value optimization, the nonoptimized reference set was compared with the optimized set of 20 b values from the first optimized acquisition in terms of mean and SD of the 4 parameters of interest (f, D*, D, K) within the ROI. The SD in the ROI consists of both underlying physiological differences and noise. Because the underlying physiological differences are constant, the SD within the ROI was used as a measure of precision. Only the fully corrected data (with applied distortion correction, intra- and intervolume registration) were used in this comparison. A Wilcoxon signed-rank test was used to test significance for both metrics and all parameters, leading to a total of 8 tests.
2.5.2 Repeatability assessment for type of registration and set size
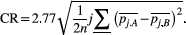 (6)
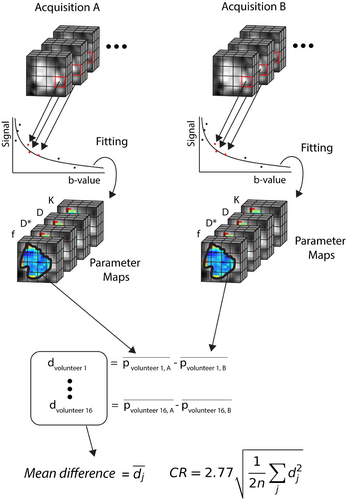
(6)where 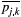 is the ROI mean of the parameter of interest for each subject j at time point k (either A or B), and n is the number of subjects. The workflow for obtaining the CR is schematically depicted in Figure 1. Then, the relative CR (relCR) is defined as
is the ROI mean of the parameter of interest for each subject j at time point k (either A or B), and n is the number of subjects. The workflow for obtaining the CR is schematically depicted in Figure 1. Then, the relative CR (relCR) is defined as 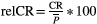 , for which
, for which 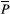 is the overall ROI mean over the 2 acquisitions across all volunteers:
is the overall ROI mean over the 2 acquisitions across all volunteers: 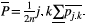 Similarly, the relative difference (relDifference) in parameter mean is defined as
Similarly, the relative difference (relDifference) in parameter mean is defined as 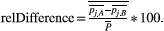
The optimized set of 30 b values was compared to the sets of 20, 15, 10, 6, and 5 in terms of relCR and relDifference. For set size assessment, only the fully corrected data (applied distortion correction, intra- and intervolume registration) were used. Wilcoxon signed-rank tests were applied to test if relDifference was statistically significantly different from 0 for all set sizes and parameters, resulting in 24 tests.
For comparison of registration methods, only the optimized set size of 30 b values was used. Comparisons in relDifference and relCR were carried out between the no registration and intervolume-only registration cases as well as between the no registration and intra- and intervolume registration cases. Wilcoxon signed-rank tests were applied to test if the relDifference was different from 0 for all registration cases and all parameters, resulting in 12 tests.
2.5.3 Assessment of swallowing artifact rejection
In case of swallowing artifact image rejection, the ROI mean before rejection was compared to the ROI mean after rejection, and a Wilcoxon signed-rank test was applied. Comparisons were done for each set size applying intra- and intervolume registration, resulting in 24 comparisons. Additionally, in case a significant difference (P ≤ .05) was found before and after swallowing artifact rejection, the mean before and after rejection was compared to that of the second optimized acquisition without swallowing to assess improvement in accuracy, leading to another 18 comparisons.
3 RESULTS
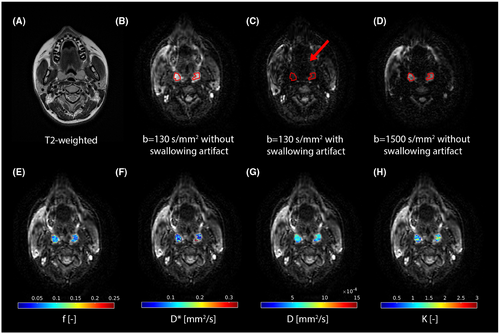
Figure 2 shows an example of the tonsil ROIs and corresponding parametric maps. One volunteer was excluded because the tonsils of this volunteer had been removed.
3.1 Optimization of b values
Table 3 shows the resulting optimized sets for 5, 6, 10, 15, 20, and 30 b values. Note that all b values are rounded to multiples of 10 to comply with the scanner’s requirements. The increase in cost function value due to rounding was less than 1%, except for the set of 5 b values where it was 1.8%. The mean and SD over all volunteers and set sizes for the 2 repeated fully registered nonswallowing acquisitions of the mean f, D*, D, and K within the ROIs were 0.12 ± 0.06 [–] for f, 0.07 ± 0.03 mm2/s for D*, 0.8·10–3 ± 0.2·10–3 mm2/s for D, and 0.73 ± 0.53 [–] for K. The obtained values of S0 were 1021 ± 136 [arbitrary units.].
| b Values [s/mm2] | |
|---|---|
| 5 b values | 0, 20, 130, 780, 1500 |
| 6 b values | 0, 20, 80, 640, 790, 1500 |
| 10 b values | 0, 20, 2×80, 570, 2×770, 780, 2×1500 |
| 15 b values | 0, 10, 2×80, 130, 570, 2×770, 2×780, 790, 4×1500 |
| 20 b values | 0, 10, 3×80, 130, 570, 2×770, 2×780, 3×790, 6×1500 |
| 30 b values | 0, 2×10, 6×80, 280, 2×750, 760, 2×770, 2×780, 5×790, 8×1500 |
Comparing the relative CRLB from the reference set with the optimized set in the simulated ground truth parameter sets, demonstrated that the reference set was expected to have a factor 2.2 higher variance than the optimized set for K. Slightly lower variance was expected in the reference set for f, D, and D* with factors 0.5, 0.59, and 0.56, respectively, compared to the optimized set.
3.1.1 Comparison of the optimized set of 20 b values with the reference set
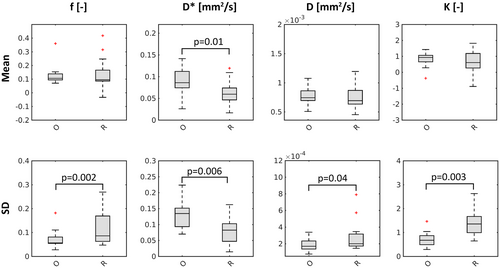
The SD for D, K, and f was significantly lower in the optimized set of 20 b values compared to the reference set that also had 20 b values (D: P = .04, K: P = .003, f: P = .002), as is shown in Figure 3. This corresponded to a 1.7, 1.5, and 2.3 times higher SD in the reference set in comparison to the optimized set in f, D, and K, respectively. The SD of D* was significantly higher in the optimized set (P = .006), corresponding with a 0.7 lower SD in the reference set versus the optimized set. On average over all parameters, the improvement in SD was a factor 1.55. A statistically significant difference in mean was only seen for D* (P = .01). A nonphysiological mean for K (K < 0) was found in 3 volunteers for the reference set and in 1 volunteer for the optimized set of 20 b values. Additionally, a nonphysiological mean for f (f < 0) was found in 1 volunteer in the reference set and in none of the volunteers in the optimized set of 20 b values. Boxplots of the difference in mean of the optimized set versus the reference set can be found in Supporting Information Figure S1.
3.1.2 The effect of the number of b values on parameter precision
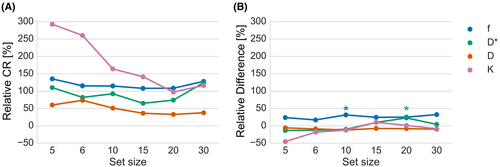
Figure 4 shows relDifference and relCR each for set size. The relCR of D was 37.7% for a set size of 30 and varied only slightly for set sizes of 20 and 15. However, for set size smaller than 15 b values, a considerable increase in relCR was observed up to 73.7% for a set size of 6. The pattern in D* was similar to D, with relCR ranging from 74.3% in the set size of 20 b values up to 110.4% in the set size of 5 b values. However, the relCR of D* in set size 30 was 124.2%, and therefore notably higher than any of the other set sizes. For f, the relCR was lowest in set size 15 and 20 and slightly higher in the other set sizes. A slight decrease from 116.4% to 97.7% in relCR was seen in set size 20 compared to 30 in K. When removing more b values from the set, relCR of K increased continuously up to 292.8% in the set of 5 b values. Significant differences in parameter mean were found in the set size 10 for f (31.6%) and the set size 20 for D* (23.0%). However, the relDifference of f was substantial with a range of 17.1% to 31.6% in all set sizes. Boxplots of the bias of set size 30 versus the other set sizes can be found in Supporting Information Figure S2.
3.2 Registration
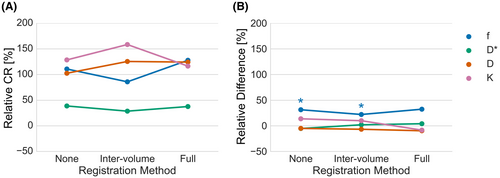
When only intervolume registration was applied, correction of severe registration errors was necessary. No registration errors were found in case intravolume registration was also applied. Figure 5 shows relDifference and relCR for each registration scenario. Intervolume-only registration showed a smaller relCR by 25% to 40% in f compared to the other scenarios. A similar effect was seen in D, where the decrease was around 10%. However, the opposite effect was seen in D* and K, where relCR increases of 1% to 23% and 30% to 42% were observed, respectively. Significant differences in parameter mean were found for no registration (31.5%) and intervolume-only registration (22.2%) for f, leaving the full registration as the only scenario without significant difference in parameter mean in any parameter. Nevertheless, the 32.5% relDifference for f in the full registration scenario was still substantial. Boxplots of the difference in mean of the fully registered set versus the other 2 registration procedures can be found in Supporting Information Figure S3. Parameter maps for each registration scenario and parameter are shown for 1 volunteer in Supporting Information Figure S4.
3.3 Swallowing artifact rejection
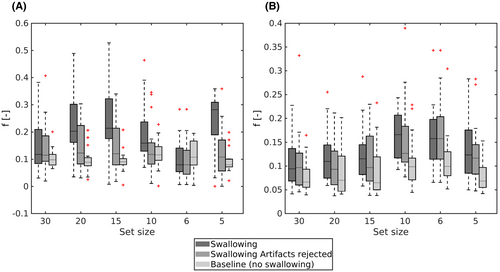
Table 4 shows the average percentage of rejected b value volumes per set size due to swallowing artifact presence. Significant differences in ROI mean between artifact-rejected and nonartifact-rejected data were found for a set size of 5 for all parameters (f: P = .001, D*: P = .007, D: P = .0005, K: P = .003), as well as in set sizes 10 (P = .01), 15 (P = .008), and 20 (P = .008) for f and set size 15 (P = .006) and 20 (P = .03) for D. In all mentioned cases, except D, a significant difference in ROI mean was observed between the nonrejected data and the second optimized acquisition. This difference was resolved after artifact image rejection, as is illustrated by Figure 6 for the mean perfusion fraction, indicating repeatability is improved after artifact image rejection. Boxplots for the other parameters can be found in Supporting Information Figure S5. Supporting Information Figure S6 contains scatter plot of the data before and after swallowing rejection plotted versus the baseline for all parameters and set sizes.
| Set size | Average amount rejected b values (%) |
|---|---|
| 30 | 9.6 |
| 20 | 15.3 |
| 15 | 15.0 |
| 10 | 12.5 |
| 6 | 4.2 |
| 5 | 16.3 |
4 DISCUSSION
Hybrid IVIM-DKI is promising for response assessment of head and neck cancer during (chemo)radiotherapy.3, 4 However, inefficient sampling of b values and motion corruption hamper precise parameter estimation in hybrid IVIM-DKI and thus diminish its potential for response assessment. In this paper, we showed that acquisition optimization and motion correction improves parameter estimation precision of the parameters of interest. Additionally, parameter repeatability metrics did not improve for sets larger than 15 b values, suggesting that 15 b values is sufficient. Possibly because image acquisition noise is not the dominant error term for larger sets. No benefit of registration was found in our data, but swallowing artifact image rejection was beneficial, especially if a larger part (>10%) of the data is affected by such artifacts. To the best of our knowledge, this is the first study that addressed combined acquisition optimization and motion correction for hybrid IVIM-DKI in head and neck.
Most b values in the optimal sets were near or above 800 s/mm2 (restricted diffusion regime), followed by b values below 200 s/mm2 (perfusion regime), whereas relatively little b values were chosen in between in the free diffusion regime. Our findings suggest that the distribution of the b values reflects the uncertainty in the main parameters that rely on each regime. D was the most reliable parameter, followed by f and D*. K relies mainly on high b values and was the least reliable parameter.
Using a higher maximum b value than 1500 s/mm2 is expected to improve the precision of K as long as the signal intensity at the maximum b value is well above the noise floor to avoid parameter estimation bias. Since the model predicts an increase in signal at very high b values, it is not expected that the optimization reaches a natural maximum b value. Instead, it would venture into b values where the kurtosis model is not valid. In this work, we chose a maximum b value of b = 1500 s/mm2 due to the limited SNR at this b value in the head and neck region at 1.5 tesla to avoid the noise floor and the related parameter estimation bias.
Previous studies on b value optimization were carried out for the monoexponential DWI model7-9 and the IVIM model.14, 15, 17 These studies have largely reported an amount of unique b values equal to the minimum of b values needed to estimate all parameters. In this work, however, sets larger than 5 b values always consisted of more than 5 unique b values. Nonetheless, in the monoexponential model Brihuega-Moreno et al9 also reported more variation in b values than the minimum needed for estimation; however, this was in the presence of large parameter ranges and without maximum b value constraint. This could be in line with this study because the parameter ranges applied in this study were quite broad, and due to inclusion of K, the b value range was also larger. Additionally, a larger distribution was seen in more complex problems, for example, in optimizing gradient strengths and directions for diffusion kurtosis imaging.28 Yet, even then discretization is seen.
The optimization technique discussed in this paper consists of a general framework and can be tailored to specific interests by weighting the parameters or even choosing another model. We chose an equal weighting for all the parameters of interest (f, D*, D, and K). However, if there is no interest in 1 specific parameter, it can be left out of the optimization. Similarly, if 1 parameter is more important than the others, a higher weight can be assigned to this parameter. Therefore, specifying the objective function for b value optimization should be adapted according to specific interests and is therefore subjective in nature.17
Standardization of DWI across imaging systems is challenging.29 The presented optimization framework depends on 2 parameters that are system-dependent: SNR and TE. The b value set is quite robust to SNR, with only about 1% variation in cost between the set optimized in this manuscript and the set optimized at an SNR of 15 to 25. Because the TE-correction primarily affects simulated SNR, it is expected that the b value set is still relatively optimal, even if the TE used and SNR achieved are slightly different across systems. Therefore, the optimal b value sets that we propose could be of interest as a first step toward standardization.
Compared to the reference b value set of 20 b values, the optimal b value set showed superior SD in f, D, and K, and slightly inferior SD in D*. This indicates that precision is improved because the underlying physiological variation was assumed to remain constant within the scan session. Interestingly, based on the relative CRLB, a higher variation of a factor 2.2 for K was expected in the reference set compared to the optimized set and a slightly decreased variation in f, D, and D* (factor 0.5, 0.59, and 0.56, respectively) in the reference set compared to the optimized set. Thus, the actual optimized set performed better than expected for D and f, showing there are more factors at play than the image noise described by the CRLB. Repeatability could not be assessed because a repeat of the reference scan was not acquired due to scan time limitations.
The repeatability of all parameters seems worse for the set of 30 b values than for the set of 20 b values. This seems counterintuitive but is likely due to longer scan time, resulting in an opportunity for motion and other effects corrupting the data that could eventually lead to worsening the parameter estimation. The registration method that we applied was not sufficient to account for all the time-related effects. Also, no clear impairment in repeatability metrics was observed for any of the parameters when moving from a set of 20 to a set of 15 b values. A tradeoff between scan time and parameter repeatability was only observed when imaging with less than 15 b values.
Repeatability is expected to improve in patients due to higher SNR often encountered in tumors than in healthy tissue for diffusion imaging. This is because healthy tissue has a lower intravoxel water content than nonnecrotic tumor tissue. Therefore, even if the measurement variation in f, D*, and K are high, they might become acceptable in patients. Especially K is expected to improve because it mainly relies on high b values where the increase in SNR has the highest impact.
No clear advantage is shown for applying inter- and intravolume registration in this work. This might be because volunteers generally lie very still, which would not necessarily be the case in patients. It might also be that once the estimation is based on a multi-b value fitting, a sufficient number of b values make the estimation robust enough regarding motion. However, a side effect of intravolume registration is smoothing of the b value images. This could lead to smoothing of underlying physiological differences and might be problematic when assessing intratumor heterogeneity.
In this study, we have shown that swallowing artifact image rejection improves the accuracy of parameter estimation. Previously, Chevallier et al30 already showed that rejection of motion corrupted volumes is beneficial for estimation of IVIM parameters. In this paper, the clearest effect is seen in the perfusion fraction, especially in set sizes 20, 15, 10, and 5. This could be because that the relative signal drop is larger in low b values (b < 200 s/mm2) than in higher b values. Furthermore, our results suggest that there is a threshold for the amount of artifacts of around 10% that need to occur before swallowing artifact image rejection is worthwhile. In set size 6 and 30, no strong swallowing artifact image rejection effect was observed, whereas in a set size of 5, 10, 15, and 20, more than 10% of data was rejected and a clear effect is seen in the perfusion fraction.
Because swallowing artifacts can severely hamper parameter estimation, swallowing artifact rejection is beneficial. An automated strategy for swallowing artifact rejection, such as proposed by Gurney-Champion et al,31 could be a relatively simple way to implement this procedure in clinical practice. As a topic for further research, the effect of swallowing artifact correction in the head and neck region could be investigated, for example, using a combined principal component analysis and temporal maximum intensity projection approach PCATMIP.32
The main limitation of this work is that it was performed on healthy volunteers for whom the SNR at high b values was relatively low compared to tumor tissue. Another limitation is that a correction for multiple comparisons was not applied. For these 2 reasons, the results should be independently validated in a larger cohort with head and neck cancer patients.
5 CONCLUSION
The effect of b value optimization, protocol efficiency, registration, and swallowing artifact image rejection on parameter precision of hybrid IVIM-DKI was assessed. Optimization of b values is recommended because it improved the precision of 3 (D, f, K) out of 4 parameters of interest compared to the reference set. The b value set of 15 images (b = 1 × 0, 1 × 10, 2 × 80, 1 × 130, 1 × 570, 2 × 770, 2 × 780, 1 × 790, and 4 × 1500) yielded the optimal tradeoff between scan time and parameter precision, with a repeatability comparable to the set of 30 in half the scan time. No clear advantage of image registration was demonstrated. However, swallowing artifact image rejection was beneficial when more than roughly 10% of the images contained artifacts.
CONFLICT OF INTEREST
This work was funded by a research grant from Elekta AB, Stockholm, Sweden. Erasmus MC Cancer Institute also has a research collaboration with Accuray Inc, Sunnyvale, California, USA.
Open Research
DATA AVAILABILITY STATEMENT
The b value optimization code that supports the findings of this study is openly available in IVIMDKI_b-value_optimization at github.com/nsijtsema/IVIMDKI_b-value_optimization, reference number d0d6fcf4058b9bd56144b884aa042f2f1a469f29.




